Abstract
Objective. The aim of the study is to fabricate stents with rabbit outgrowth endothelial progenitor cells (OECs) to facilitate their endothelial function in vitro. Methods. Rabbit OECs were isolated from peripheral blood and identified by immunofluorescence and flow cytometry. Cells proliferation and migration were measured by growth curve and modified Boyden chamber assay. Adhesion assay was performed by replating cells on fibronectin-coated dishes. VEGF, G-CSF and NO in supernatant were tested. OECs were poured on fibronectin-coated or uncoated stents. After six days, scanning electron microscopy (SEM) and inverted fluorescent microscopy observation were performed. Results. About three to four weeks after culture, OECs were characterized as adherent cells which were double positive for Dil-acLDL uptake and FITC-UEA-I binding, with high expression of CD34. They also showed high ability of proliferation, adhesion and migration properties. Compared with uncoated stents, more OECs migrated and adhered onto fibronectin-coated stents. OECs seeding onto the fibronectin coated stents could secret more cytokine and NO. Endothelialization of coated stents was visible both under the SEM and inverted fluorescent microscope. Conclusions. OECs can differentiate to endothelial lineage and possess high ability of proliferation, migration and adhesion. It is feasible to fabricate OECs-seeded stents in vitro, while the stents coated with fibronectin facilitate this endothelialization process.
Coronary artery stenting is currently the most frequently performed percutaneous coronary intervention (PCI) for the treatment of coronary artery diseases. Several multi-center clinical trials have shown that drug eluting-stents (DES) compared with bare metal stents largely reduce the incidence of in-stent restenosis (Citation1,Citation2). However, late thrombosis can be seen over the DES struts after PCI. This phenomenon is probably caused by the inhibitory effects of immunosuppressive, antimitotic and anti-inflammatory drug released from the stent on the endothelial cell adhering to the struts. On the other hand, discontinuation of dual anti-platelet therapy may increase the risk of thrombotic events (Citation3). Late thrombosis is the result of delayed reendothelialization (Citation4). Therefore, it is necessary to develop a novel stent to prevent the restenosis and thrombosis.
The endothelium is a single layer of endothelial cells lining the vascular wall and plays an integral part in maintaining vascular homeostasis. It has been shown that a functionally intact endothelium is a prerequisite for the inhibition of neointimal growth and thrombosis after PCI. However, the ability of surrounding mature endothelial cells to repair the endothelium is limited.
Accumulating evidence has shown that circulating endothelial progenitor cells (EPCs) derived from bone marrow contribute to endothelial repair (Citation5). EPCs are cell populations that have the capacity to proliferate and differentiate into mature endothelial cells. Recent studies have shown that there are two different types of EPCs from circulating mononu-clear cells, early EPCs and late EPCs or outgrowth endothelial cells (OECs) (Citation6,Citation7). Early EPCs formed colonies consisting of spindle-shaped cells with a limited proliferation potential. In contrast, OECs were characterized by cobblestone appearances and late outgrowth. Therefore, we hypothesized that OECs can be a promising cell source to fabricate the cell-seeded stent.
This study was aimed to test: 1) if it is feasible in vitro to fabricate the stents through seeding the stent with OECs isolated and expanded from peripheral blood of the rabbits; 2) if fibronectin coated stents would facilitate this endothelialization process conferred by OECs.
Methods
Materials
Three percent pentobarbital (Shanghai Reagent Factory), lymphocyte separating solution (Shanghai Huajing Biological High-technology Company), fibronectin (Sigma), fetal bovine serum (FBS, Sino-USA Joint Venture of Lan Zhou Ming Hai Bioengineering Limited Company), EGM-2 BulletKit (Clonetics), Dil-acLDL (Molecular Probes), FITC-UEA-I (Sigma), PC5-conjugated CD34 (Immunotech), FITC-CD14 (Biolegend), flow-cytometry (Beckman), vascular endothelial growth factor, (VEGF) (R&D), modified Boyden chamber (Jiangsu Province Haimen City Qilin Medical Instrument Factory), VEGF and G-CSF ELISA kit (R&D systems), NO kit (Bi Yun Tian Company), inverted fluorescent microscope (Nikon), stainless bare stent (Shanghai MicroPort Medical Co. Ltd.), scanning electron microscope, SEM (PHILIP, QUANTA-200) were used in this study.
