Abstract
Objectives. Preoperative treatment with anti-coagulants for patients with non-ST-elevation acute coronary syndromes (NSTE-ACS) exposes patients undergoing surgical revascularization to a higher risk of perioperative bleeding. The aim of this study was to compare the effect on bleeding and transfusion needs during cardiac surgery for patients treated with enoxaparin or fondaparinux. Design. Using a combined retrospective and prospective approach, we studied the outcome of 147 patients with NSTE-ACS referred to coronary artery bypass grafting in terms of bleeding, blood transfusions and other complications. Results. Eighty patients were treated preoperatively with enoxaparin, and 67 patients with fondaparinux. There was no significant difference in postoperative bleeding (532 ± 355 for enoxaparin group vs. 580 ± 300 ml for fondaparinux group, p = ns) or transfusion needs for the two groups. A subgroup analysis of the fondaparinux group showed a significantly higher amount of postoperative bleeding after 12 h for patients when preoperative treatment with fondaparinux was discontinued less than 36 h prior to surgery compared to more than 36 h. Discussion. This study suggests that preoperative treatment with fondaparinux for NSTE-ACS is as safe as enoxaparin in terms of postoperative bleeding and transfusion needs. Findings support discontinuation of fondaparinux at 36 h prior to surgery.
Recent advances in the management of patients with non-ST-elevation acute coronary syndromes (NSTE-ACS) are based on swift and accurate diagnosis, immediate medical treatment and subsequent intervention. The introduction of antithrombotic agents has shown to be beneficial for this patient group, and the present practice is to combine antiplatelet therapy with anticoagulants (Citation1,Citation2). The latest recommendations from the American College of Cardiology/ American Heart Association and European Society of Cardiology promote a combination of acetylsalicylic acid (Aspirin®), clopidogrel (Plavix®) and a low-molecular-weight heparin such as enoxaparin (Klexane®) or factor Xa-inhibitor such as fondaparinux (Arixtra®) (Citation3,Citation4). Patients with NSTE-ACS now routinely receive antithrombotic pharmacological treatment for protection from new ischemic events from the time of diagnosis to surgical revascularization (Citation5), and fondaparinux was recently given a class I recommendation in the European guidelines (Citation6,Citation7).
From a surgical perspective, introduction of new anticoagulant regimes has often led to increased bleeding during and after surgery, and has challenged surgeons and anaesthesiologists to improve patient management (Citation8,Citation9). For instance, clopidogrel was introduced by cardiologists to prevent further development of coronary thrombosis and led immediately to increased perioperative bleeding and use of blood products (Citation10–12).
Recently, fondaparinux has been introduced as a substitute for a low-molecular-weight heparin (Citation13). Fondaparinux is a selective factor Xa inhibitor that attenuates thrombin generation and fibrin formation. Fondaparinux has an elimination half-life of 17 h and can therefore be administrated once daily. The advantage of fondaparinux is the reduced bleeding complications before surgery. However, there is no antidote, the effect cannot be measured on activated partial thromboplastin time (aPTT) or activated clotting time (ACT) and the long half-life could be a problem for patients in need of acute surgery (Citation14).
The safety profile of fondaparinux regarding peri-operative bleeding complications has not been previously studied, nor when this anticoagulant agent should be discontinued in relation to start of surgery. American and European guidelines recommend that fondaparinux be discontinued 24 h prior to surgery based on its half-life (Citation4,Citation15). The aim of this study was to investigate the safety profile with specific reference to perioperative bleeding and transfusion needs for fondaparinux as compared to enoxaparin in patients with NSTE-ACS undergoing coronary artery bypass graft surgery.
Material and methods
Study design
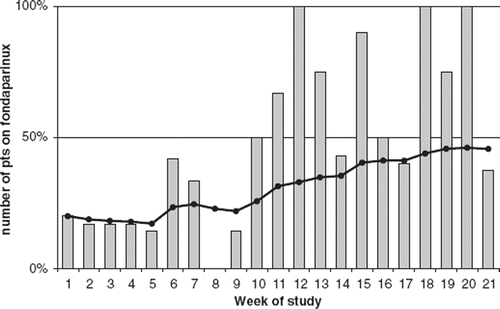
A combined retro- and prospective observational study was conducted during a period of 21 weeks. During these five months, all patients that underwent cardiac surgery at the Cardiothoracic Department at the University Hospital in Lund, Sweden, were enrolled. At the beginning of this period, Lund University Hospital was routinely using fondaparinux for NSTE-ACS, whereas referring centres used mainly enoxaparin. During the five-month period a majority of the referring centres changed to fondaparinux, thus adjusting the inclusion rate to the favour of fondaparinux at the end of the study (). The study was approved by the local ethics committee.
