Abstract
Objective. Brain natriuretic peptide (BNP) is a promising marker for heart failure diagnosis and prognosis. Although psychological factors also influence heart failure (HF) prognosis, this might be attributed to confounding by BNP. Our aim was to examine the association between multiple psychological markers using a prospective study design with repeated N-terminal pro-B-type natriuretic peptide (NT-proBNP) measurements. Design. The sample comprised 94 outpatients with systolic HF (80% men; mean age =62.2±9.3). The psychological markers (i.e., anxiety, depression, and Type D personality), assessed with the Hospital Anxiety and Depression Scale (HADS), the Beck Depression Inventory (BDI), and the Type D Scale (DS14) were assessed only at baseline. Plasma NT-proBNP levels were measured at baseline and at 9 months. Results. The prevalence of anxiety, depression, and Type D personality at baseline was 23.4% (HADS-A), 17.0% (HADS-D), 46.6% (BDI), and 21.3% (DS14), respectively. At baseline, none of the psychological risk markers were associated with NT-proBNP levels (all p >.05). In the subset of patients with scores on psychological risk markers both at baseline and at 9 months, there were no association between anxiety (p =0.44), depression (HADS-D: p =0.90; BDI: p =0.85), and Type D (p =0.63) with NT-proBNP levels using ANOVA for repeated measures. Conclusions. Our findings indicate that measures frequently used in HF to assess psychological risk markers are unconfounded by NT-proBNP. Futher studies are warranted to replicate these findings and examine whether psychological risk markers are independent predictors of prognosis in HF or an artifact that may be attributed to other biological or behavioral mechanisms.
Introduction
Heart failure (HF), which is typically identified by features such as dyspnea, fatigue, signs of fluid retention, and cardiac remodeling, is associated with considerable physical impairments, poor quality of life and increased psychological distress (Citation1,Citation2).
Despite the availability of a wide array of laboratory and radiological tests, a diagnosis of HF may initially go unnoticed. In recent year, brain natriuretic peptide (BNP) and its N-terminal prohormone (NT-proBNP), have been introduced as an additional method to facilitate a diagnosis of HF. BNP, which is now known as B-type natriuretic peptide, belongs to the natriuretic peptide family, which contributes to cardiovascular homeostasis by promoting natriuresis and diuresis, acting as vasodilators, and exerting antimitogenic effects on cardiovascular tissues. Increased BNP levels have been shown to be strong risk indicators of a poor prognosis, but also to be of value in guiding therapy to treat HF (Citation3–5).
Besides being a valuable prognostic marker for HF, evidence suggests that BNP may also influence psychological distress (e.g., anxiety and depression) by affecting the corticosterone response in the hypothalamus-pituitary-adrenal gland (HPA) axis (Citation6). However, since psychological distress is also an independent risk factor for HF prognosis on its own, psychological distress might be confounded by BNP and thus be a risk marker rather than a risk factor, with the relation between distress and poor prognosis being explained by increased BNP levels (Citation7,Citation8).
A paucity of studies has examined the association between episodic and chronic psychological distress and BNP levels, with most studies being cross-sectional and examining single psychological risk markers. Of the 10 available studies (Citation6–15), seven were conducted in HF patients (Citation7,Citation10–15). Two studies focusing on anxiety found that in patients with mild HF changes in BNP concentration were positively associated with both anxiety and state anger (Citation13,Citation15). Findings on depression (Citation7–11,Citation14) were mixed. The single study that focused on the distressed (Type D) personality found no association with BNP levels (Citation12). Thus, the evidence for an association between psychological factors and BNP and NT-proBNP is inconsistent. Knowledge of the extent to which psychological measures frequently used in HF research are associated with indicators of disease severity is important, as the prevalence of psychological symptoms may be inflated and reflect somatic disease rather than true psychological morbidity, if confounding is present.
Hence, we examined the link between NT-proBNP and the continuous and dichotomized scores of a broad range of psychological risk markers (i.e., depressive symptoms, anxiety, and Type D personality) using a prospective study design with measurements of NT-proBNP at baseline and at 9 months.
