Abstract
Objectives. Inflammation and increased blood viscosity are associated with increased risk of cardiovascular mortality. Erythrocyte sedimentation rate (ESR) and hematocrit both influence blood viscosity whereas the first also is a marker of inflammation. We aimed to investigate ESR, hematocrit and the interaction between them as predictors of cardiovascular mortality during 26 years follow-up among healthy middle aged men. Design. Four hundred and eighty eight men aged 40–59 were extensively examined in 1972–1975 and followed over a period of 26 years. Risk estimation was made in Cox proportional hazards and adjusted for age, smoking, systolic blood pressure, total serum cholesterol, and physical fitness. Results. A 2.44-fold (95% CI 1.37–4.35) adjusted risk of cardiovascular mortality was found in the highest quartile of hematocrit compared to the lowest. Among the 265 men who had an ESR <6 mm/h (median), the adjusted risk of cardiovascular mortality was 3.05-fold (95% CI 1.49–6.23) in the highest quartile of hematocrit compared to the lowest. This association was not observed among the 223 men with ESR <6 mm/h. Conclusion. Elevated hematocrit is independently associated with increased long-term risk of cardiovascular mortality in men with high ESR. Our data suggest that the combination of inflammation and blood viscosity may improve the prediction of cardiovascular risk.
Inflammation and increased blood viscosity are associated with an increased risk of cardiovascular (CV) mortality (Citation1). Erythrocyte sedimentation rate (ESR) and hematocrit (Hct) both influence blood viscosity. ESR is also an important marker of inflammation, and has been reported to be an independent predictor of long-term CV mortality (Citation2,Citation3). The association between Hct and CV mortality is complex. ESR and Hct (Citation1,Citation4) are inversely correlated to each other.
Increased whole blood viscosity has been reported to be associated with increased risk of CV morbidity and mortality. This association is, however, uncertain when adjusting for other known CV risk factors (Citation1). Hct accounts for some 75% of blood viscosity at all shear rates encountered in the vascular bed. Hct is easily measured in contrast to the more cumbersome, direct measurement of whole blood viscosity (Citation5). Theoretically, high Hct may promote atherosclerosis and thrombotic complications and thereby be associated with increased CV mortality and morbidity. Epidemiological data are, however, not conclusive (Citation1).
We have previously reported both ESR and Hct to be independent predictors of CV mortality among healthy middle-aged men (Citation3,Citation4), but little is known about a possible interaction between ESR, Hct and long-term risk of CV mortality. We therefore aimed at testing if such interactions exist by studying long-term CV mortality (26 years) in quartiles of Hct, separately for those with an ESR above and below population median (ESR = 6 mm/h). To our knowledge, no study has assessed possible interaction between inflammation and blood viscosity on long-term risk of CV disease.
Subjects and methods
Study population
From August 1972 to March 1975; 2 014 apparently healthy men working in five governmental institutions in Oslo, Norway, aged 40–59 years underwent a careful screening procedure before inclusion. They were recruited through the health care system of their companies. All were free from known or suspected heart disease, hypertensive treatment, cancer and a number of other diseases as reported in detail elsewhere (Citation6). The examination programme included clinical examination, blood tests, spirographic studies, chest x-ray, resting electrocardiogram (ECG) and a symptom-limited bicycle exercise ECG test.
Laboratory measurements and blood pressure
When the study was half completed, Hct measurements were added to the study protocol and performed in every second participant who was included. Hct was thereby measured in 488 men and was estimated by the filling disposable micro tubes, covered with dry heparin. They were filled up to the mark from a finger capillary puncture. After sealing one end, the tube was centrifuged in a Heraeus Christ Microfuge (Harz, Germany) at 20 600 × g (14 000 r.p.m.) for 3 min. Hct was read from a special chart with a magnifying lens, and given as percent of total blood volume with two significant digits. Measurements of ESR were done in all 2 014 men according to Westergreen's method.
Blood pressure was measured using a mercury sphygmomanometer. Resting blood pressure was taken after subjects had been familiarized with the laboratory and had been 5 minutes in the supine position in a quiet room.
