Abstract
Objective. Transcatheter aortic valve implantation (TAVI) offers a new treatment option for patients with severe symptomatic aortic valve stenosis, classified as “inoperable”. The purpose of the study was to reveal the association between ascertained hospital costs with the actual patient Diagnosis-Related Group (DRG). Method. We examined 50 consecutive patients who underwent either transapical TAVI, (TAVI-TA) or transfemoral TAVI (TAVI-TF) with the Edwards SAPIEN valve and CoreValve® between September 2009 and August 2011. Results. Fourty-nine patients had successful valve deployment. Seven patients died within 30 days of the operation. The mean length of hospital stay for TAVI-TA was 199 hours (range 77–362), and the mean costs for TAVI-TA were 55,690 US$. For TAVI-TF the mean length of hospital stay was 170 hours (range 49–276) and the mean costs were 52,087 US$. Conclusion. There was no significant difference between TAVI-TA and TAVI-TF patient characteristics. There was a significant discrepancy between actual hospital costs and the current Norwegian DRG reimbursement for the TAVI procedure. This discrepancy can be partly explained by excessive costs related to the introduction of a new program with new technology. Costly innovations should be considered in price-setting of reimbursement for novel technology.
Introduction
Aortic valve stenosis is the most frequently acquired heart valve disease. In the coming decades, there will be a tremendous aging of the population in developed countries and the estimated prevalence in individuals older than 75 years is 5% (Citation1–2). Conventional aortic valve replacement (AVR) is the gold standard and has yielded excellent results (Citation1–2). As the patient population gets older more co-morbidities will also develop. The European Heart Survey of patients with valvular heart disease suggests that up to 33% of subjects over the age of 75 years are not considered for surgical AVR due to age and comorbidities (Citation3). Transcatheter aortic valve implantation (TAVI) has emerged as an alternative for high risk patients where surgical treatment is not possible. The life expectancy for patients with inoperable aortic valve disease is short (Citation4–5), and the valve implants are expensive. TAVI may add a better quality of life to these patients, and help them stay home with less need for care. The cost effectiveness of the procedure has been questioned (Citation6). For hospitals it is important to calculate the actual hospital costs for the procedure to get a realistic reimbursement. In the present study the actual hospital costs were calculated.
Material and method
Material
The first 50 patients with severe aortic stenosis from September 2009 to August 2011 treated with TAVI in our institution were included. There were 31 males, mean age of 82 years (59–92). Twenty-nine patients had previously undergone heart surgery (coronary bypass) and seven had pacemaker implanted (). The mean Logistic EuroSCORE was 33 (range 7–74) and the patients were in NYHA class III/IV. The mean ejection fraction was slightly reduced 44% (20–55). The mean gradient of the aortic valve was 54 mmHg (range 33–105) and mean area was 0.6 cm² (range 0.3–0.9). The mean annular size was 23 mm (range 20–28) and the mean size of prosthesis was 25 mm (range 23–31) ().
Table I. Patient demographics.
Method
The study was approved by the Regional Ethical Committee and all patients were included after informed consent.
The procedure.
All procedures were carried out in a combined surgical and angiography room with a team of cardiac surgeons, cardiologists, anesthesiologists and a perfusionist stand-by with a primed heart-lung machine (HLM) (Citation7). All procedures were performed save one in general anesthesia. The induction was done with benzodiazepines and maintained with gas and fentanyl. But most importantly, the fluid treatment and norepinephrine infusion were mandatory to maintain the blood pressure at an acceptable level.
For the transfemoral access a “cut down” was done, identifying the common femoral artery. Between two longitudinal purse string sutures (Prolene 4–0), the introducer sheath 18/19 Fr was placed. Transapical approach was performed through a left anterolateral mini thoracotomy in 6th intercostal space.
