Abstract
Context: Tuberculosis is primarily caused by Mycobacterium tuberculosis (Mtb). Previous studies have shown that the dichloromethanic extract of Ambrosia confertiflora DC (Asteraceae) inhibited Mtb.
Objective: To isolate the compounds responsible for the mycobactericidal activity against clinical Mtb strains.
Materials and methods: The dichloromethanic extract of aerial parts of A. confertiflora was separated using chromatography columns. Mycobactericidal activity of the isolated compounds was evaluated using the Alamar Blue bioassay (128–16 μg/mL, 7 days). Cytotoxicity was tested against normal cell line L929 using the MTT ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium]) assay (100–3.125 μg/mL, 48 h). Compound structures were elucidated by 1H and 13C uni- and bidimensional NMR.
Results: Two sesquiterpene lactones (SQLs) with mycobactericidal activity were identified: santamarine and reynosin. Reynosin was the most active compound, with a minimal bactericidal concentration (MBC) of 128 μg/mL against the H37Rv, 366-2009 and 104-2010 Mtb strains and a minimal inhibitory concentration (MIC) of 64, 64, 128, 128 and 128 μg/mL against the H37Rv, 104-2010, 63-2009, 366-2009 and 430-2010 Mtb strains, respectively. Santamarine had MBCs of 128 μg/mL against the H3Rv and 104-2010 Mtb strains and MICs of 128 μg/mL against the H37Rv, 366-2009 and 104-2010 Mtb strains. We also isolated 1,10-epoxyparthenolide but only showed mycobacteriostatic activity (MIC 128 μg/mL) against the Mtb strain. Compounds were tested against the L929 cell line and the calculated selectivity index was <1.
Discussion and conclusions: This is the first report of the mycobactericidal activity of these compounds against clinical Mtb strains. It is also the first report of the isolation of 1,10-epoxyparthenolide from A. confertiflora. The anti-mycobacterial activity of A. confertiflora was attributed to the SQLs identified.
Introduction
Tuberculosis (TB) is an infectious disease that is caused by the bacillus Mycobacterium tuberculosis (Mtb) or any other member of the Mycobacterium tuberculosis complex (MTC) (Ueyama et al. Citation2014). In 2013, 6.1 million TB cases were reported by World Health Organization (WHO); among them, 5.7 million cases were newly diagnosed, and another 0.4 million were people already on treatment (WHO Citation2014). The currently recommended WHO treatment regimen for new cases of drug-susceptible TB is a six-month regimen consisting of first-line drugs with treatment success rates of at least 85%; however, drug-resistant TB (DR-TB) threatens global TB control and is a major public health concern in several countries (WHO Citation2014).
Multidrug-resistant TB (MDR-TB) is defined as strains resistant to isoniazid and rifampin, while extensively drug-resistant TB (XDR-TB) is resistant to three of the six classes of second line agents (Lawn & Wilkinson Citation2006). Additionally, there are some reports of Iranian and Indian TB patients with “totally drug-resistant” (TDR) strains that exhibit patterns of drug resistance to all first-line (isoniazid, rifampin, ethambutol, pyrazinamide and streptomycin) and second-line drugs (kanamycin, amikacin, capreomycin, ofloxacin, moxifloxacin, ethionamide and para-amino salicylic acid) against which they were tested. No changes in the current guidelines for the design of treatment regimens for patients with broad patterns of resistance have been recommended by the WHO; nevertheless, improvements in drug susceptibility testing and the release of innovative new drugs could change this position in the future (Velayati et al. Citation2009; Udwadia et al. Citation2012; WHO Citation2012).
In our previous studies, we examined the anti-mycobacterial activity of organic extracts of nine Sonoran (Mexico) medicinal plants. The most active plant of the studied group was Ambrosia confertiflora DC (Asteraceae) that exhibited a minimal inhibitory concentration (MIC) of 120 μg/mL (Robles et al. Citation2013). A. confertiflora grows in the northern and central regions of Mexico (including the Sonoran desert ecosystem), United States of America and the Caribbean territories (ITIS Citation2014; SEINet Citation2014). This perennial plant is commonly known as “estafiate” or chi’ichibo by the Mayo and Yaqui Sonoran ethnic groups and is traditionally used for intestinal parasites, stomachache, hemorrhoids and other illnesses (Johnson et al. Citation1996). The majority of the species from the Asteraceae (or Compositae) family, to which the Ambrosia genus belongs, are characterized by their high and structurally diverse sesquiterpene lactone (SQLs) content. SQLs are naturally occurring plant terpenoids that are used for taxonomic analysis (Seaman Citation1982). Many biological activities have been described for the A. confertiflora SQLs, including larvicidal, antimicrobial, antifungal, antiplasmodial and antiproliferative activities (Picman Citation1986; Pan et al. Citation2011; Robles et al. Citation2011; Duraipandiyan et al. Citation2012; de la Torre et al. Citation2013; Salazar et al. Citation2014). Earlier studies on the aerial parts of A. confertiflora reported more than 12 SQLs (Yoshioka et al. Citation1970); mycobacterial inhibition has been reported for some of these SQLs (Fischer et al. Citation1998). Due to the biological activity previously demonstrated for A. confertiflora (Robles et al. Citation2013) and the biological activity attributed to some of the SQL compounds isolated from this species (Fischer et al. Citation1998; Cantrell et al. Citation2001), we chose to study this interesting plant that grows in our country and promises to yield compounds with mycobactericidal activity.
