Abstract
Objectives. Surgical embolectomy for acute pulmonary embolism (PE) is considered to be a high risk procedure and therefore a last treatment option. We wanted to evaluate the procedures role in modern treatment of acute PE. Design. All data on patients treated with surgical embolectomy for acute PE were retrieved from our clinical database. The mortality was extracted from the Danish mortality register. Results. From October 1998 to July 2010, 33 patients underwent surgical embolectomy. All procedures were done through a median sternotomy and extracorporeal circulation. Twenty-six patients were diagnosed with a high risk PE and 7 with an intermediate risk PE and intracardial pathology. Six patients had been insufficiently treated with thrombolysis. Thirteen patients had contraindication for thrombolysis. Six patients were brought to the operating theatre in cardiogenic shock, 8 needed ventilator support, and 1 was in cardiac arrest. The postoperative 30-day mortality was 6% and during the 12-year follow-up the cumulative survival was 80% with 4 late deaths. Conclusion. Surgical pulmonary embolectomy can be performed with low mortality although the treated patients belong to the most compromised part of the PE population. The results support surgical embolectomy as a vital part of the treatment algorithm for acute PE.
Introduction
Acute pulmonary embolism (PE) can be a serious and potential life threatening condition, but covers a broad spectrum of clinical severity. Previously, patients were categorized as having acute massive, submassive, or nonmassive PE based on anatomical distribution, burden of intrapulmonary emboli and circulatory status. Acute massive PE was defined as thrombus occlusion of more than 50% of the pulmonary artery in cross section and circulatory compromise. Since thrombus burden is poorly correlated with morbidity and mortality patients are now classified with high-, intermediate-, or low-risk pulmonary embolism according to expected pulmonary embolism-related early mortality rate (Citation1).
High-risk PE is defined as clinical shock or severe hypotension, with systolic blood pressure <90 mmHg or systolic blood pressure drop >40 mmHg for more than 15 minutes. Patients with high-risk PE have a mortality of up to 70% in the first hour. Intermediate-risk PE is defined as condition without clinical shock or hypotension, but echocardiographic signs of right ventricle dysfunction and/or biochemical signs of myocardial injury. Patients with an intermediate-risk PE have a mortality of up to 15%. Patients with low-risk PE represent the group without clinical shock, echocardiographic signs of right ventricle dysfunction or myocardial injury. Their mortality is less than 1% (Citation1).
Patients with high-risk PE who survive the first hour have a 90-day mortality of 50% (Citation2–4). Overall 90-day mortality after acute PE of any kind is reported to be 15–20% (Citation5,Citation6).
According to the guidelines from the European Society of Cardiology, treatment of high-risk PE is thrombolysis or surgical embolectomy (Citation1). After thrombolysis, major bleeding is seen in about 13% and intracranial/fatal bleeding in up to 3% of the patients (Citation1,Citation6,Citation7). Furthermore, 50% of the patients with high-risk PE present with contraindications for thrombolysis () (Citation7). Surgical embolectomy has been reserved for patients with contraindications for thrombolysis (), insufficient response to thrombolysis, or patients in need for a surgical salvage embolectomy because of profound cardiogenic shock or cardiac arrest. However, definition of insufficient response to thrombolysis is not clear and there has been a reluctance to refer patients to surgical treatment for acute PE because older studies have reported a high mortality ranging from 16% to 64% depending on the patients preoperative hemodynamic status (Citation5,Citation8). Recently, two studies have shown a 30-day mortality of 6–8% in patients undergoing surgical embolectomy (Citation8,Citation9).
Table I. Contraindications for thrombolysis.
We present a retrospective analysis of patients undergoing surgical embolectomy for acute PE with up to 12-year follow-up. The results show low mortality supporting surgical embolectomy as a vital part of the treatment algorithm for acute PE.