Blood pressure was measured three times in all subjects by the same physician. On average, blood pressure was lowest at the second measurement. The second measurement was therefore used for prognostic evaluation.
Cholesterol and triglycerides were determined by standard methods (Citation7).
Death registrations
With permission granted from the Norwegian Data Inspectorate and the Norwegian Board of Health we obtained a complete update of cause-specific deaths from Statistics Norway. Statistics Norway has record of cause of death in all Norwegians who have died with a delay in completeness of 6 months. The present mortality data are complete up to December 31, 1999, and none was lost to follow-up.
CV mortality includes sudden death, myocardial infarction, stroke (cerebral infarction and haemorrhage), venous thrombosis and pulmonary embolism.
Statistical methods
The Cox model was used in a statistical analysis of the relations between risk factors and CV mortality. Besides classical CV-risk factors (age, smoking status, total cholesterol and systolic blood pressure), physical fitness was included in the model.
For age the HR associated with an increase of 10 years was calculated. We assessed the risk of CV mortality in the total group of 488 men divided into quartiles of Hct levels using Cox proportional hazard models and Kaplan Meier Plots.
We further analysed the relationship in participants divided into groups with ESR < 6 mm/h (n = 223) and ESR ≥ 6 mm/h (n = 265).The reason why we chose median ESR as cut-off was the sample size in our study; the smallest subgroup resulting from other cut-offs would be too small to allow for a valid multivariate statistical analysis.
All analyses were performed using SPPS and JMP 7.
Results
Mean Hct at baseline was 47.2%. summarizes the different baseline variables. Mean values are given separately for men with ESR above and below the population median of 6 mm/h. The 265 men with ESR above the median value were slightly older and smoked more than the 223 men with ESR below the median value. Otherwise no group differences were observed for the other variables. Quartile cut-offs for Hct were similar in the two groups ().
Table I. Baseline variables mean values and the number of individuals (n) in each group who had this variable measured at baseline. Variable are shown separately for individuals with an ESR <6 and ≥6.
Table II. Different quartiles of hematocrit, the number of men (n) within each quartile and the level of haematocrit with the standard deviation (SD). The different quartiles are divided into groups with erythrocyte sedimentation rate (ESR) below and above median (6 mm/h).
We found an association between Hct and CV mortality at 26 years of follow-up (), minimally changed after adjustment for differences in age, smoking, serum cholesterol, systolic blood pressure and physical fitness. Both ESR and physical fitness were inversely correlated to Hct (p < 0.01) whereas age, smoking habits, systolic blood pressure and cholesterol were not. (Data not shown).
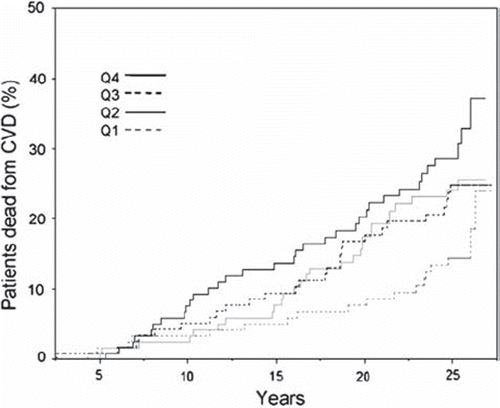
Figure 1. Kaplan-Meier curves describing risk development among 488, grouped by quartiles of hematocrit (Q1-Q4).
When both ESR and Hct were integrated in a Cox regression model, both were found to be independent predictors of CV mortality (Data not shown). We aimed, however, to emphasize on the interaction between ESR and Hct with respect to future CV mortality. Further results are therefore presented as adjusted HR for the different quartiles of Hct among individuals with ESR below and above the median rather than adjusting for ESR in the Cox regression model.
Age adjusted risk of CV mortality in the highest quartile versus the lowest quartile of Hct was 2.41 (95% CI 1.36 to 4.28). When further adjusting for smoking habits, systolic blood pressure, cholesterol and physical fitness, the risk of CV mortality in the highest quartile of Hct versus the lowest was 2.44 (CI 1.37 to 4.35).