Material and methods
Study population and design
Consecutive patients (N =94) with a diagnosis of systolic HF comprised the sample for the current study. Patients were recruited from four different centers, that is, Aarhus University Hospital (Skejby), Aarhus University Hospital (Aalborg), Aarhus University Hospital (Amtssygehuset), and Odense University Hospital. Patients were asked to complete a set of standardized and validated questionnaires at baseline, assessing the psychological risk markers, while NT-proBNP levels were assessed both at baseline and at 9 months. Inclusion criteria were: Diagnosis of systolic HF, left ventricular ejection fraction (LVEF) ≤40%, and stable on HF medication within the last 1 month prior to inclusion. Patients ≥75 years of age, unable to understand and read Danish, with clinical signs of acute infection, other life-threatening diseases, cognitive impairments, psychiatric comorbidity (except for affective disorders), or myocardial infarction within the last 2 months were excluded. The study was approved by the Medical Ethics Committees of all the participating hospitals and was conducted in accordance with the Helsinki Declaration. All patients provided written informed consent.
Measures
Demographic and clinical variables. Information on demographic and clinical variables was obtained from the patients’ medical records or from purpose-designed questions in the questionnaire. Demographic variables comprised gender, age, marital status, education, working status, smoking status, and body mass index (BMI). Clinical variables included time since HF diagnosis, etiology of HF, previous cardiac events, previous hospitalizations for HF, angina pectoris, New York Heart Association (NYHA) functional class, left ventricular ejection fraction (LVEF), presence of valvular heart disease, presence of coronary artery disease (CAD), hypertension, hypercholesterolemia, diabetes, anemia, kidney disease, comorbidities, cardiac medication (beta-blockers, calcium antagonists, nitrates, aspirin and other platelet-aggregation inhibitors, anticoagulants, angiotensin-converting-enzyme (ACE)-inhibitors, statins, diuretics, angiotensin-receptor blockers) and psychotropic medication. LVEF was measured using the Simpsons biplane method, wall motion scoring, and eyeballing depending on the patient, the echocardiographer and the available acoustic conditions.
Psychological variables
Anxiety and depressive symptoms. The Hospital Anxiety and Depression Scale (HADS) was developed by Zigmond and Snaith in 1983 to identify probable anxiety and depression disorders among patients in nonpsychiatric hospital clinics. HADS comprises two seven-item subscales, that is, Anxiety (HADS-A) and Depression (HADS-D) (Citation16). Items are answered on a four-point Likert scale from 0–3 (score range 0–21). HADS is a valid and reliable measure, with good internal consistency as demonstrated by Cronbach's α (HADS-A =.80; HADS-D =.81) (Citation16). A score of 8–10 is suggestive of the presence of the respective mood state, while a score of 11 or higher indicates probable presence of the mood disorder (Citation17). In the current study, a cut-off of ≥8 was used for both subscales to indicate the presence of anxiety and depressive symptoms (Citation16). To prevent ‘noise’ from somatic disease on the scores, all symptoms of anxiety or depression relating also to somatic disease, such as dizziness, headaches, insomnia, energy, and fatigue, are excluded from the HADS, which makes it an opportune measure to use in patients with HF (Citation18). The HADS only takes 2–5 min to complete. It has been shown to be acceptable by the population for which it was designed (Citation16–18). The HADS was administered at baseline.
The Beck Depression Inventory (BDI) is a 21-item self-report questionnaire (Citation19). It is composed of items relating to symptoms of depression such as hopelessness and irritability, cognitions such as guilt or feelings of being punished, as well as physical symptoms such as fatigue, weight loss, and lack of interest in sex (Citation20). Each item on the BDI is answered on a scale from 0 to 3. A score of 0–9 indicates that a person is not depressed, 10–18 mild to moderate depression, 19–29 moderate to severe depression, while 30–63 indicates severe depression. A higher score indicates more severe depressive symptoms. For this study, we used a cut-off of ≥10 (Citation21). The BDI can be separated into two subcomponents, that is a cognitive/affective (e.g., mood) and a somatic component (e.g., fatigue). As evidence suggests that the BDI may be confounded by indicators of somatic disease, we included the HADS not only to have a measure of anxiety but also to examine the extent of confounding of depression with NT-proBNP with these two different depression measures (Citation21). The BDI was administered at baseline.
