Abstract
Objectives. Patients with aortic stenosis (AS) develop left ventricular remodeling characterized by changes in extracellular matrix (ECM) and cardiomyocyte-hypertrophy. Aortic valve replacement (AVR) reverses this process (reverse remodeling). We examined plasma levels of interleukin-18 (IL-18) and its binding protein (IL-18BP) before and after AVR for AS since these mediators have been shown experimentally to exert effects on myocardial remodeling. Design. Plasma levels of IL-18 and IL-18BP were analyzed in 22 patients with AS undergoing AVR, preoperatively, two days, six and 12 months postoperatively. Echocardiography and functional testing were performed. Results. IL-18BP was significantly increased by 28% and 15% at two days and six months after AVR, compared to preoperative values. In contrast, IL-18 showed a later peak (increased by 24% at 12 months postoperatively) when IL-18BP was normalized. IL-18 correlated positively with deceleration time (R = 0.44) at this time-point which might indicate an association with diastolic function. Conclusions. We report for the first time that plasma IL-18 and IL-18BP are differentially regulated after AVR for AS with an early increase in IL-18BP postoperatively followed by a later peak in IL-18 at 12 months. Given the known effects of these mediators on myocardial remodeling and function, they might play a role in the reverse and remodeling process associated with AVR.
As a result of pressure overload, patients with aortic stenosis (AS) develop left ventricular remodeling. Myocardial remodeling consists of hypertrophy of car-diomyocytes combined with structural changes of the extracellular matrix including increased collagen synthesis and fibrosis. After aortic valve replacement (AVR), reverse remodeling normally follows. Within 12 to 18 months a normal left ventricular mass might be reached (Citation1, Citation2). During this process the normalization of cardiomyocyte hypertrophy occurs first, while the phase of extracellular matrix restoration might take several years (Citation3). These processes might be regulated by different mediators including cytokines and growth factors. However, little is known regarding alterations of potential mediators of remodeling and reverse and remodeling associated with AS and AVR.
Pressure overload increases wall-stress in the myocardium. The myocardium responds to stress by synthesizing various cytokines (Citation4–6). Interestingly, interleukin-18 (IL-18) and its naturally circulating binding protein (IL-18BP) have been shown to be involved in myocardial hypertrophy (Citation7–9), myocardial fibrosis (Citation10,Citation11) and myocardial dysfunction (Citation7,Citation10,Citation12). Thus, IL-18 and its binding protein IL-18BP might be of particular interest in pathophysiology of remodeling in AS and the reverse remodeling taking place after AVR. To the best of our knowledge no information exists regarding plasma levels of these potent cytokines in AS and after AVR.
Thus, the aim of the present study was to examine plasma levels of IL-18 and IL-18BP before and after AVR for AS. Furthermore, we wanted to relate these levels to the reverse remodeling process. Twenty-two patients were included in the study. Plasma IL-18 and IL-18BP were measured preoperatively, at two days, and at six and 12 months postoperatively. Additionally, echocardiography was performed at the same time-points. To evaluate the patients physical condition an estimation of NYHA class and a six minute walking test were carried out preoperatively and at six and 12 months postoperatively.
Material and methods
Patients and protocol
We included prospectively 22 patients with severe aortic stenosis (symptomatic aortic stenosis or aortic valve area <0.7 cm2 or mean aortic gradient >50 mmHg). The patients were a subgroup included in the ASSERT multicenter trial (Citation1). Only patients receiving a porcine bioprosthesis were included. The patients received either a stentless valve (Freestyle, Medtronic, Inc.) or a stented valve (Mosaic, Medtronic, Inc., Minneapolis, Minnesota, USA). Both valves are preserved with the same anti-calcification protocol and composed of the same material. In our department we use aspirin for three months and not warfarin in patients receiving a bioprosthesis. No additional valve surgery was performed in any of the patients, however, coronary artery bypass grafting (CABG) was performed in eight patients (see for patient characteristics). Blood samples were collected and echocardiographic measurements were performed preoperatively, before discharge (normally on the second postoperative day) and at six and 12 months. A trained cardiologist determined NYHA class and monitored a six minute walk test preoperatively and at six and 12 months postoperatively. Informed written consent was obtained from all patients. The study protocol was approved by the local ethical committee and conforms with the Declaration of Helsinki. We have complete follow-up data on all patients included in the study.
Table I. Patient characteristics and peroperative data.
