Abstract
Objectives. Levosimendan is an inodilator indicated for acute heart failure (AHF). Its vasodilatory and anti-ischemic effects are mediated by the opening of ATP-dependent potassium channels (KATP channels). Diabetes mellitus is common in AHF patients and sulfonylureas are often prescribed. Sulfonylureas act by blocking the KATP channels. An interaction between levosimendan and sulfonylureas has been shown in preclinical models and could be hypothesized in clinical practice. Design. We produced a pooled analysis of six randomized levosimendan trials (in total of 3004 patients of which 1700 were treated with levosimendan and 226 both with levosimendan and sulfonylureas) with the aim to study the influence of concurrent sulfonylurea treatment to the levosimendan effects. Invasive and non-invasive hemodynamics, biomarkers (BNP), adverse events related to myocardial ischemia, and survival were evaluated. Results. In our relatively small data set, we could not detect any clinically relevant interactions between the sulfonylureas and levosimendan. Similar decreases in systolic and diastolic blood pressure, pulmonary capillary wedge pressure and BNP, and similar survival and adverse event profiles were seen in sulfonylurea users and non-users exposed to levosimendan. Conclusions. Concomitant use of sulfonylureas with levosimendan does not attenuate the hemodynamic or other effects of levosimendan.
Introduction
Levosimendan is an inotrope and vasodilator developed for the treatment of acute heart failure (AHF) (Citation1). The vasodilation induced by levosimendan is mediated by the opening of ATP-dependent potassium channels (KATP channels) in the smooth muscle cells of the blood vessels (Citation2–5). Moreover, a cardioprotective and anti-ischemic effect by the drug is postulated to be mediated by the opening of KATP channels in cardiac mitochondria (Citation6) probably by suppressing calcium accumulation during the ischemic events (Citation7). Among patients hospitalized for AHF the previous diagnosis of diabetes is common and present in one third of patients (Citation8–9). Some of these patients are treated with the antidiabetic sulfonylureas which act primarily by blocking the KATP channels at the surface of the pancreatic beta cells, but have been hypothesized to have cardiovascular effects by modulating the potassium channels elsewhere (Citation10). In animal models, glibenclamide (glyburide) was shown to counteract the vasodilatory and anti-ischemic effect of levosimendan (Citation4–5,Citation11). Glibenclamide and other oral sulfonylureas could thus theoretically inhibit the effects of levosimendan on the potassium channels in the treatment of AHF patients. We performed a pooled analysis on data from six randomized levosimendan trials to evaluate whether its effects on hemodynamics, neurohormonal levels, and outcome are affected by concomitant use of sulfonylureas. No previous data on the potential interaction of levosimendan and sulfonylureas in humans have been published, to the best of our knowledge.
Methods
Post-hoc analyses from several randomized, double-blind, multicenter studies with levosimendan were performed. All studies followed the 1975 Declaration of Helsinki and informed consent was obtained prior to any study specific measures. We included studies on which we had full access to the data; which had mortality follow-up at least for 1 month post study drug infusion, and included either invasive or noninvasive hemodynamic data collection. The studies shared similar patient population (decompensated heart failure), but their endpoints, comparators and follow-up methods differed (). The concomitant medication was categorized by ATC coding and the code A10BB was used to detect sulfonylurea users. The following sulfonylureas were used: glimepiride, gliquidone, glibenclamide (glyburide), gliclazide, glipizide, and tolazamide.
Table I. Studies included in the analyses.
Invasive hemodynamic data was available in the dose-finding study (Citation12), dose-escalation and withdrawal study (Citation13,Citation14), and the LIDO study (Citation15). Patients receiving levosimendan were divided into those using sulfonylureas (SU+) concomitantly with the study drugs, and those not (SU−). Pooled hemodynamic data on pulmonary capillary wedge pressure (PCWP), cardiac output (CO), systolic blood pressure and heart rate were analyzed within the levosimendan treated patients by SU use.