Type D personality. The Type D scale (DS14) was used to assess the distressed (Type D) personality and its two constituent seven-item subscales, negative affectivity and social inhibition. Negative affectivity refers to the tendency to experience negative emotions, like anger, dysphoria, irritability, hostile feelings, depressed affect, and anxiety. Social inhibition refers to discomfort in social interactions, reticence, and lack of social poise (Citation22). Items are rated on a five-point Likert scale ranging from 0 (false) to 4 (true), with subscale scores ranging from 0–28. A cut-off of ≥10 on both subscales is used to classify patients as Type D (Citation22). The construct of Type D personality is stable when compared to the effect of gender on outcomes (Citation23). The DS14 was administered at baseline.
NT-proBNP levels
N-terminal (NT) proBNP, the biologically inactive preprohormone of BNP, which has a longer half-life than BNP and is found in plasma, was evaluated at baseline and at 9 months. Blood was obtained by venipuncture under standardized conditions (after 15 min. rest – no tourniquet used) and collected in tubes containing ethylenediamine-tetraacetic acid. Blood samples were centrifugated at 2000x for 20 min. at 4°C. Plasma was then extracted and stored at −80°C prior to testing. NT-proBNP was measured using an electrochemiluminescence immunoassay (Cobas, Elecsys 2010 Systems, Roche Diagnostics Gmbh, Mannheim, Germany) according to the manufacturer's instructions. The coefficient of variation for the NT-proBNP assay was 2–5%, and the analytical measurement range for NT-proBNP was 5–35,000 pg/mL.
Statistical analysis
Descriptive statistics were used to describe the demographic and clinical characteristics of the study sample. Student's t-test for independent samples and Pearson's correlation for parametric tests were used to examine associations between baseline psychological risk markers and NT-proBNP at baseline and at 9 months follow-up. Analysis of variance (ANOVA) with repeated measures was used to examine associations between dichotomized psychological risk markers assessed at baseline and NT-proBNP at baseline and at 9 months follow-up. NT-proBNP levels were positively skewed and logarithmic transformations were applied prior to parametric analyses. All data were analyzed using SPSS 17.0 for Windows (SPSS Inc., Chicago, Illinois). All tests were two-tailed, and a p-value <0.05 was used to indicate statistical significance.
Results
Patient characteristics
Of 190 eligible patients, 3 were omitted due to personnel error, and 65 declined participation resulting in a final response rate of 65.8% (N =122). Twenty-eight patients had either no psychological measurements or no NT-proBNP measurement and were excluded from analysis leaving 94 systolic HF patients. All study participants were outpatients at the time of recruitment. Of these patients, all (100%) had complete HADS and DS14 scores, and 76 had complete BDI scores (80.9%). The prevalence of anxiety at baseline, as measured with HADS-A, was 23.4% (22/94). The prevalence of depressive symptoms at baseline was 17.0% (16/94) with the HADS-D and 46.6% (41/88) when assessed with the BDI. Of all patients, 21.3% (20/94) had a Type D personality. Demographic, clinical and psychological baseline characteristics of the patients are shown in .
Table I. Demographic and clinical baseline characteristics of the sample.
NT-proBNP
Prior to analyses, NT-proBNP was tested for outliers and its distribution; due to its skewed distribution, the data were transformed prior to statistical analysis using natural log. These data are presented as a median with inter-quartile range (IQR) for the untransformed data and as mean (SD) for the log transformed data. NT-proBNP measurement was missing at random in some patients for logistic and practical reasons. In the total patient sample (N =122), patients with available NT-proBNP levels (baseline: n =94, 9 months: n =76) did not differ systematically from patients who did not have a NT-proBNP measurement (baseline: n =28, 9 months: n =46) on clinical, demographic and psychological characteristics, except for patients without an NT-proBNP measurement being less likely to have valvular heart disease (79.2% vs. 93.8% p =0.025) and angina pectoris (66.7% vs. 84.7% p =0.043) than patients with information on their NT-proBNP level. In the total sample, the median NT-proBNP levels were 927.0 pg/mL (IQR =386–2268 pg/mL) at baseline, while at 9 months this level declined significantly to 614.0 pg/mL (IQR =231–1229 pg/mL) (p <0.001).