Materials and methods
Plant material
Ambrosia confertiflora was collected in Hermosillo, Sonora, México, in October 2010. The plant was botanically authenticated at the Herbarium of the University of Sonora by Professor Jesús Sánchez-Escalante. A voucher specimen was deposited with the code number 17401.
Preparation of the dichloromethanic extract of A. confertiflora
The aerial parts (1 kg) were air-dried, ground to a powder and allowed to macerate in dichloromethane (CH2Cl2) (Sigma-Aldrich, St. Louis, MO) (1×; 10 L) at room temperature for 10 d. After filtration, the extract was concentrated under low pressure at 40 °C in a Yamato RE300 rotator evaporator (Yamato Scientific America, Inc., Santa Clara, CA).
Extraction and isolation of compounds
The CH2Cl2 crude extract of A. confertiflora (4 g) was chromatographed over a silica gel column (120 g, 70–230 mesh, i.d. 3.2 × 40 cm) and eluted with a gradient solvent system (elution order: 100% n-hexane, 10, 15, 40 and 70% ethyl acetate (EtOAc) in n-hexane, 100% EtOAc, 10, 20 and 50% EtOAc in methanol (MeOH), and 100% MeOH) to obtain 34 fractions (F-1 to F-34). All solvents were purchased from Sigma-Aldrich (St. Louis, MO). All fractions were subjected to evaluation for anti-mycobacterial activity against Mtb H37Rv by the microplate Alamar Blue® (AbD Serotec, Raleigh, NC) assay. The most active fractions (F12 and F22) were eluted with an n-hexane-EtOAc mixture (85:15) and used as a reference for further isolation of the most abundant compounds present in each fraction.
To diminish the amount of chlorophylls, 8 g of the dichloromethane crude extract was dissolved in methanol; then, 2 g of activated charcoal was added (Scheepers et al. Citation2011). After brief agitation and further filtration, the solvent was removed in a rotary evaporator and the residue (5.73 g) was chromatographed on a silica gel (230–400 mesh; i.d. 4.8 × 30 cm) by employing mixtures of hexane-ethyl acetate as eluents (elution order: 100% n-hexane and 5, 7.5, 10, 12.5, 15, 17.5, 20 and 60% EtOAc in n-hexane) to obtain 9 fractions (F1A-F9A). F5A (280 mg) was re-chromatographed on 2.5 g of silica gel (230–400 mesh) column chromatography (i.d. 1.5 cm ×20 cm) with n-hexane and EtOAc mixtures as the eluents (5, 7.5 and 10% EtOAc in n-hexane) to obtain 7 fractions (F5A-1 to F5A-7). F5A-1 yielded 35.1 mg (0.61%) of compound 1, and F5A-4 yielded 32.3 mg (0.56%) of compound 2. F7A (650 mg) was also fractionated on 5 g of silica gel (230–400 mesh) column chromatography (i.d. 1.5 cm ×20 cm) to obtain 10 fractions (F7A-1 to F7A-10) using n-hexane and EtOAc as the eluents (5 and 7.5% of EtOAc in n-hexane and 100% EtOAc). Fraction F7A-8 yielded 187.2 mg (3.27%) of compound 3.
Baljet reaction
To identify SQLs the Baljet reaction was used: a 1:1 mixture of picric acid (1% in ethanol) and NaOH (10% wt/vol) solutions freshly prepared was added to 2–3 mg of sample. A positive reaction was evidenced by a color shift to red or orange (Silva et al. Citation1998).
Analytical procedures
1H and 13C spectra were recorded on NMR spectrometer Varian Unity (300 MHz) (Varian Inc., Palo Alto, CA) and NMR spectrometer Bruker Avance III (100 and 400 MHz) (Bruker Corporation, Brillerica, MA) using deuterated chloroform (CDCl3) as the solvent.
Chemical shifts are given in parts per million (δ) downfield from internal tetramethylsilane (TMS). Signal patterns are indicated as follows: s, singlet; d, doublet; dd, double doublet; ddd, double double doublet and m, multiplet. Coupling constants (J) are given in hertz. 1H, 13C, distortion less enhancement by polarization transfer (DEPT), correlated spectroscopy (COSY), heteronuclear correlation (HETCOR), and heteronuclear single-quantum correlation (HSQC) coherence experiments were run using standard Varian pulse sequences. The isolated compounds 1–3, were purified by flash column chromatography on silica gel with a 70–230 mesh and 230–400 mesh using mixtures of EtOAc–hexane as the eluents.