Animals
Adult New Zealand White male rabbits weighing 3 to 3.5 kg were purchased from Shanghai Sheng Wang experimental animal nursery and maintained on a 12:12 hour light:dark cycle in an ambient temperature of 24°C and 60% humidity. Food was given 100 g per day and water was provided ad libitum. All animal procedures were performed under the approval of the Institutional Animal Care and Chinese Committee on Animal Investigation.
Blood collection
Ten rabbits were anesthetized with 3% pentobarbital administered intravenously and supplemented as needed. The femoral vein was catheterized and 40 ml blood was withdrawn into a heparinized syringe. The volume of blood was instantly replaced by an equivalent volume of normal saline. The wound was closed and protective infection with gentamycin was given for three days.
Isolation of mononuclear cells and OECs culture
Mononuclear cells were separated with lymphocyte separating solution by density-gradient centrifugation. Cells were suspended in EBM-2 with supplements consisting of hEGF, VEGF, hFGF-B, IGF-a, ascorbic acid and 20%FBS. Isolated mononuclear cells per well were seeded on fibronectin-coated six-well plates (with 1×107 cell for each well) and incubated in a 5% CO2 and >95% humidity incubator at 37°C. First medium was changed four days after plating and non-adherent cells were removed by washing with phosphate buffered saline (PBS). Thereafter, media were changed every two days. Colonies with cobblestonelike morphology appearance two to three weeks later in culture were selected as OECs and expanded over two passages.
Cell characterization
Cell staining. Cells were first incubated with Dil-acLDL (2.4 µg/ml) at 37°C for four hours and then fixed with 2% paraformaldehyde for ten minutes. After washing with PBS, cells were incubated with FITC-UEA-1 (10 µg/ml) for one hour. The cells were viewed with an inverted fluorescent microscope. Cells demonstrating double positive fluorescence were identified as differentiating EPCs.
Flow-cytometry analysis. Cells detached with 0.25% trypsin were by gently flushed repeatedly through a pipette tip. Cells (2×105) were incubated with PC5-conjugated CD34 and FITC-CD14 for 30 min at 4°C, respectively. Isotype-identical antibodies served as controls. After thorough reaction, cells were then fixed in 1% paraformaldehyde and passed through the flow cytometry.
Proliferation assay. Cells were counted from the day of plating to six weeks, once a week.
Migration assay. OECs migration was evaluated by using a modified Boyden chamber assay. Briefly, cells were detached using 0.25% trypsin and harvested in 500 µl of EBM-2 and counted. 2×104 OEC were placed in the upper chamber of a modified Boyden chamber. EBM-2 and VEGF (50 ng/ml) were placed in the lower compartment of the chamber. After 24 hours of incubation at 37°C, the lower side of the filter was washed with PBS and fixed with 2% paraformaldeyde. After staining with Giemsa solution, cells that migrated into the lower chamber were counted manually and migration ability was expressed as average cell number from three random microscopic fields (×400).
Cell adhesion assay. OECs were washed with PBS and gently detached with 0.25% trypsin. After centrifugation and resuspension in EBM-2 with supplements, identical cell number (1×105/ml) was replated on to 50 µg/ml fibronectin-coated wells and incubated for 30 min at 37°C. The adhesion ratio was calculated as the ratio of adherent cell number to total cell number.
Fabrication of OECs-seeded stents
Ten stainless stents with 2.5 mm in diameter and 10 mm in length were irradiated with ultraviolet light for two hours after which the stents were immersed into fibronectin solution for one hour and dried at room temperature. The fibronectin-coated stents were then placed into a 24-well cell culture dish with 1×106/ml OECs being added in each well. After one hour of incubation, the stent was rotated 180° around its longitudinal axis and another identical number OECs was added into the well. The stents were further incubated for six days. Another ten bare stents underwent the same procedure without fibronectin coating process for comparison.
Measurement of cytokine and nitric oxide concentration of supernatant
The two groups’ stents were transferred to another culture dish and were incubated with EBM-2 medium without supplements for 24 hours. The supernatant concentration of VEGF and G-CSF was measured with ELISA kit. NO was quantified by Griess reaction according to user's manual of the NO assay kit.
