Abstract
Objectives. The aim of this study was to retrospectively evaluate three risk scoring methods in predicting outcome after elective endovascular repair of an abdominal aortic aneurysm. Design. A Zenith stent graft was employed in 205 patients during years 2001–2005. Results. The 30-day postoperative mortality rate was 2.9%. Receiver operating characteristics (ROC) curve analysis showed that the Glasgow aneurysm score (GAS) (AUC: 0.843, p=0.004) and the Giles' score (AUC 0.815, p=0.009) had a rather large area under the curve in predicting 30-day mortality rate. The modified Leiden score was much less accurate (AUC: 0.594). The best cut-off value for the GAS in predicting 30-day mortality was 90 (0.6% vs. 17.9%, p<0.0001). Patients with a GAS ≥ 90 had a 4-year survival rate of 56.8%, whereas it was 78.5% among those with a lower GAS (p = 0.001).The best cut-off value for the Giles' score was 11 (1.3% vs. 8.3%, p<0.0001). Patients with a Giles' score ≥ 11 had a 4-year survival rate of 63.9%, whereas it was 79.0% among those with a lower score (p = 0.016). Conclusions. The GAS and Giles' risk scoring methods are good predictors of poor immediate and late outcome after EVAR.
Quantification of operative risk is of paramount importance in patients with an abdominal aortic aneurysm (AAA), as it may help in deciding whether to opt for open surgery, endovascular repair (EVAR) or conservative treatment. Risk prediction also allows a comparison between different institutions or surgeons as well as treatment methods.
In the case of EVAR, a reliable risk scoring system would be an important instrument for planning resource utilization and avoiding endovascular treatment in patients with expected high immediate postoperative mortality/morbidity or short life expectancy.
The Glasgow aneurysm score (GAS) (Citation1) and the modified Leiden score (Citation2) have been shown to perform well in predicting postoperative mortality and morbidity in patients undergoing elective open repair of an AAA (Citation3). Recently, Giles et al. (Citation4) proposed a risk scoring method from a Medicare database that included patients who underwent elective open or endovascular AAA repair. We have evaluated the predictive value of these risk scoring methods in a series of patients who underwent elective EVAR with a Zenith stent graft.
Materials and methods
From January 2000 to December 2005, 205 patients underwent endovascular repair of an intact, asymptomatic AAA employing a Zenith stent graft at the Oulu University Hospital (49), Kuopio University Hospital (99) and Helsinki University Hospital (57), in Finland. Permission to use nationwide data was obtained from the Ministry of Social Affairs and Health. The institutional review board waivered the need for their approval for this retrospective study. The clinical data were collected retrospectively from patients' records. Causes of deaths were obtained from the National Center of Statistics.
We included in this study only patients who underwent elective repair of an infrarenal AAA. Patients who underwent urgent repair of a symptomatic AAA or isolated repair of aneurysms in the iliac arteries were excluded from the study. The patients' characteristics are reported in The mean patient age was 73.3 years, with 87.3% being male.
Table I. Preoperative patients' characteristics.
Operative risk was assessed according to the Glasgow aneurysm score by adding the following weighting scores: age in years, myocardial disease +7, cerebrovascular disease +10, and renal disease (serum creatinine > 150 (μmol/L) +14 (Citation1). Myocardial disease was defined as previous documented myocardial infarction and/or coronary artery revascularization and/or ongoing angina. Cerebrovascular disease referred to stroke or transient ischemic attack (TLA).
The modified Leiden score was calculated by adding the following weighting scores: age, 0 if 70 years, 1 point for every 2.5 years of age (60 years = −4; 80 years = +4), female gender +4, history of myocardial infarction +3, chronic heart failure +8, renal disease (serum creatinine > 160 (μmol/L) +12, and pulmonary disease + 7 (Citation2). Since we did not have preoperative spirometry values of these patients, in all cases we classified pulmonary disease as a condition requiring use of corticosteroids and/or bronchodilators.
The Giles' risk score was calculated by adding the following weighting scores: age > 80 years +11, age 76–80 years +6, age 71–75 years +1, female gender +4, dialysis +9, no dialysis dependent renal failure +7, congestive heart failure +6 and peripheral vascular disease and/or cerebrovascular disease +3 (Citation4). Renal failure was defined as serum creatinine > 150 (μmol/L. Cerebrovascular diseases referred to stroke or transient ischemic attack (TIA).
The main outcome end-point measures of this study were 30-day postoperative mortality, severe immediate postoperative complications (stroke, transient ischemic attack, myocardial infarction, intensive care unit stay ≥ 5 days, respiratory failure/ pneumonia, and intestinal ischemia) and all-cause late mortality.