HPLC-ESI-MS was performed on an Agilent model 1200 HPLC coupled to an Esquire 6000 (Bruker Daltonics, Brillerica, MA) mass spectrometer with an electrospray ion source (ESI) in the positive ion mode and an ion trap. The capillary voltage was set at 124 V. The scan ranged from 50 to 1000 m/z. The HPLC was run on a Zorbax Bonus reversed phase column C18 (100 × 2.1 mm) with a particle size of 3.5 μm.
The mobile phase consisted of two solvents: water (0.1% acetic acid) and methanol. The run started with 80% water for 10 min and increased to 100% methanol at 30 min; the flow rate was 0.2 mL/min. The solvents used for HPLC analysis were HPLC grade, while all other chemicals and solvents were analytical grade.
The purification was monitored through TLC in silica gel plates 60 F254 (Merck, Kenilworth, NJ) with hexane/ethyl acetate 6:4 as mobile phase, further visualized in a UV cabinet with a 254 and 365 nm lamp (Spectroline; Fisher Scientific, Waltham, MA), and revealed with a solution of ceric sulfate.
Mycobacterium tuberculosis strains
The evaluation of bactericidal activity was performed using six clinical Mtb strains isolated and provided by the State of Sonora Public Health Laboratory (LESP-SON): 51 (2009), 63 (2009), 318 (2009), 366 (2009), 104 (2010) and 430 (2010). All of the strains were susceptible to first-line anti-tuberculosis drugs (streptomycin, isoniazide, rifampin, ethambutol and pyrazinamide). The reference strain Mtb H37Rv (sensitive to all of the first-line drugs mentioned above) provided by the National Institute of Epidemiological Diagnosis and Reference of the Mexican Ministry of Health (InDRE) and maintained at LESP-SON was used as a control strain.
Preparation of the Mycobacterium tuberculosis inoculum
A bacterial suspension was prepared in saline solution (0.85% NaCl) to achieve 1 McFarland standard adjusted by turbidimeter (Orion Aquafast AQ 4500, Thermo Scientific, Waltham, MA) measurement. A working solution was prepared with a 1:25 dilution in Middlebrook 7H9 broth (Sigma-Aldrich, St. Louis, MO) supplemented with 0.2% (v/v) glycerol and 10% (v/v) OADC (oleic acid, albumin, dextrose, catalase enrichment; Becton Dickinson, Franklin Lakes, NJ) to obtain a bacterial concentration of 3.0 × 108 colony-forming units (CFU)/mL (Robles et al. Citation2013).
In vitro anti-mycobacterial activity assay
Determination of the minimal inhibitory concentration
To evaluate the anti-mycobacterial activity, the redox microplate Alamar Blue® (AbD Serotec, Raleigh, NC) assay (MABA) was performed as described by Franzblau and collaborators (Citation1998). The assay was performed on 96-well polystyrene flat bottom plates with low evaporation cover lids. First, 200 μL of sterile distilled water was added to the perimetral wells and 100 μL of Middlebrook 7H9 broth supplemented with OADC was added to the remaining wells. The working solutions of compounds (512 μg/mL) were distributed into the first well of each row, from which a 2-fold dilution series was made using the following three wells. The final concentrations in-test ranged from 128 to 16 μg/mL. Then, 100 μL of the Mtb inoculum was added. Rifampin (Sigma Aldrich, St. Louis, MO) was used (15–9.15 × 10−4 μg/mL) as a control. Simultaneously, a 1:100 dilution prepared from the bacterial inoculum (representing the growth of 1% of the bacterial population) and a growth control were included. As negative controls, 200 μL of culture medium alone and 200 μL of the maximum tested concentration of the compounds (without bacteria) were used. The plates were incubated at 37 °C. After 5 d of incubation, 50 μL of a fresh Alamar Blue-10% Tween 80 (Sigma Aldrich, St. Louis, MO) mixture (1:1) was added to each control well. The microplate was sealed with parafilm and reincubated for 24 h at 37 °C. If the negative controls remained blue and the positive controls turned pink, the reagent mixture was added to all of the wells in the microplate. The microplates were resealed with parafilm and incubated for an additional 48 h; then, the colors of all wells were recorded. Finally, the visual MICs were defined as the lowest compound or antibiotic concentration at which no color change in the indicator was evident (Collins & Franzblau Citation1997). Three separate experiments were performed.
