Abstract
Objectives. The present study was carried out to determine whether inhalation of hydrogen (H2) gas protects myocardium against ischemia-reperfusion (I/R) injury in swine. Design. In anesthetized open-chest swine, myocardial stunning was produced by 12-minute occlusion of left anterior descending coronary artery (LAD) followed by 90-minute reperfusion in the first study. Group A inhaled 100% oxygen, and group B inhaled 2% H2 plus 98% oxygen during ischemia and reperfusion. In the second study, myocardial infarction was produced by 40-minute occlusion of LAD followed by 120-minute reperfusion. Group C inhaled 100% oxygen during ischemia and reperfusion. Group D inhaled 2% H2 plus 98% oxygen. Group E inhaled 4% H2 plus 96% oxygen. Results. The change of segment shortening (%SS) from baseline at 90 minutes after reperfusion in group B was 74 ± 13 (mean ± SD) %, which was significantly higher than that in group A (48 ± 15%). Myocardial infarct size in group E (32 ± 10%), but not in group D (40 ± 9%) was smaller than that in group C (46 ± 6%). Conclusions. Inhalation of 2% H2 gas improves myocardial stunning, and inhalation of 4% but not 2% H2 gas reduces myocardial infarct size in swine.
Introduction
The prognosis of acute myocardial ischemia has been improved dramatically with the development of highly successful approaches to restore blood flow by primary percutaneous coronary intervention (PCI) to the ischemic tissue. However, coronary reperfusion also leads to myocardial reperfusion injury by generation of reactive oxygen species (ROS) from mitochondria. The accelerated generation of ROS by reperfusion of the ischemic myocardium is a potential mediator of reperfusion injury (Citation1–3). It was demonstrated that hydrogen (H2) is a novel antioxidant agent, which specifically quenches exclusively detrimental ROS, such as hydroxy radical (•OH) and peroxynitrite (ONOO‐), and that inhalation of H2 gas confers protection in focal brain ischemia reperfusion (I/R) injury in rats (Citation4). This observation was echoed by others in different organs such as intestine (Citation5), liver (Citation6), kidney (Citation7), and heart (Citation8) in small animal models.
Myocardial stunning is defined as prolonged, but reversible post-ischemic contractile dysfunction following a brief ischemic episode lasting less than 20 minutes that does not result in necrosis or multiple completely reversible episodes of ischemia (Citation9–11). It occurs in unstable angina, exercise-induced ischemia, PCI, and open heart surgery (Citation12). It has been shown that •OH is the key mediator of stunning. The derivatives are present in stunned tissue and can be detected in the coronary effluent (Citation13). The fact that deferoxamine, which chelates Fe and thereby prevents the Fe-catalyzed reaction that generates •OH, prevents much of the stunning effect is further evidence that •OH is the mediator of stunning (Citation14,Citation15). In addition, any nitric oxide (NO) released during reperfusion can react with superoxide to form ONOO‐, another strong free radical that can cause stunning. It is not known whether H2 provides protection against myocardial stunning.
The present study was carried out to clarify whether inhalation of H2 gas during ischemia and reperfusion has cardioprotective effects against myocardial stunning or myocardial infarction in swine.
Materials and methods
All experimental procedures and protocols described in this study were approved by the Institutional Animal Care and Use Committee. Date of issue and registration number is November 9, 2007 and No. 711090631.