Plasma analyses
An antecubital vein was used for blood sampling into chilled, standard EDTA tubes (1 mg/ml blood) and kept on ice. Within 30 minutes the blood was centrifuged, the plasma fraction immediately frozen and stored at −70°C until analyzed. IL-18, and IL-18BP were analyzed using commercially available kits (R&D, USA). The IL-18 assay (catalogue no. 7620) has a detection limit of 12.5 pg/ml and does not cross-react with a multitude of recombinant cytokines. The IL-18BP assay (catalogue nr DY-119) does not cross-react with human IL-18 in concentrations less than 1 600 pg/ml. The intra- and inter-assay variations are below 10%. Twenty-one people with no known history of cardiovascular disease (mean age 68.2±2.0 years) served as healthy controls.
Echocardiography
Echocardiographic examination was performed as previously described (Citation1,Citation6) and included two-dimensional M-mode and colour Doppler. An experienced echocardiographer “blinded” to patient details analyzed all recordings. Aortic flow velocities were measured by the use of continuous-wave Doppler. Left ventricular mass index (LVMI) was calculated using the Penn Convention formula for determination of LVM and divided by body surface area. The E/A ratio was calculated as the ratio between the early maximal ventricular filling velocity and the late diastolic or atrial velocity. An E/A ratio less than 1 is traditionally accepted as being indicative of the presence of diastolic dysfunction.
Statistical analyses
Data are presented as means ± standard error of the mean (SEM) and were analyzed using SigmaPlot version 11, Jandel Scientific GmbH, Erkrath, Germany. One way ANOVA or one way repeated measures ANOVA were used where appropriate. For data that were not normally distributed, one way ANOVA on ranks was used. The Holm-Sidak or the Dunn method was used to correct for multiple comparisons. The data were logarithmically transformed to fit a normal distribution examining the relation between two continuous variables by linear regression. p ≤ 0.05 was considered statistically significant.
Results
Patient characteristics
describe patient characteristics. Peak aortic gradient (PAG), LVMI, NYHA class and walking distance improved significantly 12 months post-operatively. CRP was, as expected, transiently increased two days postoperatively. Mean creatinine values were unchanged throughout the follow-up period. Patient medication is presented in .
Table II. Patient medications.
Table III. Hemodynamic and functional data.
IL-18 measurements
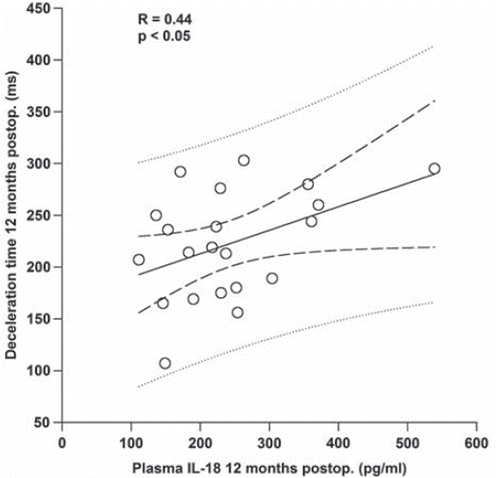
Preoperatively, mean plasma IL-18 was 192.1 ± 15.4 pg/ml which did not differ significantly from healthy controls (192.5±15.7 pg/ml). At two days and six months postoperatively respectively, plasma IL-18 was 205.4 ± 16.1 pg/ml and 216.6 ± 13.4 pg/ml. At 12 months there was a 24% increase in plasma IL-18 (238.8 ± 21.2 pg/ml; , panel A) compared to the preoperative value. Deceleration time, a measure of diastolic function of the left ventricle, correlated positively with IL-18 at 12 months postoperatively (R = 0.44, p < 0.05, ).
Figure 1. Plasma IL-18 (panel A) and IL-18 BP (panel B) levels preoperatively (PREOP), two days postoperatively (2dPO), six months postoperatively (6mPO) and 12 months postoperatively (12mPO). Data are presented as means ± SEM. * = p ≤ 0.05.
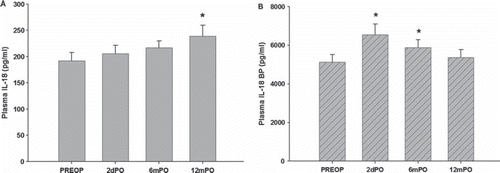
Figure 2. Correlation between left ventricular deceleration time and plasma IL-18 at 12 months postoperatively (postop.). Dashed lines show prediction and confidence intervals.
Mean LVMI was 198.3 ± 14.9 g/m2 preoperatively and decreased significantly to 119.7±7.2 g/m2 at 12 months. There was, however, no correlation between IL-18 and LVMI either preoperatively or at 12 months, neither was there any correlation between IL-18 and preoperative gradient across the aortic valve.
There were significant improvements in NYHA class and walking distance at 12 months compared to the values preoperatively, however, no correlation between the two and IL-18 levels either preoperatively or at 12 months postoperatively.