Trans esophageal guidance in addition to angiography were used in all the procedures in general anesthesia to determine the optimal position of the valve. The patients were treated consecutively first with TAVI-TA, then with TAVI-TF with the Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA). We did not make any selection for the access site until after the first 19 TAVI-TA procedures were done. Afterwards, TAVI-TF was the first choice during the next 20 procedures. The CoreValve® ReValving system (Medtronic, Minneapolis, MINN, USA) was introduced during the last part of the period (from March 29. 2011). We then had experience with the Edwards SAPIEN valve and were in need of a transcatheter heart valve system for annuli larger than 25 mm.
Altogether forty-two procedures were done with the Edwards SAPIEN System, 25 transapically and 17 transfemorally. In addition eight transfemoral approaches were done with the CoreValve® ReValving system.
Patient logistics.
All patients deemed inoperable for open surgery were discussed in the TAVI team and accepted or rejected for TAVI treatment. The patients were admitted to the Cardiothoracic general unit (TKASP) one day before the TAVI procedure for blood samples, chest x-ray and preoperative information. The procedure was performed at the Intervention Center and the patients stayed at least one night in the Cardiothoracic Intensive Care Unit (TKAI) or in the Postoperative care unit (PO). The patients who underwent cardiopulmonary bypass and had postoperatively intraaortic balloon pump (IABP) were in the intensive care unit for several days. They were brought back to the Cardiothoracic general unit (TKASP) for three days and discharged either to the Cardiology medical unit (HMSP) or to the local hospital. Because of the risk of developing heart block during the first postoperative week with the CoreValve® system, these patients stayed longer to get their heart rhythm monitored.
Cost analysis.
The analysis involved two sets of data, one based on data for the individual patient (direct costs) and one based on the overhead costs (indirect costs), with the overhead costs ultimately also allocated to the individual patient. The basic principle of the analysis was to relate as much as possible of the resources used as direct costs to the individual patient (Citation8).
All cost data were calculated in 2010-prices and converted from Norwegian Kroner (NOK) to US dollars (US$) with the exchange rate of 1 US$ = 6 NOK.
Statistical methods.
We evaluated the trend in total costs by the means of multivariate regression analyses. The trend was simply described by a variable taking the value of 1 for the first TAVI-procedure and increasing by 1 for each consecutive treatment. The different procedures were modeled by a dummy variable (0 = TAVI TF, 1 = TAVI TA). EuroSCORE was entered as a control variable to account for selection bias in the patient population. We considered P-values < 0.05 as statistically significant. SPSS 18 was used for the statistical analyses.
DRG reimbursement.
The actual DRG code is automatically created by a combination of ICD-10 diagnosis and procedure code based on the Nordic Classification of Surgical Procedures (NOMESCO) (Citation9). Based on procedure codes, main diagnosis and secondary diagnosis system algorithm allocates special DRG to each patient (Citation10). In Norway, during the study time no specific DRG for TAVI patients had yet been developed. So far all patients treated with TAVI have been classified to the same DRG as conventional surgical aortic valve replacement surgery, DRG 104. DRG 104 had in 2010 a unit price of 39,555 US$ (). There are no revenues coming from out-of-pocket payment as in-patient stays are free of charge in Norway (Citation11).
Results
The valve was successfully implanted in all patients except from one and a second valve had to be used due to malpositioning. The procedure time in the operating room was almost equal for TA and TF, however slightly longer in the TA group. This was mainly because of an initial learning curve as we started with the TA approach. On the other hand, the operating time was somewhat extended in the TF group probably due to the retrograde crossing of the stenotic aortic valve with the guide wire.
There was initially no major (> 2+) paravalvular regurgitation.
Eleven patients had periprocedural external chest compressions and of these, five patients needed partial fem-fem bypass assist for nearly one hour (mean 56 (range18–89) minutes) and four patients had IABP following the TAVI procedure.
A number of serious adverse events were observed within 30 days (). Stroke occurred in five patients (two major and three minor strokes), one patient had permanent pacemaker implanted postoperatively and two patients were reoperated due to hematothorax. Additionally, two patients had femoral artery revision due to pseudoaneurysm and arterial thrombosis/obstruction. Pericardiocenthesis due to tamponade was done in another two patients. Seven patients died within 30 days postoperatively and the over all survival was 78% (observation time 4–26 months) ().
Table II. Results.