General preparation
Forty-nine swine of either sex weighing 20–30 kg were sedated with 20 mg/kg intramuscular ketamine hydrochloride. Surgical preparation was performed as described previously (Citation16). Swine were anesthetized with 100 mg/kg intravenous alpha-chloralose and 10 µg/kg fentanyl, followed by continuous infusion of 10 mg/kg/h alpha-chloralose and 5 µg/kg/h fentanyl throughout the study period. Through a midline cervical incision, the trachea was intubated for connection to a Harvard respirator pump (Harvard Apparatus Co., South Natrick, MA, USA). Mechanical ventilation was facilitated by intermittent infusion of 0.2 mg/kg vecuronium, and adjusted to maintain arterial carbon dioxide tension (PaCO2) at 35–40 mmHg. The esophageal temperature was maintained at 36–37°C throughout the study period using a warmer blanket and a heating lamp. A catheter was inserted into the right carotid vein to administer fluid and drugs. Lactated Ringer's solution was infused at a rate of 5 ml/kg/h. Systemic anticoagulation was achieved with intravenous 750 U/kg sodium heparin followed by a continuous infusion of sodium heparin at 250 U/kg/h. Sodium bicarbonate was administered to maintain the base deficit within 5 mmol/l. A standard peripheral lead electrocardiogram was monitored continuously. Medial sternotomy was performed, and the pericardium was opened to expose the heart. The left anterior descending coronary artery (LAD) distal to the first diagonal branch was cannulated with a stainless steel cannula and perfused with blood from the left carotid artery through an extracorporeal circuit. Coronary blood flow (CBF) of the perfused area of LAD was measured with an ultrasonic flow probe (ADP17; Crystal Biotech, Hopkinton, MA, A) attached at the extracorporeal circuit. Pressure transducer-tipped catheters (PC500; Millar Instruments, Huston, TX, USA) were connected to the left ventricular (LV) chamber cannula through an incision in the apex and right internal carotid artery cannula for continuous recording of left ventricular pressure (LVP) and arterial blood pressure. The peak rate of increase in LVP (LVdP/dt max) was determined by electric differentiation of the LVP waveform. Two ultrasonic segment length transducers were implanted 10–15 mm apart in the subendocardium of the perfused area of the extracorporeal circuit and aligned such that the intercrystal axis was parallel to the direction of myocardial fiber shortening. The regional contractile function was accessed by changes in percent segment shortening (%SS). Segment length was monitored by ultrasonic amplifiers (VF-1; Crystal Biotech, Hopkinton, MA, USA). The end-systolic segment length (ESL) was determined 10 milliseconds before maximum negative LVdp/dt, and the end-diastolic segment length (EDL) was determined 10 milliseconds before the LVdP/dt first exceeded 140 mmHg/s (immediately before the onset of LV isovolemic contraction). %SS was calculated using the formula: %SS = (EDL − ESL) × 100 × 1/EDL. All hemodynamic data were continuously monitored on a polygraph and digitized through a computer interfaced with an analog-to-digital converter (HEM; Physio-Tech, Tokyo, Japan).
Experimental protocol
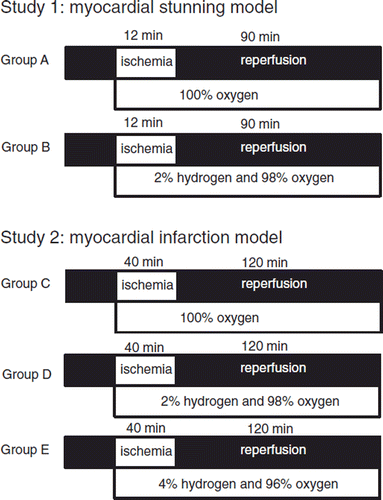
Study 1: Myocardial stunning model. shows the experimental time course. Baseline systemic and coronary hemodynamics and %SS were recorded 30 minutes after the instrumentation was completed. Nineteen swine were randomly assigned to group A or B. Group A (n = 9) received 100% oxygen during and after ischemia. Group B (n = 10) received 2% hydrogen plus 98% oxygen during and after ischemia. All swine were subjected to 12-minute ischemia with complete occlusion of the extracorporeal circuit followed by a 90-minute reperfusion. Hemodynamics and contractile function were monitored continuously throughout the experiment and recorded at the time points illustrated in (P0: baseline, R0: just before reperfusion, R5, R30, R60, and R90: 5, 30, 60, and 90 minutes after reperfusion, respectively). All swine received 2 mg/kg intravenous lidocaine at 1 minute before reperfusion. If five or more premature ventricular contractions per minute or multifocal premature ventricular contractions were observed after reperfusion, 1 mg/kg intravenous lidocaine was administered and repeatedly given if necessary. Swine with continuous ventricular fibrillation (VF) or ventricular tachycardia (VT) after reperfusion were excluded from the study. The effects on reperfusion-induced arrhythmias were evaluated with regard to the incidence of VF or VT and the total amount of lidocaine used for 10 minutes after reperfusion.