Determination of the minimal bactericidal concentration (MBC)
Mycobactericidal effects were assessed for compounds with an MIC ≤128 μg/mL as described by Molina-Salinas et al. (Citation2006). Briefly, 5 μL of the undeveloped duplicates of mycobacterial suspensions and controls were transferred to a new microplate that contained 195 μL of fresh culture medium per well. The microplates were incubated and developed with Alamar Blue as described for the MABA. The MBC corresponded to the minimum compound concentration that did not cause a color shift in cultures reincubated in fresh medium.
Cell viability assay and selectivity index
Cell viability was evaluated by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] reduction assay with some modifications (Mosmann Citation1983; Valencia et al. Citation2012) as reported by Rascón et al. (Citation2015). L929 (murine subcutaneous connective tissue) purchased from the American Type Culture Collection (ATCC, Rockville, MD) was used as the normal cell line. The selectivity index (SI) was calculated by dividing the 50% inhibitory concentration (IC50) by the MIC; if the SI was >10, the compound was considered selective (Orme et al. Citation2001).
Results
The bioassay-guided fractionation of the crude dichloromethanic extract of A. confertiflora resulted in 35 chromatographic fractions. Fractions F12 and F22 eluted with a hexane–ethyl acetate mixture (85:15) were the most active fractions, with MICs of 64 and 128 μg/mL, respectively. These fractions were used as a reference for the further isolation of the most abundant compounds present in each, where a more efficient extraction procedure (activated charcoal chlorophyl reduction of the dichloromethanic extract) was applied prior the chromatographic fractionation (Scheepers et al. Citation2011). The possible presence of SQLs in the samples was monitored through the Baljet reaction, while the confirmation of the structures was done by NMR.
Spectral identification of compounds
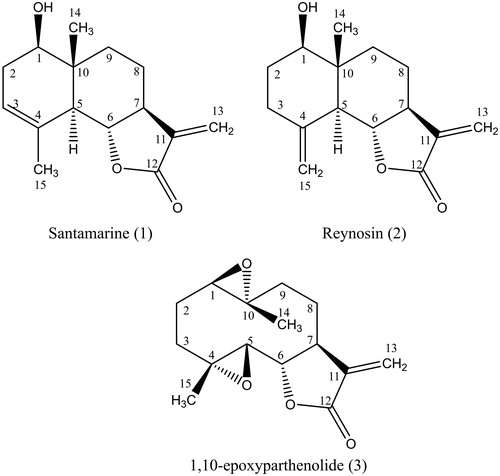
As a result of the purification process, we obtained the SQLs santamarine (1), reynosin (2) and 1,10-epoxyparthenolide (3). The structures of these isolated compounds () were determined by NMR and confirmed by comparison of the obtained spectroscopic data with data previously reported in the literature (Yoshioka et al. Citation1970; Ahmed & Abdelgaleil Citation2005; Fang et al. Citation2005).
Santamarine (1): C15H20O3, 1H NMR (CDCl3, 400 MHz) δ 3.68 (1H, dd, J = 9.9, 6.6 Hz, H-1), δ 1.98 (1H, m, H-2), δ 2.39 (1H, m, H-2), δ 5.35 (1H, m, H-3), δ 2.37 (1H, m, H-5), δ 3.95 (1H, t, J = 11 Hz, H-6), δ 2.50 (1H, m, H-7), δ 1.64 (1H, m, H-8), δ 2.05 (1H, m, H-8), δ 1.29 (1H, m, H-9), δ 2.07 (1H, m, H-9), δ 5.41 (1H, d, J = 3.1 Hz, H-13), δ 6.08 (1H, d, J = 3.2 Hz, H-13), δ 0.88 (3H, s, H-14), δ 1.84 (3H, s, H-15); 13C NMR (CDCl3, 100 MHz) δ 75.4 (C-1), δ 32.9 (C-2), δ 121.4 (C-3), δ 133.6 (C-4), δ 51.3 (C-5), δ 81.7 (C-6), δ 51.2 (C-7), δ 21.3 (C-8), δ 34.4 (C-9), δ 41.0 (C-10), δ 139.1 (C-11), δ 171.0 (C-12), δ 117.0 (C-13), δ 11.2 (C-14), δ 23.5 (C-15).
Reynosin (2): C15H20O3, 1H NMR (CDCl3, 300 MHz) δ 3.53 (1H, dd, J = 11.5, 4.6 Hz, H-1), δ 1.59 (1H, m, H-2), δ 1.85 (1H, m, H-2), δ 1.38 (1H, m, H-3), δ 2.10 (1H, m, H-3), δ 2.12 (1H, m, H-5), δ 4.03 (1H, t, J = 10.9 Hz, H-6), δ 2.54 (1H, m, H-7), δ 1.59 (1H, m, H-8), δ 2.09 (1H, m, H-8), δ 2.34 (1H, ddd, J = 13.7, 5.1, 2.1 Hz, H-9), δ 2.12 (1H, m, H-9), δ 6.09 (1H, d, J = 3.2 Hz, H-13), δ 5.41 (1H, d, J = 3.0 Hz, H-13), 0.82 (3H, s, H-14), 4.87 (1H, d, J = 0.9 Hz, H-15), 4.99 (1H, d, J = 0.9 Hz, H-15); 13C NMR (CDCl3, 300 MHz) δ 78.4 (C-1), δ 31.5 (C-2), δ 35.9 (C-3), δ 142.7 (C-4), δ 53.2 (C-5), δ 79.8 (C-6), δ 49.8 (C-7), δ 21.7 (C-8), δ 33.7 (C-9), δ 43.2 (C-10), δ 139.5 (C-11), δ 170.8 (C-12), δ 117.2 (C-13), δ 11.8 (C-14), δ 110.9 (C-15).