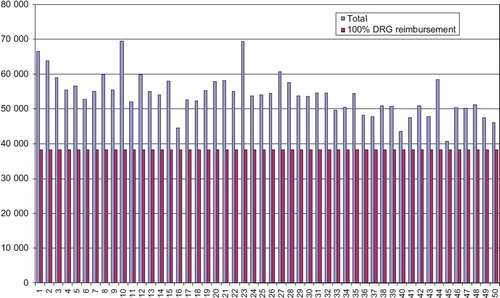
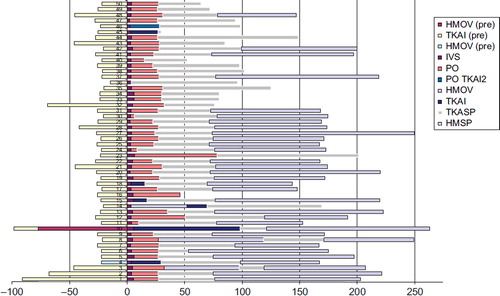
We analyzed the total hospital LOS including the time before and after TAVI procedures. Our results showed huge variations in LOS between the patients (), but we could not identify whether this was due to clinical or logistic reasons. The mean hospital LOS was 185 hours (range 49–362) for both groups. The mean costs for all TAVI patients were 55,537 US$ and the median 54,607 US$ (range 44,552–69,505).
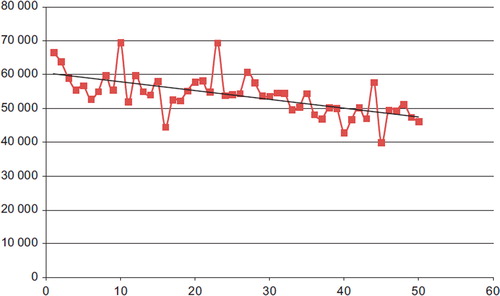
Figure 3. The correlation between the number of procedures and the cost. There is a slight negative slope in costs with the increasing number of procedures performed.
The total mean cost for TAVI patients was 55,537 US$ and the median 54,607 US$ (range 44,552–69,505). The mean cost for TAVI-TA patients was 55,690 US$ and the median 55,075 US$ (range 44,552–69,505). The mean hospital LOS was 199 hours (range 77–362).
The mean cost for TAVI-TF procedures was 52,087 US$ and the median 51,284 US$ (range 39,925–69,422). The mean LOS was 170 hours (range 49–276). The mean DRG reimbursement, weighted by 100% case mix (2010) was 39,555 US$ ().
Table III. Average Procedure cost and DRG reimbursement (mean) (US$).
From a total number of 50 patients, five were on HLM and four of these also got an IABP which added more costs to the treatment. These patients had a longer operating time and also a longer hospital stay.
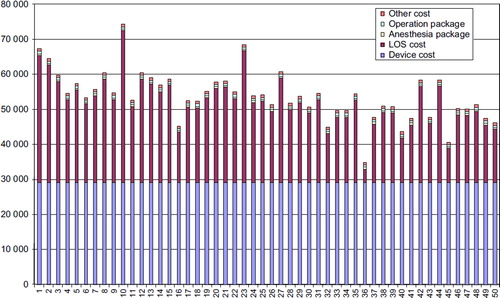
Disaggregating total costs into the different cost drivers revealed that 52% of the costs for the TAVI-TA procedures were related to the device and 34% of the cost related to LOS. Comparable figures for the TAVI-TF procedure were 55% and 33%, respectively.
The estimates from the regression analysis indicated that total costs were declining as the number of treated patients increased (). The estimate from the trend variable was ‐275 (CI: ‐388 to ‐162, p < 0.01) indicating that for each additional patient, total costs were reduced by 275 US$ (adjusted R-sq: 0.39). The effect of the EuroSCORE variable was insignificant, so was the effect of the dummy variable describing the different procedures.