Material and methods
From October 1998 through July 2010, 33 patients underwent surgical pulmonary embolectomy for treatment of acute PE. We retrospectively analyzed this cohort, which consisted of 17 men and 16 women. Median age was 55 years (range: 19–77). The median body mass index was 27.7 (range 17–37.9). Preoperatively two patients were known with hypertension, one patient with nephropathy, one patient with chronic obstructive pulmonary disease, and one patient had Factor V-Leiden mutation. Six patients had previously experienced a thrombo-embolic event, of which one had been diagnosed with a cerebral thrombo-embolism. The major predisposing factors for PE were major surgery, cancer, medical illness, and trauma ().
Table II. Predisposing risk factors for development of PE (N =33).
Symptoms and clinical signs related to PE were dyspnea (n =24), chest pain (n =6), syncope with witnessed loss of consciousness (n =16), need of preoperative inotropics (n =6), preoperative ventilatory support (n =8), and cardiac arrest (n =1). No patients were treated with ECMO preoperative.
The diagnosis was confirmed by chest CT-scan in 27 patients and echocardiography was performed in 30 patients. Echocardiographic signs of right ventricle affection, defined as the “60/60 sign,” the McConnell sign or RV overload were present in all but one patient. One patient was brought to the operating room (OR) in cardiac arrest.
The surgical indication for embolectomy was for most patients high-risk PE, contraindications for thrombolysis (), insufficient results of thrombolysis, hemodynamic instability, patent foramen ovale or thrombi in the right atrium (). Insufficient result of thrombolysis was defined as worsening or lack of improvement in circulatory status and/or right ventricle dysfunction. Mortality data were achieved from the Danish mortality register and have been updated until August 2, 2010.
Table III. Indication for surgical treatment of PE (N =33).
Surgical technique
The operative method was median sternotomy, pericardiotomy, standard central cannulation, and commencement of cardiopulmonary bypass (CPB) in normothermia. In some cases, the heart was fibrillated. The main pulmonary artery was opened and thrombus mass was retrieved with suction and Forgarty catheter. If thrombus mass was present in the heart chambers and/or in case of a patent forarmen ovale, cardioplegia was given and aorta cross clamped for intracardial access. No patients had vena cava filters inserted.
Thrombolytic therapy
All patients who received thrombolysis before surgical embolectomy had been treated accordingly to the “National Recommendations” from The Danish Society of Cardiology. In this algorithm, 100-mg Alteplase is administered intravenously over 2 hours. If the treatment was repeated, the same algorithm was used.
Statistical analysis
The data are presented as medians with range or percentages. Cumulative survival rate was calculated by using the Kaplan–Meier method. All data were processed in SPSS® statistical program (SPSS Inc., Chicago, IL, USA).
Results
The median time in CPB was 53 minutes (range: 12–133). In seven patients, intracardial access was needed, median aortic cross clamp time was 39 minutes (range: 15–62). Three patients had patent foramen ovale closures, two patients had retrieval of thrombus material from the right atrium, and two patients had removal of a myxoma. There were no pulmonary artery injuries during retrieval of the thrombotic material.
A 62-year-old woman brought to the OR in cardiac arrest and died in circulatory collapse during the attempt to wean off the CPB. All other patients survived the procedure. Within the first 30 days postoperatively, one patient died. A 77-year-old man known with prostate cancer died on postoperative day 22 in the emergency room in a community hospital due to cardiac arrest of on known origin.
Reoperation for postoperative bleeding was performed in one patient and four patients underwent surgical centesis for pericardial effusion. The patients were admitted to the intensive care unit for a median time of 2 days (range 1–34). Twenty patients could be extubated immediately after surgery. The remaining patients needed mechanical ventilation for a median time of 4 days (range 1–24). Postoperative inotropics was administered to 14 patients for a median time of 3.5 days (range 1–11) and 5 patients were in postoperative dialysis.