Study 2: Myocardial infarction model. shows the experimental time course of study 2. Baseline systemic and coronary hemodynamics and %SS were recorded 30 minutes after the instrumentation was completed. Thirty swine were randomly assigned to one of the three groups. Group C (n = 10) received 100% oxygen during and after ischemia. Group D (n = 10) received 2% hydrogen plus 98% oxygen during and after ischemia. Group E (n = 10) received 4% hydrogen plus 96% oxygen during and after ischemia. All swine were subjected to 40-minute ischemia with complete occlusion of the extracorporeal circuit followed by a 120-minute reperfusion. Hemodynamics and contractile function were monitored continuously throughout the experiment and recorded at the time points illustrated in (P0: baseline, R0: just before reperfusion, R5, R30, R60, R90, and R120: 5, 30, 60, 90, and 120 minutes after reperfusion, respectively). After starting ischemia, all swine were given intravenously 800 mg of magnesium chloride and 120 mg of amiodarone over 20 minutes to prevent cardiac arrhythmias (Citation17). Swine with continuous VF or VT after reperfusion were electrically defibrillated. Swine with continuous VF or VT after a defibrillation were excluded from the study.
Determination of infarct size
Determination of infarct size (IS) was performed as described previously (Citation17). After final hemodynamics and contractile function data collection, the LAD was occluded. The ascending aorta was cross-clamped, and 40 ml of Evans Blue solution was injected into the left atrial appendage to stain normally perfused myocardium. Immediately thereafter, 20 mmol of potassium chloride solution was injected into the same site to induce cardiac arrest. The heart was resected, and the LV was cut into six 6–8-mm slices starting 5 mm from the apex and perpendicular to the long axis. The slices were placed in 1% triphenyl tetrazolium chloride solution for 15 minutes to determine myocardial IS of area at risk (AAR). Photographs of each LV section were processed to divide each transection into three color areas of blue (unaffected myocardium), red (noninfarcted area of AAR), or gray (infarcted area), before performing computerized planimetry (ImageJ 1.34 software, NIH public domain, USA). The respective areas for each color from all six sections were normalized to the total sum of areas. The size of the myocardial AAR, as related to total area, and the myocardial IS in relation to AAR were calculated as follows:
AR [%] =∑(a gray1 + a red1…a gray6 + a red6)/ ∑(a gray1 + a red1 + a blue1…a gray6 + a red6 + a blue6)
IS [%] =(a gray1 …a gray6)/∑(a gray1 + a red1 …a gray6 + a red6)
Statistical analysis
All data were expressed as mean ± SD. One-way analysis of variance (ANOVA) for non-repeated measures followed by the Bonferroni's post hoc test was used to test for differences in baseline hemodynamics and %SS. Data within groups were analyzed with one-way ANOVA for repeated measures, and data between groups were analyzed with two-way repeated measures ANOVA followed by the Bonferroni's post hoc test. P values < 0.05 were considered statistically significant.
Results
Study 1: Myocardial stunning model
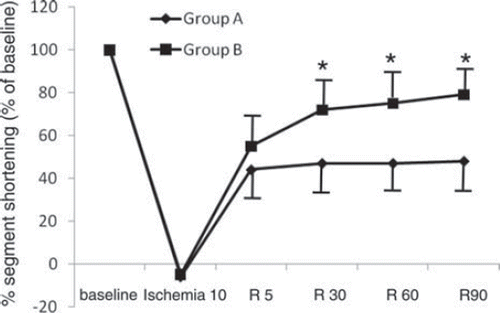
Three in group A and two in group B had continuous VF or VT after reperfusion, and they were excluded from further study. Thus, results were reported for six animals in group A and eight in group B. The incidence of VF or VT and the total amount of lidocaine used were not significantly different between the groups (data not shown). There were no significant differences in weight or sex between the groups. Arterial blood gas values and blood glucose were maintained within physiological range in all swines throughout the study period (data not shown). shows the systemic and coronary hemodynamics from baseline throughout the time course of the study 1. There were no significant differences between the groups at any measured point in heart rate (HR), mean arterial pressure (MAP), LV end-diastolic pressure (LVEDP), or LVdp/dt max. CBF increased significantly at 5 minutes after reperfusion and returned to baseline thereafter, and there were no significant differences at any measured point between the groups. Baseline values of %SS were 20 ± 5 in group A and 20 ± 4 in group B, respectively, and there was no significant difference between the groups. shows the percent changes of %SS from baseline throughout the time course of the study. All swine showed negative %SS values at 10 minutes after ischemia, which indicates bulging. The percent changes of %SS at 30-, 60-, and 90-minute reperfusion in group B (72 ± 14%, 75 ± 9%, and 79 ± 14% of baseline, respectively) were significantly higher than those in group A (47 ± 16%, 47 ± 17%, and 48 ± 15% of baseline, respectively).