IL-18 binding protein
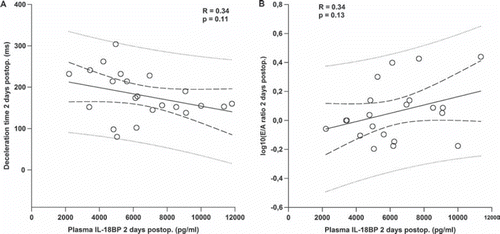
Preoperatively plasma IL-18BP in patients with aortic stenosis was 5120 ± 405 pg/ml. This was not significantly different from controls (4541 ± 637 pg/ml). At two days and six months postoperatively there was a significant increase to 6547 ± 549 and 5884±398 pg/ml, respectively (, panel B). At 12 months postoperatively IL-18BP was 5367±400 pg/ml which was no longer significantly different from the preoperative value. At six months there was a positive correlation between IL-18BP and peak aortic gradient (R = 0.54, p = 0.02) and a negative correlation between IL-18BP and aortic orifice area (R = 0.49, p = 0.04). Moreover, at two days postoperatively when IL-18BP reached the highest value, there was a borderline positive and negative correlation between IL-18BP and E/A ratio (R = 0.34 and p = 0.13) and deceleration time (R = 0.34 and p = 0.11), respectively (, panel A and B). There was no correlation between IL-18 and IL-18BP at any time-point.
Discussion
This study demonstrates that circulating plasma levels of IL-18 and IL-18BP are differentially regulated after AVR for AS. In particular, IL-18BP is significantly upregulated two days and six months after AVR, whereas IL-18 has a later peak and is increased by 24% at 12 months postoperatively when IL-18BP is back to preoperative values. IL-18 correlates positively with deceleration time at this time-point whereas IL-18BP correlates positively with peak aortic gradient at six months postoperatively.
It is well known that after operation for aortic stenosis, the hypertrophy of cardiomyocytes and left ventricular mass are almost normalized within a year, whereas myocardial fibrosis might persist for several years (Citation3). In the present study we did confirm the reduction in left ventricular mass which was almost normalized at six months. It is known that IL-18 has both hypertrophic (Citation7–9) and profibrotic (Citation10) effects in vivo. In an elegant study by Reddy and coworkers (Citation11) they reported that IL-18 stimulates fibronectin expression in human fibroblasts via NF-kappa B activation. Fibronectin is a key component of ECM and basal lamina (Citation13) and is expressed within the normal adult heart (Citation14). Fibronectin induces a pro-hypertrophic gene program and car-diomyocyte hypertrophy (Citation14–16) and is associated with expression of ECM proteins and in particular collagens (Citation17). Since IL-18 induces fibrosis (Citation10) and reduces left ventricular compliance (Citation10) it might contribute to a prolonged diastolic dysfunction (Citation10) and fibrosis after aortic valve replacement for aortic stenosis. This might also be supported by the findings in the present study of a positive correlation between IL-18 and deceleration time at 12 months postoperatively. Interestingly, we measured a significant increase in IL-18BP at two days and six months postoperatively. The naturally circulating peptide IL-18BP is known to counteract the effects of IL-18 (Citation12). Given the known hypertrophic effects of IL-18 (Citation7–9), the increase in IL-18BP in the early phase after AVR might counterbalance the hypertrophic stimulus of IL-18 in this period and thus contribute to reduction in left ventricular mass observed during this time span. There was also a borderline positive and negative correlation between IL-18BP and E/A ratio and deceleration time, respectively. This might support the suggestion that IL-18BP counteracts the negative effects of IL-18 on diastolic dysfunction although the interpretation of E/A ratio and deceleration time might be difficult in the early postoperative phase.
We did only measure IL-18 and IL-18BP in circulating plasma in the present study. The site of production of the elevated levels or what stimuli induce the production, are not known. We have, however, previously shown that IL-18 might be synthesized in the heart (Citation5). Thus, this might be a possible site of production also in the present study although further studies are needed to clarify this. In a study by Thompson and coworkers IL-18 has been shown to be transiently increased at six hours after coronary artery bypass surgery (CABG) (Citation19). We did not find any increase in IL-18 at two days following AVR in the present study. However, IL-18BP was increased both at two days postoperatively in the present study and at 24 hours following CABG (Citation19). This might suggest that the surgical procedure as such might be implicated in the early increase in IL-18BP following AVR and CABG.
In conclusion, we report for the first time that circulating plasma levels of IL-18 and IL-18BP are differentially regulated after AVR for AS. IL-18BP is significantly upregulated two days and six months after AVR, whereas IL-18 has a later peak and is increased by 24% at 12 months postoperatively. IL-18 correlates positively with deceleration time at this time-point whereas IL-18BP correlates positively with peak aortic gradient at six months postoperatively. Given the known effects of IL-18 on myocardial remodeling and function, IL-18 and it's naturally inhibiting binding protein might play a role in the remodeling associated with operation for aortic stenosis.