Mortality data up to 30 days post-infusion was available from the above-mentioned studies with invasive hemodynamics, the RUSSLAN, the SURVIVE, and the REVIVE I & II studies. In the RUSSLAN study, 504 patients with acute myocardial infarction within 5 days earlier, developing heart failure were exposed to levosimendan (Citation16). The SURVIVE study was a mortality trial in 1327 patients comparing the efficacy and safety of levosimendan and dobutamine (Citation17). The REVIVE study had a pilot phase (REVIVE I) with 100 patients and an efficacy study (REVIVE II) with 600 patients. The eligibility criteria and the outcome variables of the REVIVE I & II were essentially the same and the recruitment continued uninterrupted until the 700 patients had been included. The studies are therefore presented as one for the present analyses. We obtained the main study results (including the data on mortality) from the paper by de Lissovoy et al. (Citation18), since the full report of this Phase III study is still to be published.
In the SURVIVE and the REVIVE I & II studies adverse event data up to 30 days, and non-invasive vital signs and BNP levels up to 5 days post- infusion, were collected (). Pooled adverse event data reflecting myocardial ischemia were analyzed in SU+ and SU− patients treated with levosimendan or comparator. In addition, the vasodilatory effect by SU use was non-invasively evaluated via changes in systolic and diastolic blood pressure and BNP values.
The invasive hemodynamic studies and the RUSSLAN study only followed adverse events and non-invasive hemodynamics for 1 day post-infusion and BNP was not measured. Therefore, these studies are not included in the adverse event, non-invasive hemodynamic or BNP analyses.
Statistical analyses
Baseline demographic and clinical characteristics are presented as mean for continuous variables, and as percentage of patients for categorical variables. Comparisons between SU− and SU+ use at baseline for categorical variables and adverse events were performed using Fisher's exact test. The analysis of variance (ANOVA) was used to assess baseline differences between SU+ and SU− groups for the continuous variables. For the hemodynamic variables the changes from baseline were analyzed using analysis of covariance (ANCOVA) model with SU use at baseline and baseline value as covariate.
Cox proportional hazard model was used to evaluate the effect of SU use on mortality. Multivariate Cox proportional hazard analysis was performed to evaluate the effect of SU use on survival using Cox proportional hazard model with forward selection including significant baseline factors observed in univariate analysis. Further, propensity score adjusted and propensity score SU+/SU− matched logistic regression model analyses corroborating findings from multivariate model were performed. Multivariate logistic regression model was used to evaluate the incidence of adverse events of myocardial ischemia. The statistical analyses were performed using SAS software version 9.2 (SAS Institute Inc, Cary NC), and significance was reported at a p-value of 0.05 or less.
Results
Invasive hemodynamics
In the three studies considered in this analysis (Citation11,Citation12,Citation14), 296 patients received levosimendan. Of these, about 15% had ongoing SU use during levosimendan infusion. The baseline hemodynamic characteristics in the SU+ and SU− patients were similar (). The mean (±SD) levosimendan dose was similar; 21.4 (13.2) mg vs. 22.6 (15.2) mg in SU+ and SU− groups, respectively (p = 0.767). Levosimendan decreased PCWP and increased CO similarly in SU+ and SU− groups (). Also, no statistically significant differences were seen in the changes of systolic blood pressure and heart rate by SU use ().
Table II. Change in hemodynamic variables [mean (SD)] after a 24-hour infusion of levosimendan by the use of sulfonylurea treatment in studies with invasive hemodynamic assessment (Citation12–15).
Mortality
About 13% of the patients in the studies had ongoing SU treatment. This ratio was similar in both the levosimendan and comparator groups. The baseline characteristics of all subgroups are presented in . The doses of levosimendan in SU+ and SU− groups adjusted per BMI were similar in the two groups (p = 0.116).
Table III. Baseline characteristics in all studies (Citation12–18) combined (in sulfonylurea users and non-users and in levosimendan- and comparator-treated patients).
Surprisingly, the SU+ group had a statistically significantly lower mortality than the SU− group in the combined analysis (6.6% vs. 11.1%; hazard ratio 0.585, 95% confidence interval 0.392–0.847) (). There were, however, statistically significant differences at baseline in several risk factors generally known to be linked with poor outcome (). In addition to diabetes mellitus; hypertension, BMI, and baseline blood glucose were higher in the SU+ group and the patients were older. On the other hand, baseline heart rate and BNP were lower and systolic blood pressure higher and more patients had ongoing ACE-inhibitor/angiotensin receptor blocker and aldosterone antagonist in the SU+ group. Male gender was more prevalent in the SU− group. Of these factors, increased heart rate, decreased systolic blood pressure, older age, higher BMI, the non-use of ACE-inhibitor/angiotensin receptor blocker and aldosterone antagonist remained statistically significant predictors of increased mortality. After adjusting for these variables, the 31-day mortality in SU+ and SU− groups was no longer statistically different or the significance was markedly reduced, depending on the analysis method used (). BNP and blood glucose level were not included in the models as these variables were not obtained in all studies.