Inverted fluorescent microscope of OECs-seeded stents
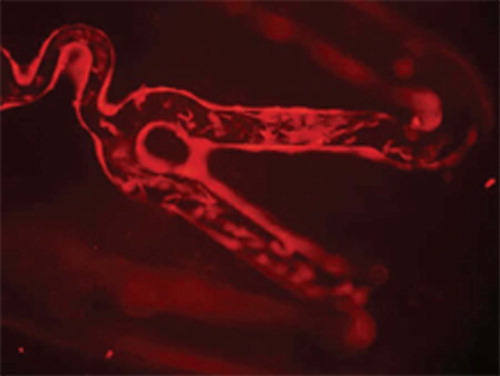
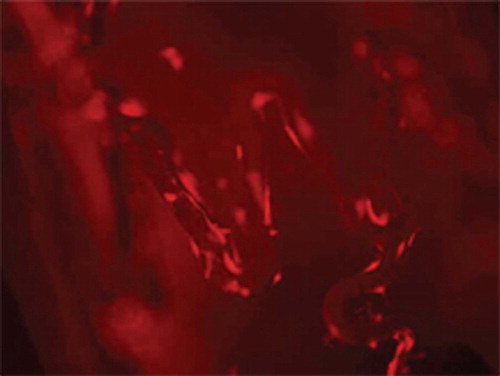
Three of ten stents from each group were placed into a 24-well cell culture dish, OECs suspension of positive Dil-acLDL staining was added to the wells. Under the inverted fluorescent microscope, cells with red fluorescence adhering to the surface of stents were counted in three random microscopic fields (×400).
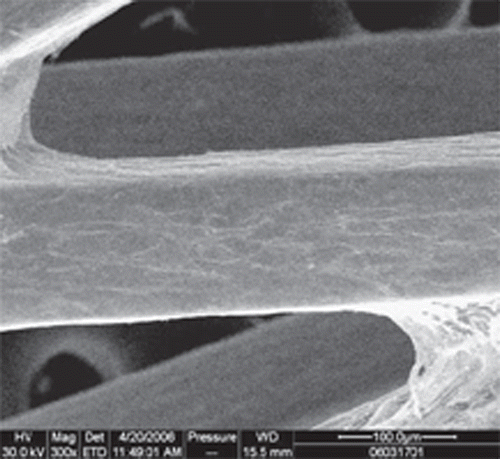
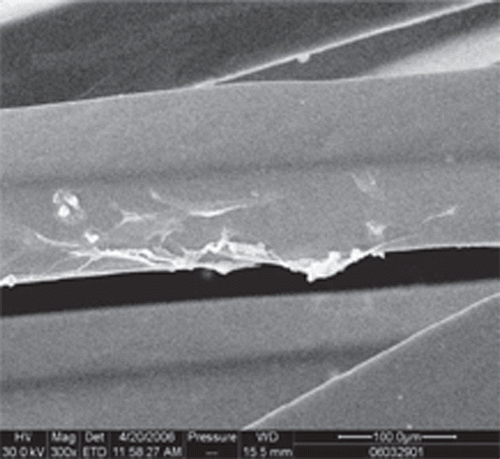
Scanning electron microscope of OECs-seeded stents
After six days of incubation, cell-seeded stents were fixed with 2% glutaraldehyde for one hour. Then they were treated with 1% osmium tetroxide for one hour, followed by dehydration with gradual increased concentrations of acetone. Finally, samples were dried and sputtered with gold before analysis.
Statistical analysis
All data are presented as mean±SEM. Comparisons between two groups were performed using independent-sample t test. Probability values of p < 0.05 were considered as statistically significance.
Results
Characterization of OECs
Early EPCs were present at one week. However, cobblestone-like OECs were only found at about two to three weeks after plating. These OECs exhibited the ability of take-up Dil-acLDL and binding to FITC-UEA-I with strong expression of CD34 (87.4±0.9%), yet negative staining for CD14 (1.5±0.3%).
Compared with early EPCs, we found OECs also had a higher ability of proliferation. After two weeks culture, OECs could proliferate in exponential growth. From two to six weeks, OECs have amplified 104 fold in vitro. However, OECs demonstrated equivalent ability of adhesion (94.6±1.0% vs. 91.3±1.7%, p > 0.05, n = 8) and migration (25.8±2.4 vs. 22.8±2.5, p > 0.05, n = 8) compared to early EPCs.