Patient inclusion
For all patients admitted for CABG, data from patient's records was registered prospectively in a database. Preoperative coagulation status, anticoagulation treatment and length of treatment were registered together with postoperative bleeding. This data was combined with data from the internal quality registry at our clinic. Furthermore, blood transfusion data was collected from the hospital blood bank registry.
Patient exclusion
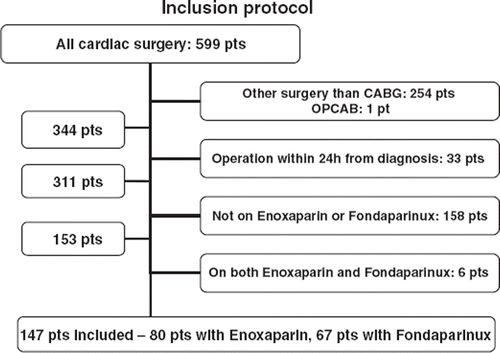
Patients operated acutely (<24 h after decision to proceed to surgery) were excluded, since they often had been treated with GP IIb/IIIa inhibitors and/or represented an inhomogeneous group.
Patients who were not treated preoperatively with enoxaparin or fondaparinux were excluded from the study, regardless of whether they had stable angina or had other reasons for not receiving anticoagulant therapy. Patients who received both enoxaparin and fondaparinux during hospitalization were also excluded, as were patients who underwent off-pump coronary artery by-pass grafting ().
Database management
Data was collected from four principal sources. The data collected preoperatively was complemented by data from the internal quality database. For transfusion of blood products, data from the local blood bank registry was added to our database. Where data was missing, patient records was read to complete the database. In any case of data mismatch between different data sources, the data was manually controlled by assessing the patient's records for direct information.
Administration of enoxaparin or fondaparinux
Treatment with anticoagulants for NSTE-ACS was performed according to guidelines (Citation3,Citation4). Patients treated with enoxaparin were given 1 mg/kg subcutaneously twice daily up to a maximum of 100 mg twice daily. Patients treated with fondaparinux were given 2.5 mg subcutaneously once daily in the evening regardless of weight. Adjustment of dose was done according to guidelines for patients with renal failure.
Surgery and post-operative care
Surgery was performed in a standardized manner, where the left internal mammary artery (LIMA) was routinely harvested first and the patient then heparinized before pericardiectomy and division of LIMA. After cross clamping of the aorta either cold St. Thomas crystalloid cardioplegia or cold blood cardioplegia was administered in the aortic root. Perfusion was performed with either a Stöckert S3 Heart-Lung machine with a Dideco Compactflo Evo Oxygenator (All Sorin Group SpA, Milano, Italy), or a Jostra-Maquet HL20 Heart-Lung machine with a Quadrox oxygenator (Maquet Critical Care, Solna, Sweden). The use of tranexamic acid was left to the discretion of the individual surgeon. During the patient's stay in the ICU, extra protamine, tranexamic acid or desmopressin were administered based on coagulation analyses or clinical judgments. No pre-defined limits for transfusion or reoperation for bleeding were used during the study, and indication for transfusion of blood products was determined by the physician in charge of the ICU or the ward.
Statistics
A student's t-test was used for group comparisons, where numbers were large. If numbers were small and the distribution was skewed, a Wilcoxon test was used. For correlation analysis a univariate linear regression was used. Unless otherwise stated, numbers are presented as mean ± 1 standard deviation.
Results
The study included a total of 147 patients divided into two groups, those treated preoperatively with enoxaparin (n = 80) and those treated preoperatively with fondaparinux (n = 67). There were no significant differences observed in the preoperative status between the two groups based on risk evaluation scores, renal function, neurological disease and functional performance class (). Peripheral arterial vascular disease was significantly greater in the enoxaparin group compared to the fondaparinux group (21. 3% vs. 9%, p = 0.041).
Table I. Preoperative patient characteristics.