Statistical analysis
Statistical analysis was performed using SPSS statistical software (SPSS 16.0.1, Chicago, Illinois 60606, USA). Receiver operating characteristic (ROC) curve analysis was used to estimate the area under the curve of continuous variables in predicting immediate outcome. Univariate analysis was performed with the Fisher's exact test. The best cut-off value for each risk score was identified as the one with the highest sensitivity, specificity, accuracy and odds ratio. Long-term survival was estimated with the Kaplan-Meier's test and the log-rank test. A value of p<0.05 was considered statistically significant.
Results
The mean preoperative aneurysm size was 62±10 mm (median 60 mm, range 38–97 mm). The anaesthetic technique was epidural in 159 patients (77.6%), general in 45 patients (22.0%) and local in one patient (0.5%). Bifurcated grafts were used in 197 of 205 patients (96.1%), aortouni-iliac grafts in seven patients (3.4%) and a tubular graft in one patient (0.5%). The mean follow-up period was 2.46±1.66 years (median 2.37, range 0–6.21).
The 30-day mortality rate of this series was 2.9% (6/205). Two patients died from myocardial infarction, one from colon ischemia and peritonitis, one from intraoperative bleeding. One patient succumbed to pulmonary artery perforation and intrathoracic haemorrhage after insertion of a Swan-Ganz catheter. One patient developed renal and liver failure as well as bleeding from a gastric ulcer. Severe complications occurred in 9.3% of the patients. The postoperative complications are summarized in
Table II. Postoperative complications.
The ROC curve analysis showed that the GAS (AUC: 0.843; 95% C.I. 0.627–1.058, p=0.004) and the Giles' score (AUC: 0.815; 95% C.I. 0.635–0.995, p=0.009), but not the modified Leiden score (AUC: 0.594; 95% C.I. 0.342–0.846, p=0.434), had a rather large area under the curve in predicting 30-day mortality rate (). The highest GAS quartile (30-day mortality: 1.9% (1/53), 0% (0/49), 0% (0/50), and 9.4% (5 /53), Fisher's exact test, Monte C arlo method: p=0.015) and the Giles' score quartile (30-day mortality: 0% (0/47), 1.9% (1/53), 1.8% 1 (57) and 8.3% (4 /48), Fisher's exact test, Monte Carlo method: p = 0.11) showed an increased risk of immediate postoperative mortality after EVAR.
Figure 1. The ROC curve analysis showed that the GAS and the Giles' score, but not the modified Leiden, had a rather large area under the curve in predicting 30-day mortality rate.
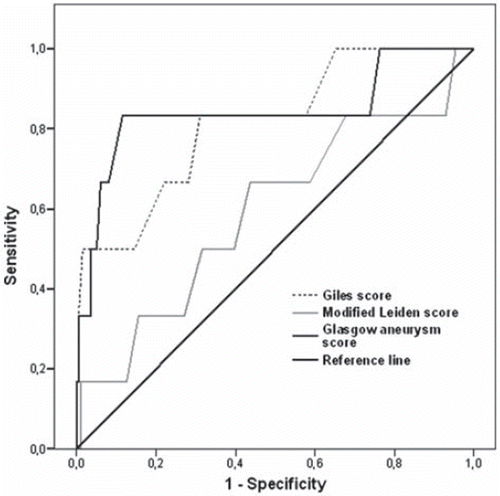
The GAS (AUC: 0.700; 95% C.I. 0.582–0.819, p=0.004) and the Giles' score (AUC: 0.643; 95% C.I. 0.510–0.776, p=0.040), but not the modified Leiden score (AUC: 0.533, 95% C.I. 0.396–0.670, p=0.638), were also predictive of severe postoperative complications.
The best cut-off value for the GAS in predicting 30-day mortality was 90 (0.6% vs. 17.9%,p<0.0001, sensitivity 83.3%, specificity 88.4%, accuracy 88.3%). Patients with a GAS ≥ 90 had a 4-year survival rate of 56.8%, whereas it was 78.5% among those with a lower GAS (p=0.001).
The best cut-off value for the Giles' score in predicting 30-day mortality was 11 (1.3% vs. 8.3%, p<0.0001, sensitivity 66.7%, specificity 77.9%, accuracy 77.6%). Patients with a Giles' score ≥ 11 had a 4-year survival rate of 63.9%, whereas it was 79.0% among those with a lower score (p=0.016).
Discussion
Preoperative estimation of operative risk plays a major role in selecting patients for EVAR and it is mainly based on the clinician's perception of risk. It is essential to objectively estimate whether the risk of postoperative mortality is likely be higher than the short-term risk of aneurysm rupture. There is a need for a simple and reliable risk scoring system, which can be easily calculated at the bed side. Many AAA-specific scoring systems have been developed (Citation1,Citation2, Citation4–7). Most of these scoring systems were primary validated for accurate prediction of outcome after elective open repair of an abdominal aortic aneurysm. The GAS has also been evaluated as a predictor of survival following EVAR (Citation8–10) and the Giles' score was developed as a predictive model of perioperative mortality after EVAR (Citation4). We have identified and evaluated three possibly suitable risk scoring methods for EVAR in this study.