Secretion function of stents
Six days after the stents were incubated with OECs, the VEGF, G-CSF and NO concentrations in the supernatant of medium were higher in fibronectin coated stents (VEGF: 150.08±11.31 µmol/L; G-CSF: 45.74±3.73 µmol/L; NO: 61.59±2.22 µmol/L) than non fibronectin-coated stents (VEGF: 26.15±2.26 µmol/L; G-CSF: 10.67±0.66 µmol/L; NO: 13.58±1.05. p < 0.05, respectively, n = 7), suggesting that OECs from fibronectin coated stents can secret more cytokine and NO.
Inverted fluorescent microscope and SEM of OECs-seeded stents
Compared with uncoated stents, more OECs marked with Dil-acLDL adhered on the surface of the stents ( and ) (27.80±4.26 vs. 6.10±3.07 cells/×400 field, p < 0.05). SEM examination showed that more OECs covered the stent struts ( and ).
Discussion
Late local thrombus formation over the DES struts has been observed in clinical practice (Citation8). It has been largely related to delayed reendothelialization. Endothelial denudation and/or injury can also induce phenotypic alteration of vascular smooth muscle cells (SMCs). The stimulated SMCs migrate into and proliferate in intima and produce a large amount of extracellular matrix. These sequential events result in neointimal hyperplasia and restenosis. Animal experiments have shown that there exists an inverse relationship between endothelial integrity and SMC proliferation (Citation9). Therefore, rapid reendothelialization may suppress the process of thrombus formation and restenosis.
There are a number of techniques aimed to facilitate the reendothelialization process. Among them all, seeding stents with cells by which they were conferred endothelial function became an attractive strategy. However, research work targeting on cells-seeded stents has been still in the exploratory stage and several technical issues needed to be resolved before its clinical applications. The way to enhance adherence of cells to stent is the key point to facilitate the endothelialization process of the stent. Selecting cells with a higher ability of adhesion and proliferation can make it easier for the cells to stick onto the stent and rapidly cover the stent surface. Different methods have been tried to improve the functions of cells, e.g., selection of OECs with comparable adherence and proliferation, drug (like statin) therapy or local transfer VEGF-2 to cells. At the same time, stents coated with special material, such as fibronectin, gelatin, cRGD (Citation10), specific antibody (Citation11), etc., may contribute to the fabrication.
It has been shown in an in vitro study that planting proliferative endothelial cells onto the stents can accelerate the process of reendothelialization (Citation12). However, endothelial cell-seeding methods have not resulted in extensive utilization largely because of difficulties in collecting cells.
Since Asahara et al. (Citation13) successfully isolated EPCs from adult peripheral blood, the past few years have witnessed a rapid expansion of our knowledge of EPCs. Compared with endothelial cells, EPCs can be obtained from peripheral blood with a minimized invasive procedure. In addition, EPCs has greater potential for multilineage differentiation. Under certain conditions, EPCs could differentiate towards endothelial lineage. Moreover, it has been suggested that this group of cells have the ability of proliferation, migration, adherence and secretion. Recent data suggested that there existed two types of EPCs, early EPCs and late EPCs (or OECs). The early EPCs were spindle-shaped with heterogeneous behavior and hence a limited proliferative potential. Whereas, OECs were relatively homogeneous and cobblestone-like revealing a higher proliferation potential. Based on these cell biological properties, OECs could serve as a more suitable cell source for tissue engineering.
In this study, we isolated mononuclear cells from the peripheral blood of rabbits. Under the specific circumstances, OECs appeared in cultures after two to three weeks of plating, demonstrating the capacity of take-up Dil-acLDL and binding to FITC-UEA-I. They also expressed high level of CD34, yet with much lower level of CD14 which was, in contrast, highly expressed in early EPCs. These are the characters by which OECs are identified in vitro. Although the number in peripheral blood was quite small, OECs could be expanded rapidly in vitro. Compared with early EPCs, we found OECs had higher ability of proliferation. After two weeks of culture, OECs could proliferate in exponential growth. From two to six weeks in culture, OECs could be amplified 104 fold in vitro. In addition, OECs showed high ability of adhesion and migration, which was equivalent to early EPCs. More importantly, these OECs also can secret VEGF, G-CSF and NO.