Table IV. Mortality during 31-days post-infusion in individual studies and all studies combined.
The overall 31-day mortality in the levosimendan- and comparator-treated groups was similar in the pooled analysis both among the SU− patients and SU+ patients ().
Myocardial ischemia
No statistically significant differences (unadjusted or adjusted) were seen in the frequency of adverse events of myocardial ischemia in the SU+ and SU− groups ().
Table V. Adverse events of myocardial ischemia* during 31-days post-infusion in SURVIVE and REVIVE I & II.
AEs reflecting myocardial ischemia were reported numerically less frequently in patients treated with levosimendan vs. comparator in the combined SURVIVE and REVIVE I & II studies both among the SU− patients (10.8% vs. 12.9%, p = 0.18) and among the SU+ ones (9.2% vs. 12.5%, p = 0.57) ().
Blood pressure and BNP
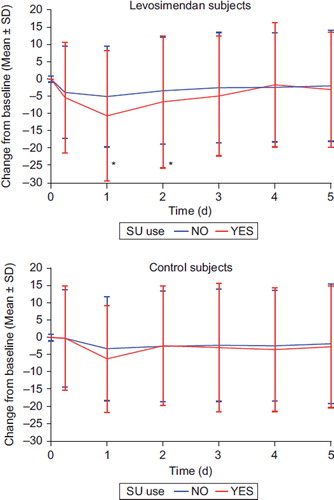
The changes in systolic blood pressure and BNP in the SURVIVE and REVIVE I & II studies combined, according to the SU use, are presented in and . Overall, both systolic blood pressure and diastolic blood pressure (data for diastolic blood pressure not shown in Figures) and BNP decreased more in the levosimendan-treated patients. In the levosimendan-treated patients, systolic blood pressure decreased statistically significantly more in the SU+ group compared to SU− group. No difference in the diastolic blood pressure or BNP response by sulfonylurea use was noted in the levosimendan- or in the comparator-treated patients.
Discussion
Our results suggesting that sulfonylurea users in clinical studies on decompensated heart failure have a trend towards lower mortality compared to non-users were unexpected. Sulfonylurea users are diabetic patients and diabetes mellitus alone is a risk factor for increased mortality in heart failure (Citation19). Further, sulfonylureas could theoretically expose patients to myocardial ischemia via their potassium channel-blocking effects (Citation20). Sulfonylurea treatment is associated with significantly higher mortality than metformin treatment in diabetic patients with heart failure (Citation21). After adjusting for baseline risk factors, the statistical significance in mortality was either lost or was greatly reduced, depending on the analysis method. The less severe risk factor profile thus probably explains the numerically lower mortality in the sulfonylurea user group.
The overall finding in our analysis, in relation to the interaction between levosimendan and sulfonylureas, was that concomitant use of sulfonylureas does not have any clinically significant effect on the clinical responses to levosimendan.
Firstly, the invasive hemodynamic data show that the beneficial effects of levosimendan on cardiac output and pulmonary capillary wedge pressure are similar in sulfonylurea users and non-users.
Secondly, similar patterns were seen in levosimendan and comparator groups with regard to mortality and myocardial ischemia adverse events, which further suggests that there is not any negative interaction between levosimendan and sulfonylureas on outcome variables.
Thirdly, there was a significantly greater decrease in systolic blood pressure and similar decrease in diastolic blood pressure and BNP in the sulfonylurea users in levosimendan-treated patients. It can thus be concluded that the vasodilatory effects of levosimendan treatment are not attenuated by the concomitant use of sulfonylureas.
Levosimendan has an active metabolite (OR-1896) which evokes clinically significant effects (Citation22). Unlike levosimendan, which has an elimination half-life of about 1 hour, the half-life of OR-1896 is about 75–80 hours allowing cardiovascular effects to persist up to 7–9 days after discontinuation of a 24-hour infusion of levosimendan (Citation23–25). The pharmacological and therapeutic effects of OR-1896 are mediated by the same mechanisms of action as those of levosimendan; i.e. a positive inotropic effect by calcium sensitization (Citation26) and a vasodilatory effect via opening of KATP cannels (Citation11).