The GAS and Giles' risk scoring methods proved to be good predictors of postoperative mortality. Both scores reached an area under the receiver operating characteristics curve of larger than 0.80. Previous studies have reported lower predictive values for the GAS and Giles' risk scoring methods (Citation4,Citation8–10). Herein, the modified Leiden score failed to show any significant predictive value. We do not have any explanation why this score did not perform well in this series. We can just speculate that it may perform well in lower risk patients (Citation3), but not in such high-risk patients. Indeed, marked differences exist in the variables included in these risk scoring methods and their prognostic weights.
The GAS seems to be the most feasible and usable, as there are only four variables involved, which are available for every patient requiring elective aneurysm repair. The GAS has also been previously shown to be a good predictor of mortality after both open and endovascular repair of an abdominal aortic aneurysm (Citation8,Citation9). On the other hand, Bohm et al. showed in their study that the GAS was a poor predictor of early mortality and morbidity following EVAR (Citation10). Patterson et al. (Citation11) also concluded in their review article, that there is no existing score that consistently predict the risk associated with EVAR.
The present study showed that the best cut-off value for the GAS was higher than previously reported (90 vs. 86.5 / 86.6) (Citation8,Citation9). Such a high cut-off value confirms the known fact that EVAR is associated with low postoperative mortality, despite increased operative risk. However, we must remember that identification of cut-off values is not easy and reliable, and herein we have chosen them according to their best sensitivity, specificity, accuracy and odds ratios. Despite these limitations, such cut-off values have been shown to also predict the intermediate survival of these patients rather well.
There are a few limitations in this study. Besides the retrospective nature of this study, we did not have all the details of the definition criteria of the Giles' risk scoring method.
In conclusion, the GAS and Giles' risk scoring methods proved to be good predictors of poor immediate and late outcome after EVAR. The GAS seems to be the most reliable risk scoring method and most easy to use at the bed side.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We conclude, that none the authors have identified a potential conflict of interest.
References
- Samy AK, Murray G, MacBain G. Glasgow aneurysm score. Cardiovasc Surg. 1994;2:41–4.
- Kertai MD, Steyerberg EW, Boersma E, Bax JJ, Vergouve Y, van Urk H, . Validation of two risk models for perioperative mortality in patients undergoing elective abdominal aortic aneurysm surgery. Vasc Endovascular Surg. 2003;37:13–21.
- Nesi F, Leo E, Biancari F, Bartolucci R, Rainio P, Satta J, . Preoperative risk stratification in patients undergoing elective infrarenal aortic aneurysm surgery: Evaluation of five risk scoring methods. Eur J Vasc Endovasc Surg. 2004;28: 52–8.
- Giles KA, Schermerhorn ML, O'Malley AJ, Cotteril P, Jhaveri A, Pomposelli FB, . Risk prediction for perioperative mortality of endovascular vs open repair of abdominal aortic aneurysms using the Medicare population. J Vasc Surg. 2009.
- Eagle KA, Coley CM, Newell JB, Brewster DC, Darling RC, Straus HW, . Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110:859–66.
- Steyerberg EW, Kievit J, De Mol Van Otterloo JC, van Bockel JH, Eijkemans MJ, Habbema JD. Perioperative mortality of elective abdominal aortic aneurysm surgery. A clinical prediction rule based on literature and individual patient data. Arch Intern Med. 1995;155:1998–2004.
- Vanzetto G, Machecourt J, Blendea D, Fagret D, Borrel E, Magne JL, . Additive value of thallium single-photon emission computed tomography myocardial imaging for prediction of perioperative events in clinically selected high cardiac risk patients having abdominal aortic surgery. Am J Cardiol. 1996;77:143–8.
- Baas AF, Janssen KJ, Prinssen M, Buskens E, Blankensteijn JD. The Glasgow Aneurysm Score as a tool to predict 30-day and 2-year mortality in the patients from the Dutch Randomized Endovascular Aneurysm Management trial. J Vasc Surg. 2008;47:277–81.
- Biancari F, Hobo R, Juvonen T. Glasgow Aneurysm Score predicts survival after endovascular stenting of abdominal aortic aneurysm in patients from the EUROSTAR registry. Br J Surg. 2006;93:191–4.
- Bohm N, Wales L, Dunckley M, Morgan R, Loftus I, Thompson M. Objective risk-scoring systems for repair of abdominal aortic aneurysms: Applicability in endovascular repair? Eur J Vasc Endovasc Surg. 2008;36:172–7.
- Patterson BO, Holt PJ, Hinchliffe R, Loftus IM, Thompson MM. Predicting risk in elective abdominal aortic aneurysm repair: A systematic review of current evidence. Eur J Vasc Endovasc Surg. 2008;36:637–45.