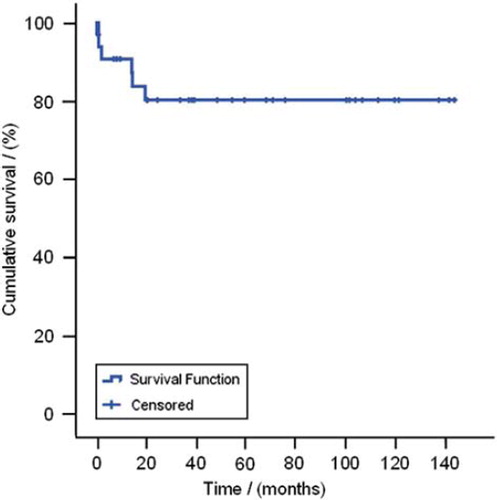
During the median follow-up of 5.2 years (range: 15 days–11.8 years) there were four late deaths. shows the cumulative survival rate after pulmonary embolectomy. Five-year cumulative survival was 80%. The causes of death reported to the Danish mortality register were: C. coli, PE, gliosarcoma and sarcoma.
Discussion
The principal finding of this study is that surgical pulmonary embolectomy can be performed with low perioperative morbidity and 30 day mortality as low as 6%. This is well in-line with recent reports, in which 30 day mortality was 6–8% (Citation2,Citation3,Citation5,Citation8–10). In our material, one patient in cardiac arrest underwent salvage embolectomy and could not be saved. Salvage pulmonary embolectomy is known to have a very high mortality rate due to the poor preoperative condition (Citation2,Citation5,Citation8). Carvalho et al. reported a 85% mortality in seven patients undergoing salvage pulmonary embolectomy (Citation5). Salvage operations were defined as those performed on patients in cardiogenic shock, receiving cardiopulmonary resuscitation or with recent history of cardiac arrest.
Depending on the severity of the acute pulmonary embolism patients are treated with heparin alone, thrombolysis or surgical embolectomy. A meta-analysis of randomized trials comparing thrombolysis versus heparin could not demonstrate a significant benefit of thrombolysis in patients with acute PE. However, a subgroup analysis of patients treated with thrombolysis for high-risk PE showed significantly reduction in the composite outcome of recurrent PE and death (Citation11). In contrast, data from “The International Cooperative Pulmonary Embolism Registry” (ICOPER) including data from 2454 patients from 52 hospitals in 7 counties were not able to show that thrombolysis reduced 90 day mortality or recurrence of PE in patients with high-risk PE (Citation3,Citation4,Citation12).
According to the current guidelines of the European Society of Cardiology, only patients in cardiogenic shock or hypotension should be offered other treatment than heparin/oral anticoagulation (Citation1). It has been estimated that this accounts of about 5% of all PE patients (Citation12). Most of these patients are treated with thrombolysis and surgical embolectomy has been reserved for patients who are hemodynamically unstable had failed thrombolytic therapy or contraindication for thrombolysis. Although surgery is performed in the most compromised patients, recent reports have shown encouraging results. Today few high-risk PE patients are offered pulmonary embolectomy as first line treatment. Thrombolysis versus surgical embolectomy has never been compared in a randomized trial. In a retrospective study Gulba et al. analyzed 37 patients with high risk PE, 13 patients were treated surgically and 24 patients were treated with thrombolysis. All surgical treated patients had their vena cava inferior clipped. Patients were followed for 5 years. In this study, patients undergoing thrombolysis had a higher mortality and increased risk of recurrence of PE than surgically treated patients (Citation13). Whether patients with right ventricle dysfunction but hemodynamic stabile (intermediate risk PE) should be offered thrombolysis or embolectomy is also unclear. With a more aggressive treatment, the number of patients developing cardiogenic shock may be reduced as well as the number of patients presenting with chronic thromboembolic pulmonary hypertension may decrease.
The risk of long term complications for patients treated for acute high risk and intermediate risk PE has been investigated by Meneveau et al. Between 1990 and 1999, 249 patients diagnosed with acute PE and treated with thrombolysis were followed. During a mean follow-up of 5 years, 12% had a recurrent PE. The investigation also showed that patients with pulmonal hypertension after thrombolysis had a higher mortality as well as up to 25% of the patients only had partial resolution of the PE after thrombolysis, which again was related to increased mortality. They conclude that residual thrombi mass after thrombolysis could suggest that the patient should be offered invasive treatments (Citation14). In a study of patients with massive PE and unsuccessful thrombolysis, rescue surgical embolectomy resulted in better in-hospital outcomes compared to repeat thrombolysis. The mortality was 7% after embolectomy compared to 38% after repeat thrombolysis (Citation15). In our study, 18% had undergone unsuccessful thrombolysis and 50% of these were given repeat thrombolysis before transfer to surgical embolectomy. In this perspective, it could be advocated that patients with large thrombus burden and pulmonary hypertension should be offered surgical treatment in an earlier stage of the treatment algorithm.