1,10-Epoxyparthenolide (3): C15H20O4, ESI-MS, m/z 287.1 [M + Na]+, m/z 551.3 [2M + Na]+; 1H NMR (CDCl3, 400 MHz) δ 2.86 (1H, dd, J = 10.8, 1.6 Hz, H-1), δ 1.60 (1H, m, H-2), δ 2.22 (1H, m, H-2), δ 1.43 (1H, m, H-3), δ 2.23 (1H, m, H-3), δ 2.91 (1H, d, J = 8.9 Hz, H-5), δ 3.94 (1H, t, J = 8.9 Hz, H-6), δ 2.75 (1H, m, H-7), δ 1.51 (1H, m, H-8), δ 2.22 (1H, m, H-8), δ 1.24 (1H, m, H-9), δ 2.48 (1H, dd, J = 14.2, 7.7 Hz, H-9), δ 5.63 (1H, d, J = 3.3 Hz, H-13), δ 6.34 (1H, d, J = 3.7 Hz, H-13), δ 1.40 (3H, s, H-14), δ 1.35 (3H, s, H-15); 13C NMR (CDCl3, 100 MHz) δ 63.7 (C-1), δ 26.0 (C-2), δ 35.1 (C-3), δ 60.5 (C-4), δ 64.6 (C-5), δ 81.8 (C-6), δ 47.7 (C-7), δ 24.0 (C-8), δ 40.1 (C-9), δ 60.7 (C-10), δ 138.9 (C-11), δ 168.8 (C-12), δ 121.3 (C-13), δ 17.1 (C-14), δ 17.5 (C-15).
HPLC-ESI-MS
An HPLC-ESI-MS analysis was performed to determine the purity and molecular weight of compound 3 (not reported previously in A. confertiflora). The chromatogram showed that compound 3 was obtained with a retention time of 10.9 min and a percentage purity of 99.4%.
In vitro mycobactericidal activity assay
The isolated SQLs santamarine (1), reynosin (2) and 1,10-epoxyparthenolide (3) were tested for their mycobactericidal activity and inhibitor effect against six clinical strains and the reference strain Mtb H37Rv.
Reynosin was the most active compound, with an MBC of 128 μg/mL and MICs ranging from 64 to 128 μg/mL. Santamarine exhibited a MBC and a MIC of 128 μg/mL, while 1,10-epoxyparthenolide remained inactive against the six clinical strains (). Compounds 1 and 2 presented MBCs of 128 μg/mL against the reference strain (Mtb H37Rv), but compound 3 did not. Compounds 1, 2 and 3 exhibited a MIC of 128, 64 and 128 μg/mL, respectively.
Table 1. Antimycobacterial effects from compounds 1–3 against six sensitive Mycobacterium tuberculosis clinical strains and M. tuberculosis H37RvTable Footnotea.
Cell viability assay and selectivity index
Compounds 1, 2 and 3 exhibited IC50 values against L929 of 20.2 ± 1.9, 33.2 ± 0.4 and 8.7 ± 0.3 μg/mL, respectively, and their selectivity indices ranged from 0.07 to 0.52 ().
Table 2. Cytotoxicity and selectivity index of compounds 1–3.
Discussion
Natural products represent an interesting and promising option in the search for new anti-tubercular agents. Terrestrial plants have been the object of chemical studies, and their extracts commonly yield secondary metabolites of terpenoid origin (Copp Citation2003), such as those here reported.
Over 50 sesquiterpenes with natural and synthetic origins showing anti-mycobacterial MICs ranging from 2 μg/mL to >128 μg/mL were reported by Cantrell and collaborators (2001). Previously, santamarine, reynosin and 1,10-epoxyparthenolide presented inhibitory activity against Mtb H37Rv at MICs of 64, 64 and 128 μg/mL, respectively (Fischer et al. Citation1998), which were similar to the MICs obtained in our study (128, 64 and 128 μg/mL, respectively). These results are comparable to ours; the difference in the activity of santamarine can be explained by the fact that the Mtb H37Rv strains are not identical. We found no bibliographic report of the mycobactericidal activity of these compounds against clinical strains of Mtb or Mtb H37Rv. Therefore, we performed this study to determine whether these SQLs also presented bactericidal activity. Our findings demonstrated that compounds 1 and 2 exerted mycobactericidal activity; in contrast with some frontline anti-TB drugs (i.e., ethambutol) and second line drugs (i.e., ethionamide, p-amino salicylic acid and cycloserine) that are only bacteriostatic (Coll Citation2009).