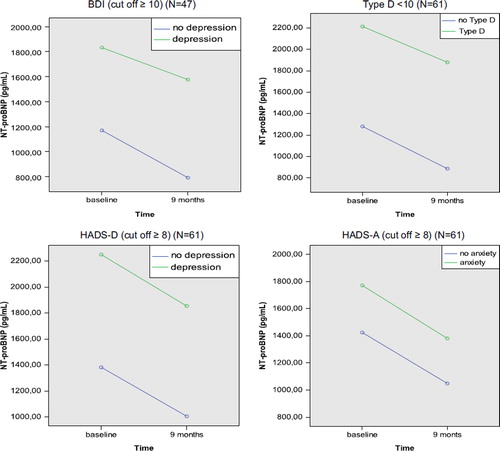
Relationship between anxiety, depression, and Type D personality and NT-proBNP levels (unadjusted analysis)
presents associations between the psychological risk markers (i.e., symptoms of anxiety, depression, and Type D personality) assessed at baseline and NT-proBNP levels measured at baseline and at 9 months follow-up. Patients with a Type D personality had higher NT-proBNP levels than patients without Type D at baseline and follow-up, but these differences were not statistically significant. There was almost no difference in NT-proBNP levels between patients with and without anxiety and depression at baseline. The results did not change when calculating Pearson's rho with the continuous scores of the HADS-A (r = −.109, p =0.296), HADS-D (r =0.027, p =0.796), BDI (r =0.101, p =0.385), and DS14 (SI: r =0.130, p =0.210; NA: r =0.013, p =0.903) in relation to NT-proBNP at baseline and the HADS-A (r = −.065, p =0.581), HADS-D (r = −.064, p =0.586), BDI (r =0.109, p =0.445) and DS14 (SI: r =0.085, p =0.474;NA: r =0.002, ρ =0.987), in relation to NT-proBNP at follow-up. Neither of the subcomponents of the BDI at baseline (BDIb N =86, df =1) and follow-up (BDIf N =47, df =1) were significantly related to NT-proBNP using the total continuous scores of the affective (BDIb r =0.057, p =0.631; BDIf r =0.063, p =0.670, respectively) and somatic items (BDIb r =0.153, p =0.187; BDIf r =0.156, p =0.275, respectively) (data not shown). Note that baseline NT-proBNP levels were negatively correlated with anxiety scores, and follow-up NT-proBNP levels were negatively correlated with anxiety and depression scores, as measured by the HADS-A and HADS-D.
. Association between baseline psychological risk markers (i.e., symptoms of anxiety and depression and Type D personality) and NT-proBNP levels (pg/ml) and log NT-proBNP at baseline and at 9 months follow-up*.
In a secondary analysis, ANOVA with repeated measures was performed with the psychological risk markers (i.e., dichotomous scores (presence/absence) of anxiety, depressive symptoms, and Type D personality) entered as the between-subjects factors in four separate analyses with log NT-proBNP at baseline and at 9 months as the outcome. Of the total number of patients in the analysis (N =94), 61 (64.9%) had available data on the HADS or DS14 in combination with NT-proBNP measurements at baseline and at follow-up, while 47 (50%) had available data on the BDI in combination with NT-proBNP at baseline and at follow-up. The DS14 ((N =61, df =1) F =0.236; p =0.63), HADS-A ((N =61, df =1) F =0.603; p =0.44), HADS-D ((N =61, df =1) F =0.016; p =0.90), and BDI ((N =47, df =1) F =0.035; p =0.85) showed no significant interaction with NT-proBNP levels over time (). Moreover, the affective ((N =47, df =1) F =0.095; p =0.45) and somatic (N =47, df =1) F =1.032; p =0.15) subcomponents of the BDI were also not significantly related to NT-proBNP levels, nor were the NA ((N =61, df =1) F =0.749; p =0.28) and SI (N =61, df =1) F =0.058; p =0.18) subscales of the DS14.
Given the absence of a significant main effect between psychological risk markers and NT-proBNP in unadjusted analysis, it makes little sense to perform analysis of covariance (ANCOVA) with repeated measures to examine the potential confounding of clinical and demographic characteristics on the relationship between psychological risk markers and NT-proBNP levels.