No differences were observed in basic hemato-logical parameters, preoperative antiplatelet or anticoagulant regimes (besides treatment with enoxaparin and fondaparinux) (). There were no difference in anti-platelet therapy between groups; almost all patients received preoperative treatment with aspirin, and almost half of the patients were treated with clopidogrel. Preoperative coagulation status showed no differences in platelet count, INR or aPTT Duration of treatment with the anticoagulant agent differed, with three days longer treatment received on average in the enoxaparin group compared to the fondaparinux group (13.3 ± 8.1 vs. 10.4 ± 6.2, p = 0.023). Time in hours from last dose of anticoagulant to surgery was shorter for enoxaparin compared to fondaparinux (22.6 ± 8.7 vs. 41.4 ± 16.8, p = 0.0001).
Table II. Preoperative anticoagulants.
There was no difference in postoperative bleeding from chest tube drainages after 12 h (532 ± 355 mL for the enoxaparin group vs. 580 ± 300 mL for the fondaparinux group, p = ns) and total bleeding also did not differ between the two groups (). There was one reexploration for bleeding in each group. There were no differences in the frequency of transfusion of blood products. Packed red blood cell (RBC) units were given to both groups in equal amounts. Fresh frozen plasma and platelets were also given to both groups in equal amounts (). The amount of pharmacological pro-coagulants (tranexamic acid, anti thrombin III, desmopressin, pro-thrombin and factor VII) did not differ between groups (). Fibrinogen was not given to any patient.
Table III. Outcome – Bleeding and transfusion
No significant difference was observed in basic outcome variables during the postoperative course (). No patient developed stroke in either group. One patient developed renal failure in the enoxaparin group, whereas no patient in the fondaparinux group developed renal failure. One patient was treated with postoperative dialysis in the enoxaparin group. No patient developed postoperative mediastinitis that required surgical treatment. There was no 30 day mortality in either group.
Table IV. Outcome – Postoperative results.
A univariate regression analysis was performed with the pre-op characteristics ( and ) and independent variables and bleeding after 12 hours and risk of erythrocyte transfusion as dependent variables. Treatment with NSAID was positively correlated with bleeding at 12 hours (r=0.16, p<0.05), whereas platelet count, BMI and female gender was negatively correlated to bleeding at 12 hours (r=-0.21, p<0.05; r=-0.19, p<0.05 and r=-0.22, p<0.01 respectively). Body weight and BMI were negatively correlated to risk of erythrocyte transfusion (r=-0.21, p<0.01 and r=-0.21, p<0.05). Additive Euroscore was positively correlated to risk of erythrocyte transfusion (r=0.21, p<0.05).
To investigate the effect of time from discontinuation of fondaparinux to surgery, the fondaparinux group was divided into two subgroups depending on whether fondaparinux had been discontinued before or after 36 h prior to surgery. There was increased bleeding after 12 h in patients (n = 12) who had fondaparinux discontinued less than 36 h prior to surgery as compared to patients (n = 55) who had fondaparinux discontinued more than 36 h before surgery (729 ± 309 mL vs. 547 ± 290 mL, p = 0.039). There were no significant difference in total bleeding (969 ± 399 mL vs. 795 ±431 mL, p = 0.146).
Discussion
This study set out to determine the safety of fondaparinux as compared to enoxaparin from a cardiac surgical perspective. The results from the study imply that there are no differences in outcome in terms of bleeding, blood transfusion, reoperation for bleeding and standard outcome variables between the two drugs. The study did however find a difference in bleeding depending on whether fondaparinux had been discontinued 36 h before surgery. This increased bleeding for the fondaparinux group was seen in bleeding after 12 h but not in total postoperative chest tube bleeding. A possible explanation for this could be that the chest tube drainage during the first 12 postoperative hours is more prone to reflect the true amount of bleeding contrary to the more serous content including pleural effusion after the first postoperative hours.
Postoperative blood loss and transfusion of blood components are risk factors known to be associated with decreased long-term survival following coronary artery bypass grafting surgery (Citation16). However, adequate anti-ischemic protection before surgery is of crucial importance in preventing thrombus-related events (Citation2). Fondaparinux has been tested thoroughly in the OASIS-5 and OASIS-6 trials (Citation17,Citation18) and found to be superior to enoxaparin for patients with NSTE-ACS in terms of the composite endpoint of death, myocardial infarction, refractory ischemia or major bleeding by day 9. Our study shows that postoperative bleeding and transfusion of blood components did not differ between anticoagulant agents suggesting the non-inferiority of fondaparinux compared to enoxaparin, which further supports the use of fondaparinux in patients with NSTE-ACS.