Acknowledgements
The authors are grateful to the staff at Department of Cardiothoracic Surgery and Department of Cardiology at Ullevål University Hospital, Oslo, Norway and Clinical Trials and Evaluation Unit, Royal Brompton Hospital, London, UK. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology (Citation18). The study was supported by research grants from Medtronic, Inc., from Center for Heart Failure Research, University of Oslo and The Ingegerd and Viking Olov Bjørk Scholarship received by Professor Theis Tønnessen.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Perez de AD, Lees B, Flather M, Nugara F, Husebye T, Jasinski M, . Randomized comparison of stentless versus stented valves for aortic stenosis: Effects on left ventricular mass. Circulation. 2005;112:2696–702.
- Lund O, Kristensen LH, Baandrup U, Hansen OK, Nielsen TT, Emmertsen K, . Myocardial structure as a determinant of pre- and postoperative ventricular function and long-term prognosis after valve replacement for aortic stenosis. Eur Heart J. 1998;19:1099–108.
- Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989;79:744–55.
- Tønnessen T, Giaid A, Saleh D, Naess PA, Yanagisawa M, Christensen G. Increased in vivo expression and production of endothelin-1 by porcine cardiomyocytes subjected to ischemia. Circ Res. 1995;76:767–72.
- Woldbaek PR, Tønnessen T, Henriksen UL, Florholmen G, Lunde PK, Lyberg T, . Increased cardiac IL-18 mRNA, pro-IL-18 and plasma IL-18 after myocardial infarction in the mouse; a potential role in cardiac dysfunction. Cardiovasc Res. 2003;59:122–31.
- Neverdal NO, Knudsen CW, Husebye T, Vengen OA, Pepper J, Lie M, . The effect of aortic valve replacement on plasma B-type natriuretic peptide in patients with severe aortic stenosis–one year follow-up. Eur J Heart Fail. 2006;8:257–62.
- Woldbaek PR, Sande JB, Strømme TA, Lunde PK, Djurovic S, Lyberg T, . Daily administration of inter-leukin-18 causes myocardial dysfunction in healthy mice. Am J Physiol Heart Circ Physiol. 2005;289:H708–H714.
- Chandrasekar B, Mummidi S, Claycomb WC, Mestril R, Nemer M. Interleukin-18 is a pro-hypertrophic cytokine that acts through a phosphatidylinositol 3-kinase-phosphoinositide-dependent kinase-1-Akt-GATA4 signaling pathway in car-diomyocytes. J Biol Chem. 2005;280:4553–67.
- Colston JT, Boylston WH, Feldman MD, Jenkinson CP, de la Rosa SD, Barton A, . Interleukin-18 knockout mice display maladaptive cardiac hypertrophy in response to pressure overload. Biochem Biophys Res Commun. 2007;354:552–8.
- Platis A, Yu Q, Moore D, Khojeini E, Tsau P, Larson D. The effect of daily administration of IL-18 on cardiac structure and function. Perfusion. 2008;23:237–42.
- Reddy VS, Harskamp RE, van Ginkel MW, Calhoon J, Baisden CE, Kim IS, . Interleukin-18 stimulates fibronectin expression in primary human cardiac fibroblasts via PI3K-Akt-dependent NF-kappa B activation. J Cell Physiol. 2008;215:697–707.
- Dinarello CA. Novel targets for interleukin 18 binding protein. Ann Rheum Dis. 2001;60(Suppl 3):iii18–iii24.
- Weber KT, Brilla CG, Janicki JS, Reddy HK, Campbell SE. Myocardial fibrosis: Role of ventricular systolic pressure, arterial hypertension, and circulating hormones. Basic Res Cardiol. 1991;86(Suppl 3):25–31.
- Farhadian F, Contard F, Corbier A, Barrieux A, Rappaport L, Samuel JL. Fibronectin expression during physiological and pathological cardiac growth. J Mol Cell Cardiol. 1995;27:981–90.
- Chen H, Huang XN, Stewart AF, Sepulveda JL. Gene expression changes associated with fibronectin-induced cardiac myocyte hypertrophy. Physiol Genomics. 2004;11;18:273–83.
- Ulrich MM, Janssen AM, Daemen MJ, Rappaport L, Samuel JL, Contard F, . Increased expression of fibronectin isoforms after myocardial infarction in rats. J Mol Cell Cardiol. 1997;29:2533–43.
- Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, . Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res. 2000;86:846–53.
- Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–50.
- Scanders J, Brull D, Cooper J, Thompson SR, Novick D, Stock CJ, . Free interleukin (IL)-18 levels, and the impact of IL18 and IL18BP genetic variation, in CHD patients and healthy men. Arterioscler Thromb Vasc Biol. 2007;27:2743–9.