Discussion
In the current study, we investigated whether common psychological risk markers in HF are confounded by disease severity, as measured by NT-proBNP. Although NT-proBNP is not a standalone marker of HF prognosis and mortality, caused mainly by the large intra-individual canges in concentrations which quesions its specifity for HF, it has earned an important status of contributing to prognosis in HF in combination with other clinical measures. This is in part due to many HF patients having preserved LVEF but also due to the assessment of functional class being strongly influenced by symptoms of depression and having a poor inter-rater reliability (Citation24). Our results demonstrated that none of the psychological risk markers examined (i.e., anxiety, depression, and Type D personality) were confounded by levels of NT-proBNP.
Previous studies examining the relationship between psychological risk markers and markers of HF used either BNP (Citation7,Citation10–13,Citation15) or NT-proBNP (Citation8,Citation9). Although these markers are not directly comparable in terms of their levels, it is possible to compare the overall results of the studies. Six out of the nine studies dedicated to this topic found a significant association between BNP or NT-proBNP and anxiety or depression. The two studies examining more than one psychological risk marker found a significant relation with anxiety or depression, but not both (Citation9,Citation13). In relation to depression, the studies of Gottlieb et al. and Van den Broek et al., which used the largest sample sizes of respectively 2322 and 4332 (HF and non-HF) individuals, showed that BNP levels did not predict BDI scores in multivariable analyses (Citation10,Citation14), suggesting that depression and (NT-pro)BNP are independent and additive predictors that may adversely affect HF progression via independent pathophysiological pathways. The study of Pelle et al. found no relation between BNP and Type D personality nor between the Type D subdomains negative affectivity and social inhibition and BNP levels (Citation12). Half of these studies used a cross-sectional study design (Citation8–10,Citation12), having only a (NT-pro)BNP measurement at baseline. It has been argued in the literature that the inter-individual biological variation in BNP and NT-proBNP is so high that it is beneficial to increase the number of assays over time to reach a better estimate of a patient's homeostatic set point (Citation25). Hence, the prospective design of our study with assessments of NT-proBNP both at baseline and at 9 months follow-up and the examination of a broader range of psychological risk markers, including both episodic (i.e., anxiety and depression) and chronic (i.e., Type D personality) markers is a strength in comparison to some of the current literature on the relationship between psychological markers and potential confounding by HF disease severity.
The study of Parissis et al. reported a remarkably high prevalence of patients with depressive symptoms in their study sample (62%) using the BDI and Zung SDS (Citation7). In our study sample, the BDI also showed a considerably higher prevalence of depression (46.6%) than found by the HADS-D (17.0%). However, we found neither a significant relation between NT-proBNP and depressive symptoms when measured with the BDI, nor between NT-proBNP and depressive symptoms when measured with the HADS-D. In the other studies that found a significant relationship between NT-proBNP and depression, analyses were performed with severely depressed patients (Citation8,Citation9,Citation11). Other limitations of the previous studies were the use of a self-report questionnaire for depression for which there was no designated cut-off for the severity of depression, and the inclusion of patients with less severe cardiac impairments (LVEF ≥30%) (Citation11,Citation15).
In contrast to some previous studies, we did not find a significant association between NT-proBNP levels and psychological risk markers. This indicates that the psychological measures used in the current study may not be confounded by disease severity, as measured by NT-proBNP, increasing the likelihood that they reflect true psychological morbidity rather than underlying disease severity in patients with HF. Simultaneously, this finding also points to the complexity of the relationship between psychological risk markers and HF severity, especially with respect to depression which shows the most contradicting results. It is possible that the relationship between BNP and emotional distress (e.g., depression) is dependent on the subgroup of HF patients, and on the severity of psychological distress and symptoms. Previous studies in other HF populations show prevalence rates of depression of 21.5% (range: 19.3%–33.6%) (Citation26), for anxiety up to 40% (Citation2), and for Type D between 19–44% with a lower percentage in Northern and Western European country clusters (24%) (Citation27) that are in concordance with our findings. However, it seems prevalence rates of emotional distress have been shown to vary greatly among HF subgroups (Citation2), which might in part explain our null funding between psychological risk markers and NT-proBNP levels. An unresolved issue also pertains to the question of the ‘chicken and the egg’. Although most studies indicate that the relation between emotional distress and disease severity might be bidirectional, HF is more often assumed to be the cause of emotional distress than the other way around (Citation10).