Reynosin and santamarine are germacranolide-type SQLs. These SQLs were first identified in A. confertiflora and Chrysanthemum parthenium (L.) Pers. (Asteraceae), respectively (Romo de Vivar & Jiménez Citation1965; Yoshioka et al. Citation1970). In contrast, 1,10-epoxyparthenolide was isolated from Anthemis melampodina Delile (El-Alfy et al. Citation1989) and an epoxydation derivative prepared from parthenolide (El-Feraly & Benigni Citation1980); however, this was the first report of its presence in A. confertiflora. These SQLs present many biological activities. For example, reynosin has been previously reported to exhibit cytotoxic activity over the myeloid leukemia cell lines HL-60 and U937 (Rivero et al. Citation2003), a protective effect (at low concentrations) on thioacetamide-induced mouse hepatocyte damage (Lim et al. Citation2013) and antiprotozoal activity (Schmidt et al. Citation2009). Santamarine has presented anti-inflammatory (Choi et al. Citation2012), anticancer (Ma et al. Citation2009) and trypanocidal activities (Asaruddin et al. Citation2003). Finally, 1,10-epoxyparthenolide has demonstrated antifungal effects (Ahmed & Abdelgaleil Citation2005), and its herbicidal activity has been evaluated (Macías et al. Citation1999).
The anti-mycobacterial activity of the A. confertiflora batch collected for our study was attributed to SQLs 1, 2 and 3 isolated in the biodirected assay. Importantly, other SQLs previously isolated from A. confertiflora (i.e., parthenolide, deacetylconfertiflorin and confertiflorin) have also been demonstrated to possess anti-mycobacterial activity, with MICs of 16, 128 and 128 μg/mL, respectively (Yoshioka et al. Citation1970; Fischer et al. Citation1998; Cantrell et al. Citation2001); however, we did not isolate these SQLs in our sample. This discrepancy could be explained by the results of Seaman and Mabry (Citation1979), who reported that some Ambrosia species from the Sonoran Desert varied their SQL chemistry depending on the collection site.
To determine the selectivity of our molecules, a cytotoxicity assay was performed in the L929 cell line. The SI for all of the samples was <1, which indicated that 1, 2 and 3 possessed very low selectivity towards the normal cell line; 1 and 2 were about 6 and 4 times more toxic to L929 cell line than to Mtb strains. This was definitely not a desirable effect; unfortunately, adverse effects produced by anti-tuberculosis drugs are quite common, and some of them can be life-threatening (e.g., nephrotoxicity, cardiotoxicity, gastrointestinal toxicity and central nervous system toxicity) (Ramachandran & Swaminathan Citation2015).
Previous studies concluded that there was a clear dependence between the anti-mycobacterial activity and the presence of the α-methylene γ-lactone group (Picman Citation1986), this activity can be improved by chemical modifications (Cantrell et al. Citation2001).
Reynosin and santamarine were bactericidal compounds; however, 1,10-epoxyparthenolide remained inactive against all of the clinical strains tested (with the exception of the reference Mtb H37Rv strain). None of the compounds tested had a mycobactericidal activity higher than rifampin (positive control).
As noted above, it is important to investigate medicinal plants and identify active compounds against Mtb because even when they do not have a direct ethnobotanical use for respiratory diseases, they can yield interesting compounds with anti-mycobacterial activities (Robles et al. Citation2013). Organic extracts of medicinal plants may guide the future isolation and anti-mycobacterial evaluation of these active principles.
Based on our findings, we propose that SQLs might serve as structural templates for drug design in the search for new anti-mycobacterial compounds. However, chemical modifications must be done in order to increase its mycobactericidal activity and selectivity. Moreover, investigation into the exact bactericidal mechanism underlying action of these SQLs and studies of molecular docking with target enzymes (Maruthi & Suresh Citation2013) still need to be performed and might be the key to their development.
Conclusion
In the present investigation, we demonstrated that the SQL reynosin and santamarine displayed a mycobactericidal effect against two of the six clinical isolates tested and the reference Mtb H37Rv strain in addition to their previously reported inhibitory activity. While the SQL 1,10-epoxyparthenolide remained inactive against all of the strains tested, this is the first report of its presence in A. confertiflora. The results shown herein also confirm that the mycobactericidal activity of A. confertiflora is attributed mainly to SQL molecules.