New catheter-directed techniques are evolving, where local thrombolysis can be administered or suction embolectomy can be performed. The value of these methods seems promising and could be a relevant alternative to the known treatment modalities (Citation16).
There is only a very limited knowledge on how the survived patients perform after they have been treated with thrombolysis or surgical embolectomy. There are no or very limited published data on how many of the treated patients have chronic dyspnoea or physical limitations, chronic pulmonary hypertension, repeat pulmonary embolisms, or impaired right ventricle function in relation to the received treatment.
As long as no well-designed randomized trial has been conducted with adequate long-term follow-up the most optimal treatment for high- and intermediate risk PE patients remains unclear.
PE is a potential lethal condition with a high mortality despite modern treatment. Few patients with acute PE are offered surgical embolectomy, although our and other studies have shown the treatment is safe and with good long-term prognosis. Patients with unsuccessful thrombolytic treatment of PE should be transferred to centers with cardiac surgery facilities to be evaluated for rescue surgical embolectomy.
Patients with acute high- and intermediate-risk PE should be evaluated and treated in a multidisciplinary medical and surgical setup where clinical condition, thrombus burden, and cardiac status can guide the optimal treatment of the individual patient.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, . Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;18:2276–2315.
- Dauphine C, Omari B. Pulmonary embolectomy for acute massive pulmonary embolism. Ann Thorac Surg. 2005;4:1240–44.
- Samoukovic G, Malas T, De varennes B. The role of pulmonary embolectomy in the treatment of acute pulmonary embolism: a literature review from 1968 to 2008. Interact Cardiovasc Thorac Surg. 2010;11:265–70.
- Kucher N, Rossi E, De RM, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006;4:577–82.
- Carvalho EM, Macedo FI, Panos AL, Ricci M, Salerno TA. Pulmonary embolectomy: recommendation for early surgical intervention. J.Card Surg. 2010;3:261–66.
- Aklog L, Williams CS, Byrne JG, Goldhaber SZ. Acute pulmonary embolectomy: a contemporary approach. Circulation. 2002;12:1416–19.
- Digonnet A, Moya-Plana A, Aubert S, Flecher E, Bonnet N, Leprince P, . Acute pulmonary embolism: a current surgical approach. Interact Cardiovasc Thorac Surg. 2007;1:27–9.
- Leacche M, Unic D, Goldhaber SZ, Rawn JD, Aranki SF, Couper GS, . Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg. 2005;5:1018–23.
- Kadner A, Schmidli J, Schonhoff F, Krahenbuhl E, Immer F, Carrel T, . Excellent outcome after surgical treatment of massive pulmonary embolism in critically ill patients. J Thorac Cardiovasc Surg. 2008;2:448–51.
- Doerge HC, Schoendube FA, Loeser H, Walter M, Messmer BJ. Pulmonary embolectomy: review of a 15-year experience and role in the age of thrombolytic therapy. Eur J Cardiothorac Surg. 1996;11:952–7.
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;6:744–9.
- Goldhaber SZ, Visani L, De RM. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;9162:1386–9.
- Gulba DC, Schmid C, Borst HG, Lichtlen P, Dietz R, Luft FC, . Medical compared with surgical treatment for massive pulmonary embolism. Lancet. 1994;8897:576–7.
- Meneveau N, Ming LP, Seronde MF, Mersin N, Schiele F, Caulfield F, . In-hospital and long-term outcome after sub-massive and massive pulmonary embolism submitted to thrombolytic therapy. Eur Heart J. 2003;15:1447–54.
- Meneveau N, Seronde MF, Blonde MC, Legalery P, Didier-Petit K, Briand F, . Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest. 2006;4:1043–50.
- Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV, . Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;11:1431–40.