Among the 265 men with ESR $6 mm/h at baseline, the risk of CV mortality in the highest versus the lowest quartile of Hct () was 3.05 (CI 1.49 to 6.23) when adjusting for the above mentioned risk factors () Among the 223 individuals with ESR < 6 mm/h at baseline, no risk association was seen when comparing Hct quartiles ().
Figure 2. Kaplan-Meier curves describing risk development among 265 men with erythrocyte sedimentation rate above 6 mm/h, grouped by quartiles of hematocrit (Q1–Q4).
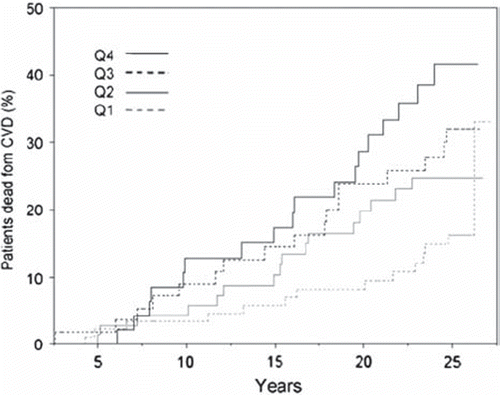
Table III. Numbers of CV deaths in within each quartile (Q1Q4) of hematocrit and the adjusted hazard ratio (HR) compared to the quartile with the lowest hematocrit (Q1). Data are shown separately for individuals with an erythrocyte sedimentation rate (ESR) below and above median (6 mm/h).
We also wanted to evaluate if there was an association between haemoglobin (Hb) and CV mortality in our cohort. No such association was found.
We further analyzed the association between Hct, ESR and CV mortality when dividing Hct into tertiles instead of quartiles and with different cut-off of ESR than median. These ways of analyzing the association did not change the results markedly.
Discussion
ESR in the high range may reflect ongoing inflammatory processes in the body of which atherosclerosis represents one such inflammatory process. Conceivably a high ESR therefore may have the same prognostic significance regarding future CV-events as has been reported for high sensitivity CRP which is a highly sensitive marker of inflammation. The association between inflammation and CV morbidity and mortality risk has mostly been studied in the recent 20 years using high sensitive c-reactive protein (hs-CRP). Increased hs-CRP has been shown to be associated with an increased risk of CV morbidity and mortality in both epidemio-logical and interventional studies (Citation8,Citation9). Because the CRP test is a relatively new method, studies assessing its predictive abilities have relative short follow-up periods, although one study had up to 18 years [Citation8,Citation10–13]. CRP reacts faster to inflammation, is more specific, and returns faster to baseline than ESR and is thought by many to be a more reliable inflammation test than ESR. It is questionable whether this notion is correct when investigating the association between chronic, low grade inflammation represented by atherosclerosis and long-term risk of CV mortality. Although ESR may be a less specific sign of inflammation than CRP, ESR carries possible additional information. ESR is influenced by the number and size of erythrocytes, fibrinogen, immunoglobulins, lipoproteins and other acute phase proteins, especially those increasing red blood cell aggregation. Plasma-, and thereby whole blood viscosity is influenced by some of the same factors (Citation14). The association between ESR and CV mortality in this cohort has been reported previously (Citation3). In a large population-based cohort study from Reykjavik, including 16 673 men and women (Citation2), ESR within the normal range was an independent long-term predictor of coronary events. The increased risk after baseline measurement of ESR was stable for up to 25 years in men.
We have previously reported that Hct appeared to be an independent predictor of CV mortality at 10–16 years follow-up in this population of 488 apparently healthy men. After adjusting for differences in age, plasma cholesterol, systolic blood pressure, ESR and smoking habits, an increase of two SDs of Hct was associated with a 2.9-fold increased risk of CV mortality at 10 years and a 1.9-fold at 16 years of follow-up (p < 0.05) (Citation4). At 26 years of follow-up, an increase in Hct of 2 SDs was associated with a 1.83 (1.25–2.68) fold increase in risk of CV mortality (p = 0.02) when adjusted for the same risk factors.