Acknowledgements
The authors wish to thank to: InDRE for providing the Mtb H37Rv reference strain and the LESP-SON for allowing us to work in their BSL-3 laboratory and for all the support and facilities provided; Chemist María del Rosario Aguayo Verdugo and her work team at LESP-SON for the Mtb clinical strains; Professor Jesús Sánchez-Escalante from the Herbarium of the UNISON for the authentication of A. confertiflora; MSc. Heriberto Torres-Moreno for his support in the cytotoxicity evaluation; and Chemist Eréndira García Ríos and MSc. Lucía del Carmen Márquez Alonso for HPLC analysis. This research was supported by the Mexican Council of Science and Technology (CONACyT) PDCPN 2013-17215469.
Disclosure statement
The authors report no declarations of interest.
References
- Ahmed SM, Abdelgaleil SAM. 2005. Antifungal activity of extracts and sesquiterpene lactones from Magnolia grandiflora L. (Magnoliaceae). Int J Agr Biol. 7:638–642.
- Asaruddin MR, Honda G, Tsubouchi A, Nakajima-Shinada J, Aoki T, Kiuchi F. 2003. Trypanocidal constituents from Michelia alba. Nat Med. 57:61–63.
- Cantrell CL, Franzblau SG, Fischer NH. 2001. Antimycobacterial plant terpenoids. Planta Med. 67:685–694.
- Choi HG, Lee DS, Li B, Choi YH, Lee SH, Kim YC. 2012. Santamarin, a sesquiterpene lactone isolated from Saussurea lappa, represses LPS-induced inflammatory responses via expression of heme oxygenase-1 in murine macrophage cells. Int Immunopharmacol. 13:271–279.
- Coll P. 2009. Fármacos con actividad frente a Mycobacterium tuberculosis. Enferm Infecc Microbiol Clin. 27:474–480.
- Collins LA, Franzblau SG. 1997. Microplate Alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 41:1004–1009.
- Copp BR. 2003. Antimycobacterial natural products. Nat Prod Rep. 20:535–557.
- De la Torre RYC, Martínez EFR, Flores SAE, Waksman DTN, Salazar AR. 2013. Larvicidal and cytotoxic activities of extracts from 11 native plants from northeastern Mexico. J Med Entomol. 50:310–313.
- Duraipandiyan V, Al HNA, Ignacimuthu S, Muthukumar C. 2012. Antimicrobial activity of sesquiterpene lactones isolated from traditional medicinal plant, Costus speciosus (Koen ex.Retz.) Sm. BMC Complement Altern Med. 12:13.
- El-Alfy TS, Shehata AH, Koheil MA, Dahmy SA. 1989. Constituents of Anthemis melampodina growing in Egypt. Fitoterapia. 60:556–558.
- El-Feraly FS, Benigni DA. 1980. Sesquiterpene lactones of Laurus nobilis leaves. J Nat Prod. 43:527–531.
- Fang F, Sang SM, Chen KY, Gosslau A, Ho CT, Rosen RT. 2005. Isolation and identification of cytotoxic compounds from Bay leaf (Laurus nobilis). Food Chem. 93:497–501.
- Fischer NH, Lu T, Cantrell CL, Castañeda-Acosta J, Quijano L, Franzblau SG. 1998. Antimycobacterial evaluation of germacranolides in honour of Professor G.H. Neil Towers 75th birthday. Phytochemistry. 49:559–564.
- Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 36:362–366.
- ITIS. 2014. Integrated Taxonomic Information System [cited 2015 Jun 21]. Available from: http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value =36522
- Johnson J, Moreno SF, López R. 1996. Compendio fitoquímico de la medicina tradicional herbolaria de Sonora. Sonora: Universidad de Sonora Press.
- Lawn SD, Wilkinson R. 2006. Extensively drug resistant tuberculosis. Br Med J. 333:559–560.
- Lim S, Lee SJ, Nam KW, Kim KH, Mar W. 2013. Hepatoprotective effects of reynosin against thioacetamide-induced apoptosis in primary hepatocytes and mouse liver. Arch Pharm Res. 36:485–494.
- Ma G, Chong L, Li Z, Cheung AH, Tattersall MH. 2009. Anticancer activities of sesquiterpene lactones from Cyathocline purpurea in vitro. Cancer Chemother Pharmacol. 64:143–152.
- Macías FA, Galindo JCG, Castellano D, Velasco RF. 1999. Natural products as allelochemicals. 11. Sesquiterpene lactones with potential use as natural herbicide models (I): trans,trans-germacranolides. J Agric Food Chem. 47:4407–4414.
- Maruthi PP, Suresh KC. 2013. Antimycobacterial drug design: homology modeling and docking studies on Mycobacterium tuberculosis alanine racemase. Drug Discov. 3:27–36.
- Molina-Salinas GM, Ramos MC, Vargas J, Mata-Cárdenas BD, Becerril-Montes P, Said-Fernández S. 2006. Bactericidal activity of organic extracts from Flourensia cernua DC against strains of Mycobacterium tuberculosis. Arch Med Res. 37:45–49.