Other factors that must be addressed when evaluating an anticoagulant drug for use in this setting is the risk of heparin-induced thrombocytopenia (HIT). A multicenter study showed that fondaparinux had no effect on platelet activation in the presence of heparin-dependent antibodies (Citation19). Fondaparinux has also been used as an alternative anticoagulant in HIT patients (Citation20,Citation21), although a case of delayed-onset HIT has been reported with the use of fondaparinux (Citation22). The price of fondaparinux is also lower than that of enoxaparin in several markets, making it a more cost-effective antithrombotic agent (Citation23). Fondaparinux has replaced enoxaparin as the drug of choice for treating patients with NSTE-ACS at many cardiac centres in Sweden, and the data of the present study provides no cause to discontinue the widespread switch from enoxaparin to fondaparinux.
The study has a few weaknesses. First, it was not powered to detect minor differences in outcome between the two groups. However, with the fairly large cohort of 147 patients in the study, a clinically relevant difference would be detected. In addition, there were small demographic differences between groups. Patients with enoxaparin were treated for a slightly longer time period, most likely because they often came from other hospitals, and therefore waited longer for surgery than did in-house patients. From our data it is difficult to determine whether this affected results. Besides this finding, the differences between groups could be considered negligible. Fondaparinux is eliminated by the kidneys, and is contraindicated if creatinine clearance is lower than 30 mL/min. This study only had S-creatinine as a marker for renal function, and patients with an impaired renal clearance were not studied separately. It should also be noted that elimination of enoxaparin occurs both by liver metabolism and renal excretion. When studying outcome in bleeding, the 12 hour bleeding slightly favors enoxaparin and total bleeding favors fondaparinux. Therefore, no obvious trend can be detected. The same applies for transfusion of blood components in both groups. Also, it is a non-randomized study. A randomized study would be difficult to design as our centre receives patients from several referring hospitals in southern Sweden, and these centres have different approaches to what anticoagulant agent to choose. Instead we used a study protocol where the inflow of patients from the referring centres shifted over time from enoxaparin in favour of fondaparinux (). In this way, we avoided selection bias and studied a patient population that was very similar to the patients referred in everyday practice.
Discontinuation of fondaparinux has been recommended at 24 h prior to surgery because the half-life of fondaparinux is 17 h. Given that the drug often is administered in the evening, our local recommendations are to discontinue fondaparinux at least 36 h before surgery. The patients with the most postoperative bleeding had fondaparinux discontinued less than 36 h prior to surgery. This supports the recommendation to discontinue fondaparinux at least 24 h (Citation3,Citation4) before cardiac surgery, or, as in our study, at 36 h before cardiac surgery.
Every anticoagulant anti-ischemic effect must be measured against the risks of bleeding complications associated with surgery. Surgeons are well familiar with the dramatic increase in bleeding and incidence of reexploration that occurred when clopidogrel was introduced. This increased bleeding and incidence of blood product transfusion was later confirmed in several independent studies (Citation10,Citation11). Therefore, any new anticoagulant regime, regardless of its effectiveness, will be met by skepticism by surgeons. Some studies have also shown that preoperative use of aspirin and clopidogrel was not associated with excessive postoperative bleeding or incidence of reoperation (Citation24,Citation25), but this is contrary to the widespread experience of surgeons. This furthermore emphasizes the need for objective evaluation of every new anticoagulant drug in relation to surgical considerations.
In the OASIS-5 trial, fondaparinux was compared to enoxaparin, and shown to have similar short-term efficacy but reduced major bleeding by 48% and 30-day mortality by 17% (Citation13). This has led to The European Society of Cardiology (ESC) recommendation of fondaparinux in their guidelines from 2007 for NSTE-ACS (Citation4). Our study suggests that fondaparinux discontinued 36 h prior to cardiac surgery has the same result as enoxaparin does in bleeding, blood product transfusion and incidence of reoperation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Low-molecular-weight heparin during instability in coronary artery disease, Fragmin during Instability in Coronary Artery Disease (FRISC) study group. Lancet. 1996;347:561–8.