The increased risk of CV mortality associated with a high Hct at baseline appears to be of the same magnitude after 26 years of follow-up as after 16 years, and only moderately weaker than after 10 years. In epidemiological studies, it is often observed that the predictive power of a risk factor measured at baseline is weakened with time. This might be due to other risk factors coming into play with increasing age. Now, we have a 10-year longer follow-up, and still find that Hct seems to be a powerful predictor of CV mortality. Few studies have had such a long time of follow-up.
The prognostic information that Hct carries after 26 years of follow-up is markedly more powerful when measured in individuals with an ESR above median. This was also the case after 16 years of follow-up, even though the main objective of that report was not to investigate the possible interaction of ESR and Hct (Citation4). Importantly, in individuals with an ESR below median (6 mm/h), Hct did not predict CV mortality after 26 years of follow-up.
Although causation never can be proved from epidemiological data, several observations and theoretical considerations support the hypothesis that increased Hct, plasma viscosity and inflammation may increase CV morbidity and mortality both by promoting thrombotic complications and accelerating atherosclerosis (Citation1,Citation4,Citation9,Citation15). Combining ESR and Hct in a prognostic model therefore appears to be sound from a pathophysiological point of view.
Theoretically, high Hct may have a negative effect on blood flow by increasing blood viscosity in a nonlinear way (Citation4,Citation5). Blood viscosity (which increases steeply with increasing Hct) is an important determinant of oxygen transport and delivery, and high viscosity is particularly harmful in ischemic myocar-dial and brain tissues (Citation16–18). A high number of erythrocytes may also increase platelet adhesiveness and deposition on the endothelium. This can cause shortening of bleeding time and via these mechanisms could possibly increase the risk of thrombotic complications (Citation19). High Hct has been suggested to accelerate atherogenesis, explained by e.g. increased serum lipids (Citation20) and deposition of platelets and large plasma proteins on the endothelium (Citation21–23).
Interestingly, a high Hct has been shown to be associated with development of diabetes mellitus type two (Citation24) which is an important risk factor of CV. In our study, new onset diabetes was numerically higher in the highest quartile of Hct versus the lowest (Data not shown), but the difference was not statistically significant. High Hct might also be a result of subclinical atherosclerotic disease or neurohormal activation that leads to changed microcirculation and hemoconcentration rather than a risk factor (Citation25). This does not however change the longitudinal finding that high Hct seems to predict CV mortality.
Mean Hct, conventional CV risk factors and the burden of atherosclerosis vary amongst the different populations studied, and may to some extent explain different findings. In high endemic populations of atherosclerosis (e.g. Norway) the ability to observe an effect of high Hct should be favoured. Similarly, authors from Holland were able to detect highest incidence of acute myocardial infarctions among those with the highest Hct; no such association was found among men of Japanese ancestry from Honolulu (Citation4).
Our study has a long period of follow-up and detailed registration of cause of death. The participants were all apparently healthy at baseline as judged by history and clinical examination. Furthermore an extensive and thorough baseline survey was done to exclude possible participants with intercurrent or chronic disease. This would serve to limit the amount of confounding factors of Hct and ESR levels.
Our observations and results are limited to 488 apparently healthy men aged 40–59 years. As mentioned above, the 488 men who had Hct measured were every second man included in the study of 2 014 men after the study was about halfway completed. We can therefore not rule out selection bias. The 488 men who had Hct measured were about 2 years older, had a slightly higher blood pressure and poorer physical fitness than the rest (n = 1526) of the 2 014 men. Percentage of smokers and cholesterol level were equal. When dividing these men into quartiles of Hct and further into subgroups with ESR above and below median, there are relatively few men in each subgroup. Further studies are needed to investigate whether high Hct, alone, or Hct combined with an ESR ≥ 6 mm/h is associated with increased CV mortality in other age groups, among women and in the setting of co-morbidities.