Table 1. Hemodynamic data in study 1.
Table II. Hemodynamic data in study 2.
Study 2: Myocardial infarction model
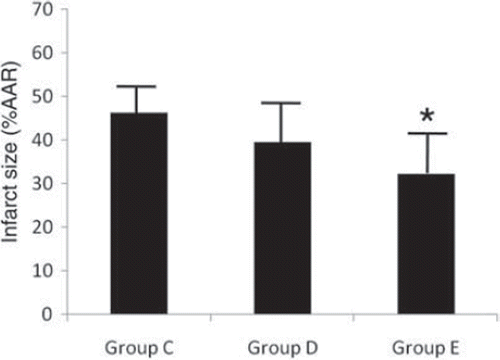
Seven animals were excluded from the analysis. Two swine in group C, two swine in group D and one swine in group E had VF or VT after reperfusion. One swine in group D and one swine in group E were excluded from the analysis because of failed myocardial staining. Thus, results are reported for seven animals in group D and eight in groups C and E. shows the systemic and coronary hemodynamics throughout the time course in study 2. There were no significant differences between the groups at any measured point in HR, MAP, LVEDP, LVdp/dt max, CBF, or %SS. shows myocardial IS in relation to AAR. The AAR for myocardial infarction as related to total LV area was 26 ± 4%, 27 ± 5%, and 28 ± 3% in groups C, D, and E, respectively. There were no significant differences among the groups. Myocardial IS in group E (32 ± 10%) was significantly lower than that in group C (46 ± 6%). During the first 10 minutes of reperfusion, electrical cardioversion was necessary in 10 animals because of sustained VT, with equal distribution over the groups.
Discussion
The present results show that inhalation of 2% H2 gas during I/R improves the recovery from myocardial stunning, and that inhalation of 4% but not 2% H2 gas reduces myocardial IS in swine.
The accelerated generation of ROS by reperfusion of ischemic myocardium is a potential mediator of reperfusion injury. Unfortunately, however, therapy to reduce ROS during early reperfusion failed to relieve this pathological cascade of oxidative damage after reperfusion injury. There are a number of possible reasons for this result. First, ROS in ischemia-reperfused hearts may have the dual roles (Citation18,Citation19). The majority of detrimental effects associated with lethal reperfusion injury are attributed to •OH and ONOO‐, the highly reactive oxygen species. By comparison with these, superoxide anion radical (O2‐•) and hydrogen peroxide (H2O2) have less oxidative energy and, paradoxically are implicated as crucial signaling components in the establishment of favorable tolerance to oxidative stress upon ischemia–reperfusion (Citation20,Citation21). Thus, the inhibition of both pathways might offset the beneficial effect. A second major limitation for exogenous antioxidant substances is a fundamental difficulty in successfully delivering the drug to myocardial tissue at risk, and poor cellular permeability.
On the other hand, it was demonstrated that H2 selectively reduces the detrimental ROS, •OH, and ONOO‐, and that H2 is rapidly transported and can reach “at risk” ischemic myocardium before CBF of the occluded infarct-related artery is reestablished. Since the H2 molecule is electrically neutral and much smaller than the oxygen molecule, it easily penetrates membranes and enters cells and organelles such as a nucleus and mitochondria.
About 50%–70% of the stunning effect to a burst of O2-derived free radicals liberated during the first few minutes of reperfusion with arterial blood (Citation22). In the present study, inhalation of 2% H2 gas improved regional contractile function at I/R area, whereas it did not affect HR, MAP, LVEDP, LVdp/dt max, or CBF. Thus, it is unlikely that systemic and coronary hemodynamics played any role in the present results.
In addition to ROS reduction, some possible mechanisms involved in the beneficial effect of H2 have been postulated. Sun et al. (Citation23) demonstrated that hydrogen-rich saline administered at 5 minutes before the reperfusion reduced myocardial IS and plasma and myocardial malondialdehyde in the rat LAD occlusion model. They also showed that hydrogen-rich saline inhibited activation of down-stream caspase-3 and reduced apoptosis of cardiomyocytes. Zhang et al. (Citation24) demonstrated that hydrogen-rich saline decreased neutrophil infiltration, 3-nitrotyrosine level, and expression of intracellular adhesion molecule 1 and attenuated the increase of proinflammatory cytokines in the rat LAD occlusion model. Thus H2 would have anti-apoptotic and anti-inflammatory effects.