Data showed that cells used in graft or stent were mostly early EPCs or mixed EPCs. Shirta et al. (Citation14) fabricated two types of EPCs-seeded intravascular stents. One was a photocured gelatin-coated metallic stent, and the other was a microporous thin segmented polyurethane film-covered stent on which photocured gelatin was coated. Both devices were seeded ex vivo with expanded EPCs obtained from canine peripheral blood. They also tried to devise an EPCs-seeded tubular hybrid tissue (Citation15). Besides canine EPCs, human EPCs could be also seeded and cultured on a small-diameter compliant graft made of microporous segmented polyurethane film (Citation16).
On account of their high proliferating activity, we think OECs can be a more promising candidate of cell source in tissue engineering than early EPCs. High proliferation properties of OECs can decrease cells loss due to flow effects and balloon expansion. In the present study, using rabbit animal model which was widely accepted for studying atherosclerosis and rest-enosis, we first showed that stainless stents pretreated with fibronectin were covered with more OECs after six-day culture, which also was associated with high concentration of cytokine and NO, assumed to be secreted by the OECs seeding on to the stents.
In conclusion, OECs could serve in the future as a promising cell source for the development of tissue engineering. Fibronectin could made cells easily adhere onto the stents. Fabrication of endothelial function with OECs may be a novel therapeutic device of reendothelialization, resulting in the prevention of late local thrombosis and restenosis.
Acknowledgements
This work was supported by a grant from Shanghai Science and Technology Commission Foundation (05JC14031). We would like to thank Technician Liu Yi-Wen, Technician He Yi-Qing, Technician Yang Si, Director Ding Zaixian, Technician Ding Weikang for offering relevant experimental facilities and technical support. Also we would like express our sincere thanks to Dr. Wei Zhu for critically reading the manuscript and English revision.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Schofer J, Schlütera M, Gershlick AH, Wijns W, Garcia E, Schampaert E, . Sirolimuseluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: Double-blind, randomized controlled trial (E-SIRIUS). Lancet. 2003;363(9390):1093–9.
- Hong MK, Mintz GS, Lee CW, Song JM, Han KH, Kang DH, . Palitaxel coating reduces in-stent intimal hyper-plasia in human coronary arteries: A serial volumetric intravascular ultrasound analysis from the Asian Paclitaxel-eluting Stent Clinical Trial (ASPECT). Circulation. 2003;107:517–20.
- Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med. 2006;354:483–95.
- McCormick C. The search for endothelium friendly stents. Med Device Technol. 2007;18:30:32–3.
- Shantsila E, Watson T, Lip GYH. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–52.
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, . Characterization of two types of endothelial progenitor cells and their different cell biologic behaviors and contribution to neoangiogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–93.
- Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, . Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells. Circulation. 2005;112:1618–27.
- Kerner A, Gruberg L, Kapeliovich M, Grenadier E, . Late stent thrombosis after implantation of a sirolimus-eluting stent. Catheter Cardiovasc Interv. 2003;60:505–8.
- Losordo DW, Isner JM, Diaz-Sandoval LJ. Endothelial recovery: The next target in restenosis prevention. Circulation. 2003;107:2635–7.
- Blindt R, Vogt F, Astafieva I, Fach C, Hristov M, Krott N, . A novel drug-eluting stent coated with an integrin-binding byclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J Am Coll Cardiol. 2006;47:1786–95.
- Aoki J, Serruys PW, van Beusekom Heleen, Ong AT, McFadden EP, Sianos G, . Endothelial progenitor cell capture by stents coated with antibody against CD34. J Am Coll Cardiol. 2005;45:1574–9.
- Conte MS, VanMeter GA, Akst LM, Clemons T, Kashgarian M, Bender JR, . Endothelial cell seeding influences lesion development following arterial injury in the cholesterol-fed rabbit. Cardiovasc Res. 2002;53:502–11.
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, . Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7.
- Shirota T, Yasui H, Shimokawa H, Matsuda T. Fabrication of endothelial progenitor cell(EPC)-seeded intravascular stent devices and in vitro endothelialization on hybrid vascular tissue. Biomaterials. 2003;24:2295–302.
- Shirta T, Yasui H, Matsuda T. Intralumenal tissue-engineered therapeutic stent using endothelial progenitor cell-inoculated hybrid tissue and in vitro performance. Tissue Engineering. 2003;9:473–85.
- Shirota T, He H, Yasui H, Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: Proliferative and anti-thrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9:127–36.