Conclusion
Our data suggest that elevated hematocrit is associated with an increased risk of dying from CV independent of conventional CV risk factors in middle aged, apparently healthy men followed over 26 years. This is amplified by slightly elevated ESR. Our data suggest that the combination of inflammation and blood viscosity may improve the prediction of cardiovascular risk.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Danesh J, Collins R, Peto R, Lowe GD. Haematocrit, viscosity, erythrocyte sedimentation rate: Meta-analyses of prospective studies of coronary heart disease. Eur Heart J. 2000;21:515–20.
- Andresdottir MB, Sigfusson N, Sigvaldason H, Gudnason V. Erythrocyte sedimentation rate, an independent predictor of coronary heart disease in men and women: The Reykjavik Study. Am J Epidemiol. 2003;158:844–51.
- Erikssen G, Liestol K, Bjornholt JV, Stormorken H, Thaulow E, Erikssen J. Erythrocyte sedimentation rate: A possible marker of atherosclerosis and a strong predictor of coronary heart disease mortality. Eur Heart J. 2000;21:1614–20.
- Erikssen G, Thaulow E, Sandvik L, Stormorken H, Erikssen J. Haematocrit: A predictor of cardiovascular mortality?. J Intern Med. 1993;234:493–9.
- de Simone G, Devereux RB, Chien S, Alderman MH, Atlas SA, Laragh JH. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation. 1990;81:107–17.
- Erikssen J, Enge I, Forfang K, Storstein O. False positive diagnostic tests and coronary angiographic findings in 105 presumably healthy males. Circulation. 1976;54:371–6.
- Erikssen J, Skrede S. Serum lipids and latent coronary insufficiency. Scand J Clin Lab Invest. 1977;37:243–50.
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, . Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–610.
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, JrKastelein JJ, . Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207.
- Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, . C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: Results from the MONICA (MonitoringTrends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42.
- Mendall MA, Strachan DP, Butland BK, Ballam L, Morris J, Sweetnam PM, . C-reactive protein: Relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur Heart J. 2000;21:1584–90.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9.
- Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11.
- Somer T, Meiselman HJ. Disorders of blood viscosity. Ann Med. 1993;25:31–9.
- Lowe G, Rumley A, Norrie J, Ford I, Shepherd J, Cobbe S, . Blood rheology, cardiovascular risk factors, and cardiovascular disease: the West of Scotland Coronary Prevention Study. Thromb Haemost. 2000;84:553–8.
- Fuchs J, Weinberger I, Rotenberg Z, Erdberg A, Davidson E, Joshua H, . Plasma viscosity in ischemic heart disease. Am Heart J. 1984;108:435–9.
- Thomas DJ, Marshall J, Russell RW, Wetherley-Mein G, du Boulay GH, Pearson TC, . Effect of haematocrit on cerebral blood-flow in man. Lancet. 1977;2:941–3.
- Wells R. Microcirculation and coronary blood flow. Am J Cardiol. 1972;29:847–50.
- Duke WW. The relation of blood platelets to hemorrhagic disease. By W.W. Duke. JAMA. 1983;250:1201–9.
- Bottiger LE, Carlson LA. Hemoglobin, plasma lipids, and coronary heart disease. Am Heart J. 1973;86:842–4.
- Baumgartner HR, Turitto V, Weiss HJ. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. II. Relationships among platelet adhesion, thrombus dimensions, and fibrin formation. J Lab Clin Med. 1980;95:208–21.
- Turitto VT, Weiss HJ. Red blood cells: Their dual role in thrombus formation. Science. 1980;207:541–3.
- Turitto VT, Weiss HJ, Baumgartner HR. The effect of shear rate on platelet interaction with subendothelium exposed to citrated human blood. Microvasc Res. 1980;19:352–65.
- Nakanishi N, Suzuki K, Tatara K. Haematocrit and risk of development of Type 2 diabetes mellitus in middle-aged Japanese men. Diabet Med. 2004;21:476–82.
- Strand A, Gudmundsdottir H, Hoieggen A, Fossum E, Bjornerheim R, Os I, . Increased hematocrit before blood pressure in men who develop hypertension over 20 years. J Am Soc Hypertension. 2007;1:400–6.