VF and VT often occurred at reperfusion in an in vivo model of myocardial infarction and stunning. Therefore, we administered magnesium chloride and amiodarone in the myocardial infarction model, and lidocaine in the myocardial stunning model. Consequently, there were no significant differences among groups in the incidence of VF or VT.
In summary, inhalation of 2% H2 gas during I/R improves the recovery from myocardial stunning, and inhalation of 4% but not 2% H2 gas reduces myocardial IS in anesthetized open-chest swine.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–7.
- Bolli R, Patel BS, Jeroudi MO, Lai EK, McCay PB. Demonstration of free radical generation in ‘‘stunned” myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 1998:82;476–85.
- Hoek TV, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86:541–8.
- Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, . Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature Med. 2007;13:688–94.
- Buchholz BM, Kaczorowski DJ, Sugimoto R, Yang R, Wang Y, Billiar TR, . Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant 2008;8:2015–24.
- Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S, . Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–4.
- Shingu C, Koga H, Hagiwara S, Matsumoto S, Goto K, Yokoi I, . Hydrogen-rich saline solution attenuates renal ischemia-reperfusion injury. J Anesth. 2010;24:569–74.
- Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, . Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30–5.
- Bolli R, Zughaib M, Li XY, Tang XL, Sun JZ, Triana JF, . Recurrent ischemia in the canine heart causes recurrent bursts of free radical production that have a cumulative effect on contractile function. A pathophysiological basis for chronic myocardial “stunning”. J Clin Invest. 1995;96: 1066–84.
- Kim SJ, Ghaleh B, Kudej RK, Huang CH, Hintze TH, Vatner SF, . Delayed enhanced nitric oxide-mediated coronary vasodilation following brief ischemia and prolonged reperfusion in conscious dogs. Circ Res. 1997;81;53–9.
- Sun JZ, Tang XL, Park SW, Qiu Y, Turrens JF, Bollo R, . Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J Clin Invest. 1996;97:562–76.
- Kim SJ, Depre C, Vatner SF. Novel mechanisms mediating stunned myocardium. Heart Fail Rev. 2003;8:143–53.
- Sun JZ, Kaur H, Halliwell B, Li XY, Bolli R. Use of aromatic hydroxylation of phenylalanine to measure production of hydroxyl radicals after myocardial ischemia in vivo: direct evidence for a pathogenetic role of the hydroxyl radical in myocardial stunning. Circ Res. 1993;73:534–49.
- Bolli R, Patel BS, Zhu WX, O’Neill PG, Hartley CJ, Charlat ML, . The iron chelator desferrioxamine attenuated postischemic ventricular dysfunction. Am J Physiol. 1987;253:H1372–H80.
- Farber NE, Vercellotti GM, Jacob HS, Jacob HS, Pieper GM, Gross GJ, . Evidence for a role of iron-catalyzed oxidants in functional and metabolic stunning in canine heart. Circ Res. 1988;63:351–60.
- Shibata I, Yoshitomi O, Use T, Ureshino H, Cho S, Maekawa T, . Administration of the Rho-kinase inhibitor fasudil before ischemia or just after reperfusion, but not 30 min after reperfusion, protects the stunned myocardium in swine. Cardiovasc Drugs Ther. 2008;22:293–8.
- Baumert JH, Hein M, Gerets C, Baltus T, Hecker KE, Rossaint R, . The Effect of Xenon Anesthesia on the Size of Experimental Myocardial Infarction. Anesth Analg. 2007; 105:1200–06.
- Flaherty JT, Pitt B, Gruber JW, Heuser RR, Rothbaum DA, Burwell LR, . Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction, Circulation. 1994;89:1982–91.
- Richard VJ, Murry CE, Jennings RB, Reimer KA. Therapy to reduce free radicals during early reperfusion does not limit the size of myocardial infarcts caused by 90 min of ischemia in dogs, Circulation. 1988;78:473–80.
- Penna C, Rastaldo R, Mancardi D, Raimonds S, Cappello S, Gattullo D, . Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation, Basic Res Cardiol. 2006;101:180–9.
- Downey JM, Cohen MV. Cohen, A really radical observation—a comment on Penna . Basic Res Cardiol. 2006; 101:190–1.
- Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–9.
- Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang JH, . Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats.” Exp Biol Med (Maywood). 2009; 234:1212–19.
- Zhang Y, Sun Q, He B, Xiao J, Wang Z, Sun X, . Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol. 2011;148:91–5.