Another important consideration is pointed out by Gottlieb et al., who suggest that emotional distress is more strongly related to subjective HF indices, such as NYHA functional class, than to objective indices, such as LVEF and BNP (Citation10). The findings by Scherer et al. concur with this notion, as there was a significant correlation between NYHA functional class and anxiety and depression, as measured by the HADS in primary care HF patients (Citation10,Citation28). Since BNP is not based on a patient's (or a clinician's) perception of disease severity, this could explain why BNP did not predict emotional distress in our study, as in the sample of Gottlieb et al. Furthermore, this might indicate that depression influences the perception of severity of disease to a greater extent rather than severe HF causing depression (Citation10). Taken together, it is possible that HF symptoms in patients improve by addressing psychological problems, and that the combination of the presence of emotional distress together with BNP levels may have an additive prognostic influence in HF patients, as already mentioned by Parissis et al. (Citation7).
The potential limitations of our study merit consideration. Since this study only analyzed the relationship between NT-proBNP and psychological risk markers, we cannot make any statements on whether any association between repeated NT-proBNP measures and psychological measures could directly contribute to the observed relationship between psychological measures and poor outcomes for HF. Unfortunately, we also did not have information on exercise, diet, medical adherence, heart rate variability, and socio-economic status, which might have influenced our results. By excluding HF patients older than 75 years, the mean age of our sample was relatively low compared to a general HF outpatient population, which potentially limits the generalizability of the results. Furthermore, this exclusion criterion has reduced the total sample size and most probably a lower percentage of women within this sample. However, since the risks of cognitive deficits and the burden of filling in questionnaires at several time points is expected to be increased with increasing age, the validity of patients’ answers to the questionnaires is less likely to have been compromised. Furthermore, for only 75% of the patient sample, NT-proBNP measurements were available at baseline and at 9 months. For the assessment of anxiety and depression, we used a self-report measure rather than a clinical diagnostic interview. Hence, we have no information as to whether NT-proBNP is related to a clinical diagnosis of anxiety and depression. Nevertheless, even minimal symptoms, as assessed with self-report measures of depression, have been related to prognosis in cardiac populations (Citation29).
In conclusion, we found no relationship between any of the psychological risk markers assessed (i.e., anxiety, depressive symptoms (both with the HADS-D and the BDI), and Type D personality) and NT-proBNP levels using a prospective study design with the assessment of NT-proBNP levels both at baseline and 9-months follow-up in our sample of systolic HF outpatients. However, we have to keep in mind that until we have gained more insight into the determinants that govern the high intra-individual levels of BNP and NT-proBNP, we have to be careful in drawing conclusions in relation to these outcomes with psychological risk markers (Citation25). Although more large-scale studies are warranted to investigate and replicate BNP and its relation to anxiety, depression and Type D, these preliminary results are promising in that they show that measures frequently used in HF to assess psychological risk markers seem to be unconfounded by NT-proBNP. This suggests that screening for and treating depression in HF might have additional prognostic benefits to current standard care and management.
Acknowledgments
We would like to thank Dr. Aage Nørgaard, Dr. Jens Berning, Bente Mortensen, Linda Lund, Anne Marie Laustsen, Britta Rosborg Wegener, Janne Milton, Jane Petersen, Helle Arnsted, and Charlotte Anker for their involvement in the study.
This research was in part supported by the Danish Heart Foundation (grant no. 03-2-9-9-22092 and 06-4-B505-22283F) and with a VIDI grant (91710393) from the Netherlands Organisation for Health Research and Development (ZonMw), the Hague, the Netherlands, to Dr. Susanne S. Pedersen.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–442.
- Konstam V, Moser DK, De Jong MJ. Depression and anxiety in heart failure. J Card Fail. 2005; 11:455–63.
- Lee SC, Stevens TL, Sandberg SM, Heublein DM, Nelson SM, Jougasaki M, . The potential of brain natriuretic peptide as a biomarker for New York Heart Association class during the outpatient treatment of heart failure. J Card Fail. 2002;8:149–54.
- Mehra MR, Maisel A. B-type natriuretic peptide in heart failure: diagnostic, prognostic, and therapeutic use. Crit Pathw Cardiol. 2005;4:10–20.
- Tsuchida K, Tanabe K. Plasma brain natriuretic peptide concentrations and the risk of cardiovascular events and death in general practice. J Cardiol. 2008;52:212–23.