Discussion
Treatment by TAVI introduced either transfemorally or transapically was offered to patients with inoperable aortic stenosis as a way of introducing the method in our hospital. We have documented the costs related to the TAVI-TA and TAVI-TF procedures which were useful steps to evaluate the procedures’ cost efficiency. So far, we were only able to compare the procedures’ total costs with the actual DRG reimbursement during the establishment of a TAVI program at our institution. To our knowledge this is the first published study with an evaluation of the total patient costs for TAVI procedures.
It is worthwhile to notice that the device cost contributed to over 50% of the total costs both for the TAVI-TA and TAVI-TF procedures (). There were no differences in costs for the TA and TF procedures in our material, as we did a consecutive study with no clinical selection for TA or TF. One should expect a difference if there was a selection, as the TA patients tended to be sicker (Citation12). They had more peripheral vessel disease.
Figure 2. Accumulated clinical pathway for each patient. Information of LOS in hours at different clinical department levels. Zero is the time of the procedure. On the left: elapsed time prior to TAVI. On the right: elapsed time following TAVI. HMOV, Cardiological intensive care unit; HMSP, Cardiological unit; IVS, Intervention Center; PO, Post operative care unit; TKAI, Cardiothoracic intensive care unit; TKop, Cardiothoracic Operating room; TKASP, Cardiothoracic general unit.
Existing clinical studies have shown cost- effectiveness in using TAVI, however there are few studies documenting prospective hospital costs and reimbursement of TAVI procedures. A study from Thomas M et al (Citation13), demonstrates early experience data from SOURCE registry, both for the TA and TF approaches showing that one-year survival in high-risk and inoperable patients was high. Another study by Watt M et al (Citation6) indicates that TAVI procedures are cost-effective treatments and have good long-term survival. The result from Watt M et al was similar to our study as it showed that the majority of TAVI costs were related to the surgical procedures which included the procedure and device cost, ICU costs and total in-hospital costs. In the future there will probably be more competitors in the market, which might reduce device cost and thus increase cost efficiency.
Recently published were one year data from a study of randomized patients with severe, symptomatic aortic stenosis and high surgical risk to either TAVI (348 patients) or surgical aortic valve replacement SAVR (351 patients). This study showed that the TAVI procedure was cost- effective compared with SAVR (Cohort A of PARTNER), presented by Matthew R. Reynolds, Nov.10th 2011 at the Transcatheter Cardiovascular Therapeutics (TCT) conference. However the procedure also contributed to cost- effectiveness as depending on whether TAVI was performed via the femoral artery or transapically through a small incision in the chest. Overall, the TAVI–TF resulted in a difference of 2,496 US$ compared with SAVR, with the total TAVI-TF cost at 71,955 US$ and the total SAVR cost at 74,452 US$.
There are three possible reasons for costs to be higher in our study than in those reported:
First, we believe our cost-estimating method is more accurate. By directly measuring costs at patient level we can avoid general cost estimates regarding for example blood product or use of operating theater that are often required by other cost methods. The drawback of our method is that it is labor intensive. The consequence of this is that only a few hospital studies rely on the direct measurement method.
Secondly, our results should be considered in the light of the fact that we are analyzing a medical innovation. Costs at this stage of the innovation are associated with lower volume, steep learning curves, heavy investments and increases in manpower.
Third, logistic and geographic influence: We tend to keep the patients in our hospital for a longer period of time if transportation to the local hospital is long and complicated.
If we see the TAVI patients altogether, we observe a reduction in LOS, procedure time and cost. There was no big difference in operation time for the TA and TF cases. One should expect longer operation time with the TA than TF access because of the surgical procedure with thoracotomy and exposure of the apex. In this study the operation time was almost similar between TA and TF, because it was more time-consuming to cross the aortic valve retrograde than antegrade. Our results indicated a learning curve effect both on clinical outcomes and costs. The reason was probably due to the fact that the first treated patients had to stay longer than medically necessary due to lack of experience in our hospital and in local hospitals. Wendler O et al (Citation14) had in the SOURCE registry a study which analyzed the first two years data of TAVI. It revealed that there were fewer technical complications and a reduction in conversion to conventional surgery in the last cases, probably reflecting a similar learning curve effect. Lurz P. et al (Citation15) reported a decrease in procedural complication rate from 6% in the first cohort to 2.9% in the second cohort of patients for percutaneous pulmonary valve implantation. A study from Gurvitch et al (Citation16) also documented improved outcome with experience and device development.