- Long-term low-molecular-mass heparin in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. FRagmin and Fast Revascularisation during InStability in Coronary artery disease. Investigators. Lancet. 1999;354:701–7.
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr., . ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157.
- Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, . Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–660.
- Harrington RA, Becker RC, Cannon CP, Gutterman D, Lincoff AM, Popma JJ, . Antithrombotic therapy for non-ST-segment elevation acute coronary syndromes: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133:670S–707S.
- Bassand JP, Hamm C. Guidelines for anticoagulant use in acute coronary syndromes. Lancet. 2008;372:532–3; author reply 3–4.
- Bassand JP. The place of fondaparinux in the ESC and ACC/AHA guidelines for anticoagulation in patients with non-ST elevation acute coronary syndromes. Eur Heart J Suppl. 2008:C22–C29.
- Gulbins H, Malkoc A, Ennker IC, Ennker J. Preoperative platelet inhibition with ASA does not influence postoperative blood loss following coronary artery bypass grafting. Thorac Cardiovasc Surg. 2009;57:18–21.
- Kincaid EH, Monroe ML, Saliba DL, Kon ND, Byerly WG, Reichert MG. Effects of preoperative enoxaparin versus unfractionated heparin on bleeding indices in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2003;76:124–8; Discussion 8.
- Purkayastha S, Athanasiou T, Malinovski V, Tekkis P, Foale R, Casula R, . Does clopidogrel affect outcome after coronary artery bypass grafting? A meta-analysis. Heart. 2006;92:531–2.
- Kunadian B, Thornley AR, Tanos M, Dunning J. Should clopidogrel be stopped prior to urgent cardiac surgery? Interact Cardiovasc Thorac Surg. 2006;5:630–6.
- Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: A multicenter analysis. J Am Coll Cardiol. 2008;52:1693–701.
- Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, . Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354:1464–76.
- Turpie AG. Selective factor Xa inhibition with forndaparinux: From concept to clinical benefit. Eur Heart J Suppl 2008;10:C1–C7.
- JL A. ACC/AHA 2007 Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction-executive summary. J Am Coll Cardiol. 2007;50:652–726.
- Kuduvalli M, Oo AY, Newall N, Grayson AD, Jackson M, Desmond MJ, . Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2005;27:592–8.
- Mehta SR, Granger CB, Eikelboom JW, Bassand JP, Wallentin L, Faxon DP, . Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: Results from the OASIS-5 trial. J Am Coll Cardiol. 2007;50:1742–51.
- Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, . Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: The OASIS-6 randomized trial. JAMA. 2006;295:1519–30.
- Savi P, Chong BH, Greinacher A, Gruel Y, Kelton JG, Warkentin TE, . Effect of fondaparinux on platelet activation in the presence of heparin-dependent antibodies: A blinded comparative multicenter study with unfractionated heparin. Blood. 2005;105:139–44.
- Neykova A. The HIT treatment in a cardiac surgery patient. Int J Cardiol. 2009 (in press).
- Everett BM, Yeh R, Foo SY, Criss D, Van Cott EM, Laposata M, . Prevalence of heparin/platelet factor 4 antibodies before and after cardiac surgery. Ann Thorac Surg. 2007;83:592–7.
- Alsaleh KA, Al-Nasser SM, Bates SM, Patel A, Warkentin TE, Arnold DM. Delayed-onset HIT caused by low-molecular-weight heparin manifesting during fondaparinux prophylaxis. Am J Hematol. 2008;83:876–8.
- Sculpher MJ, Lozano-Ortega G, Sambrook J, Palmer S, Ormanidhi O, Bakhai A, . Fondaparinux versus Enoxaparin in non-ST-elevation acute coronary syndromes: Short-term cost and long-term cost-effectiveness using data from the Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators (OASIS-5) trial. Am Heart J. 2009;157:845–52.
- Kim JH, Newby LK, Clare RM, Shaw LK, Lodge AJ, Smith PK, . Clopidogrel use and bleeding after coronary artery bypass graft surgery. Am Heart J. 2008;156:886–92.
- Shim JK, Choi YS, Oh YJ, Bang SO, Yoo KJ, Kwak YL. Effects of preoperative aspirin and clopidogrel therapy on perioperative blood loss and blood transfusion requirements in patients undergoing off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2007;134:59–64.