- Wiedemann K, Jahn H, Kellner M. Effects of natriuretic peptides upon hypothalamo-pituitary-adrenocortical system activity and anxiety behaviour. Exp Clin Endocrinol Diabetes. 2000;108:5–13.
- Parissis JT, Nikolaou M, Farmakis D, Bistola V, Paraskevaidis IA, Adamopoulos S, . Clinical and prognostic implications of self-rating depression scales and plasma B-type natriuretic peptide in hospitalised patients with chronic heart failure. Heart. 2008;94:585–9.
- Politi P, Minoretti P, Piaggi N, Brondino N, Emanuele E. Elevated plasma N-terminal ProBNP levels in unmedicated patients with major depressive disorder. Neurosci Lett. 2007;417:322–5.
- Bunevicius R, Varoneckas G, Prange AJ Jr., Hinderliter AL, Gintauskiene V, Girdler SS. Depression and thyroid axis function in coronary artery disease: impact of cardiac impairment and gender. Clin Cardiol. 2006;29:170–4.
- Gottlieb SS, Kop WJ, Ellis SJ, Binkley P, Howlett J, O’Connor C, . Relation of depression to severity of illness in heart failure (from Heart Failure And a Controlled Trial Investigating Outcomes of Exercise Training [HF- ACTION]). Am J Cardiol. 2009;103:1285–9.
- Lesman-Leegte I, van Veldhuisen DJ, Hillege HL, Moser D, Sanderman R, Jaarsma T, . Depressive symptoms and outcomes in patients with heart failure: data from the COACH study. Eur J Heart Fail. 2009;11:1202–7.
- Pelle AJ, van den Broek KC, Szabo B, Kupper N. The relationship between Type D personality and chronic heart failure is not confounded by disease severity as assessed by BNP. Int J Cardiol. 2009;5:82–3.
- Tsuchihashi-Makaya M, Kato N, Chishaki A, Takeshita A, Tsutsui H. Anxiety and poor social support are independently associated with adverse outcomes in patients with mild heart failure. Circ J. 2009;73:280–7.
- Van den Broek KC, deFilippi CR, Christenson RH, Seliger SL, Gottdiener JS, Kop WJ, . Predictive value of depressive symptoms and B-type natriuretic peptide for new-onset heart failure and mortality. American Journal of Cardiology. 2011;1:723–9.
- Laederach-Hofmann K, Roher-Gubeli R, Messerli N, Meyer K. Comprehensive rehabilitation in chronic heart failure–better psycho-emotional status related to quality of life, brain natriuretic peptide concentrations, and clinical severity of disease. Clin Invest Med. 2007;30:E54–E62.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
- Snaith RP, Zigmond AS. The hospital anxiety and depression scale. Br Med J (Clin Res Ed). 1986;292:344.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984; 40:1365–7.
- Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics. 2002;43:386–93.
- Denollet J. DS14: standard assessment of negative affectivity, social inhibition, and Type D personality. Psychosom Med. 2005;67:89–97.
- Habra ME, Linden W, Anderson JC, Weinberg J. Type D personality is related to cardiovascular and neuroendocrine reactivity to acute stress. J Psychosom Res. 2003;55:235–45.
- Ho JE, Levy D. B-type natriuretic peptide testing in the general population: are we ready for prime time? J Am Coll Cardiol. 2010;55:2148–9.
- Bruins S, Fokkema MR, Romer JW, Dejongste MJ, van der Dijs FP, van den Ouweland JM, . High intraindividual variation of B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with stable chronic heart failure. Clin Chem. 2004;50:2052–8.
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–37.
- Kupper N, Pedersen SS, Hofer S, Saner H, Oldridge N, Denollet J, Cross-cultural analysis of Type D (distressed) personality in 6222 patients with ischemic heart disease: A study from the International HeartQoL Project. Int J Cardiol. 2011 Nov 10. [Epub ahead of print].
- Scherer M, Stanske B, Wetzel D, Koschack J, Kochen MM, Herrmann-Lingen C, . Psychosocial co-symptoms in primary care patients with heart failure. Herz. 2006;31:347–54.
- Pedersen SS, Denollet J, de Jonge P, Simsek C, Serruys PW, van Domburg RT, . Brief depression screening with the PHQ-2 associated with prognosis following percutaneous coronary intervention with paclitaxel-eluting stenting. J Gen Intern Med. 2009;24:1037–42.