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63.
- Orme I, Secrist J, Anathan S, Kwong C, Maddry J, Reynolds R, Poffenberger A, Michael M, Miller L, Krahenbuh J, et al. 2001. Search for new drugs for treatment of tuberculosis. Tuberculosis drug screening program. Antimicrob Agents Chemother. 45:1943–1946.
- Pan E, Gorka AP, Alumasa JN, Slebodnick C, Harinantenaina L, Brodie PJ, Roepe PD, Randrianaivo R, Birkinshaw C, Kingston DGI. 2011. Antiplasmodial and antiproliferative pseudoguaianolides of Athroisma proteiforme from the Madagascar dry forest. J Nat Prod. 74:2174–2180.
- Picman AK. 1986. Biological activities of sesquiterpene lactones. Biochem Syst Ecol. 14:255–281.
- Ramachandran G, Swaminathan S. 2015. Safety and tolerability profile of second-line anti-tuberculosis medications. Drug Saf. 38:253–269.
- Rascón VLA, Jiménez EM, Velázquez CCA, Garibay EA, Medina JLA, Gámez MN, Robles ZRE. 2015. Antiproliferative and apoptotic activities of extracts of Asclepias subulata. Pharm Biol. 53:1741–1751.
- Rivero A, Quintana J, Eiroa JL, López M, Triana J, Bermejo J, Estévez F. 2003. Potent induction of apoptosis by germacranolide sesquiterpene lactones on human myeloid leukemia cells. Eur J Pharmacol. 482:77–84.
- Robles RE, Coronado EW, Velazquez CA, Ruiz BE, Navarro NM, Garibay EA. 2013. In vitro anti-mycobacterial activity of nine medicinal plants used by ethnic groups in Sonora, Mexico. BMC Complement Altern Med. 13:329.
- Robles RE, Velázquez CCA, Garibay EA, Gálvez RJC, Ruíz BE. 2011. Antimicrobial activity of Northwestern Mexican plants against Helicobacter pylori. J Med Food. 14:1280–1283.
- Romo de Vivar A, Jiménez H. 1965. Structure of santamarine, a new sesquiterpene lactone. Tetrahedron. 21:1741–1745.
- Salazar AR, Waksman MN, Flores SAE, de la Torre RYC. 2014. Use of Ambrosia confertiflora extracts as larvicide agents. Mex. Pat. Appl. MX 2012012779 A20140521.
- Scheepers JC, Malan SF, Du PJL, Van DS. 2011. The high performance liquid chromatography (HPLC) analysis of ultraviolet (UV) irradiated chlorophyll a and secondary plant compounds. Afr J Biotechnol. 10:16976–16985.
- Schmidt TJ, Nour AMM, Khalid SA, Kaiser M, Brun R. 2009. Quantitative structure – antiprotozoal activity relationships of sesquiterpene lactones. Molecules. 14:2062–2076.
- Seaman FC. 1982. Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot Rev. 48:121–595.
- Seaman FC, Mabry TJ. 1979. Sesquiterpene lactones and species relationships among the Shrubby Ambrosia Taxa. Biochem Syst Ecol. 7:105–114.
- SEINet. Southwest Environmental Information Network. 2014. Ambrosia confertiflora [cited 2015 Jun 21]. Available from: http://swbiodiversity.org/seinet/taxa/index.php?taxon=Ambrosia confertiflora.
- Silva GL, Lee IS, Kinghorn AD. 1998. Special problems with the extraction of plants. In: Cannell RJP, editor. Natural products isolation. Methods in biotechnology. New Jersey: Humana Press. p. 340.
- Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C. 2012. Totally drug-resistant tuberculosis in India. Clin Infect Dis. 54:579–581.
- Ueyama M, Chikamatsu K, Aono A, Murase Y, Kuse N, Morimoto K, Kudoh S. 2014. Sub-speciation of Mycobacterium tuberculosis complex from tuberculosis patients in Japan. Tuberculosis. 94:15–19.
- Valencia D, Alday E, Robles R, Garibay-Escobar A, Gálvez-Ruíz JC, Salas-Reyes M, Jiménez-Estrada M, Velázquez-Contreras E, Hernández J, Velázquez C. 2012. Seasonal effect on propolis chemical composition and biological activities of Sonoran propolis. Food Chem. 131:645–651.
- Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, ZiaZarifi AH, Hoffner SE. 2009. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest. 136:420–425.
- WHO, World Health Organization. Global tuberculosis report 2012 [cited 2015 Jun 21]. Available from: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf .
- WHO, World Health Organization. Global Tuberculosis Report 2014 [cited 2015 Jun 21]. Available from: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf .
- Yoshioka H, Renold W, Fischer NH, Higo A, Mabry TJ. 1970. Sesquiterpene lactones from Ambrosia confertiflora (Compositae). Phytochemistry. 9:823–832.