Further research should also address the cost effectiveness of the TAVI program. Although TAVIs appear as clinically effective as other SAVR procedures; it is unlikely they will be cost-effective unless costs decrease or the benefits of their use increase.
Conclusion
We have shown significant discrepancy between actual hospital costs and current Norwegian DRG reimbursements for the TAVI procedure. This discrepancy can be partly explained by the excessive costs related to the device, and partly to the introduction of a new program with new technology. In the future we hope to be able to show the overall costs for the patients also in a macro economic perspective. Nowadays the hospitals lose money for every procedure and there is a need for appropriate DRG reimbursements.
Acknowledgements
The authors acknowledge the contribution of nursing staff at the Department of Cardiothoracic Surgery, and Department of Cardiology and Intervention Center at Rikshospitalet Oslo University Hospital.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, . A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43.
- Bloomstein LZ, Gielchinsky I, Bernstein AD, Parsonnet V, Saunders C, Karanam R, . Aortic valve replacement in geriatric patients: determinants of in-hospital mortality. Ann Thorac Surg. 2001;71:597–600.
- Nkmo VT, Gaudin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M.Burden on valvular heart diseases: A population-based study. Lancet. 2006;368:1005–11.
- Sprigings DC, Jackson G, Chambers JB, Monaghan MJ, Thomas SD, Meany TB, Jewitt DE.Balloon dilatation of the aortic valve for inoperable aortic stenosis. BMJ. 1988;297: 1007–11.
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, . PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98.
- Watt M, Mealing S, Eaton J, Piazza N, Moat N, Brasseur P, . Cost-effectiveness of transcatheter aortic valve replacement in patients ineligible for conventional aortic valve replacement. Heart. 2012;98:370–6.
- Fosse E, Hol PK, Samset E, Elle OJ, Røtnes JS, Bjørnstad P, . Integrating image-guidance into the cardiac operating room. Min Invas Ther & All Technol. 2000;9:403–9.
- Mishra V, Geiran O, Fiane AE, Sørensen G, Andresen S, Olsen EK, . Costs and reimbursement gaps after implementation of third-generation left ventricular assist devices. J Heart Lung Transplant. 2010;29:72–8.
- Classification of surgical procedures, clinical procedure code system guidelines Trondheim: permission from Nordic Medico-Statistical Committee NOMESCO Classification of Surgical Procedures (NCSP), version 1.14.:2009. http://www.helsedirektoratet.no/vp/multimedia/archive/00269/Ncsp_1_14_269099a.pdf.
- Nordic Centre for Classifications in Health Care- Uppsala Sweden - Nord DRG 2010 NOR Classic (Norway). http://www.norddrg.net/norddrgmanual/NordDRG_2010_NOR/index.htm.
- Activity based financing price list 2010. Oslo Ministry of Health and Care services. http://www.helsedirektoratet no/vp/multimedia/archive/00274/Regelverk_innsatsst_ 274039a.pdf".
- Rodés-Cabau J, Dumont E, Boone RH, Larose E, Bagur R, Gurvitch R, . Cerebral embolism following transcatheter aortic valve implantation: comparison of transfemoral and transapical approaches. J Am Coll Cardiol. 2011;57: 18–28.
- Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, . One-Year Outcomes of Cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2011;124:425–33.
- Wendler O, Walther T, Schroefel H, Lange R, Treede H, Fusari M, . The SOURCE Registry: what is the learning curve in trans-apical aortic valve implantation?Eur J Cardiothorac Surg. 2011;39:853–9.
- Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, . Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–72.
- Gurvitch R, Tay EL, Wijesinghe N, Ye J, Nietlispach F, Wood DA, . Transcatheter aortic valve implantation: lessons from the learning curve of the first 270 high-risk patients. Cathet Cardiovasc Interv. 2011;78:977–84.