Our present results suggest that concomitant sulfonylurea use has no significant impact not only on the hemodynamic effects of levosimendan, but also on the effects of its active metabolite OR-1896. The effects of levosimendan infusion on systolic and blood pressure and BNP were not attenuated in sulfonylurea users either in the first 24 hours (when only levosimendan is present), or during the follow-up to 5 days (when OR-1896 is predominant).
The follow-up time of survival in the studies we included in our meta-analysis varied from 1 to 6 months. No relevant change in the mortality effect to the 1-month data was seen. Our findings are supported by a recent meta-analysis by Landoni et al. (Citation27) which shows that the inter-study mortality effect of levosimendan does not change significantly.
The seemingly contradictory results of our present findings to those seen in animal models on the concomitant use of sulfonylureas and levosimendan, can be explained by the differences in the sulfonylurea concentrations. In preclinical models, several times higher concentrations of sulfonylureas were used compared to those achieved in clinical practice.
The limitations of the study are its retrospective nature and the relatively low number of patients for outcome analyses. However, for the hemodynamic and neurohormonal variables, the sample size is sufficient.
Over the last years, new oral antidiabetics with mode of action independent of KATP channels have been introduced and they have partly overcome sulfonylureas in clinical practice (Citation28). These include thiazolidinediones, glucagon-like peptide–1 (GLP-1) receptor agonists and dipeptidyl peptidase–4 (DPP-4) inhibitors. There is no preclinical evidence or theoretical suspicion that these newer agents would interact with levosimendan.
Conclusion
Concomitant use of sulfonylureas with levosimendan does not attenuate the hemodynamic or other effects of levosimendan.
Declaration of interest: The work was sponsored by Orion Pharma, the pharmaceutical company owning the rights for intravenous levosimendan. Matti Kivikko, Piero Pollesello and Pasi Pohjanjousi are employees of Orion Pharma. Markku Nieminen, Wilson Colucci, John Teerlink and Alexandre Mebazaa have earlier received honoraria from Orion Pharma. The authors alone are responsible for the content and writing of the paper.
This work was funded by Orion Pharma, Finland.
References
- Follath F. Newer treatments for decompensated heart failure: focus on levosimendan. Drug Des Devel Ther. 2009;3: 73–8.
- Usta C, Eksert B, Gölbasi I, Bigat Z, Ozdem S. The role of potassium channels in the vasodilatory effect of levosimendan in human internal thoracic arteries. Eur J Cardiothorac Surg. 2006;30:329–32.
- Höhn J, Pataricza J, Petri A, Tóth GK, Balogh A, Varró A, Papp JG. Levosimendan interacts with potassium channel blockers in human saphenous veins. Basic Clin Pharmacol Toxicol. 2004;94:271–3.
- Kaheinen P, Pollesello P, Levijoki J, Haikala H. Levosimendan increases diastolic coronary flow in isolated guinea-pig heart by opening ATP-sensitive potassium channels. J Cardiovasc Pharmacol. 2001;37:367–74.
- Kersten JR, Montgomery MW, Pagel PS, Warltier DC. Levosimendan, a new positive inotropic drug, decreases myocardial infarct size via activation of K(ATP) channels. Anesth Analg. 2000;90:5–11.
- Pollesello P, Papp Z. The cardioprotective effects of levosimendan: preclinical and clinical evidence. J Cardiovasc Pharmacol. 2007;50:257–63.
- Murata M, Akao M, O’Rourke B, Marbán E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca(2+) overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circulation. 2001;89: 891–8.
- Burger AJ, Tsao L, Aronson D. Prognostic impact of diabetes mellitus in patients with acute decompensated heart failure. Am J Cardiol. 2005;95:1117–9.
- Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, . EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27: 2725–36.
- Lefer DJ, Nichols CG, Coetzee WA. Sulfonylurea receptor 1 subunits of ATP-sensitive potassium channels and myocardial ischemia/reperfusion injury. Trends Cardiovasc Med. 2009;19:61–7.
- Erdei N, Papp Z, Pollesello P, Edes I, Bagi Z. The levosimendan metabolite OR-1896 elicits vasodilation by activating the K(ATP) and BK(Ca) channels in rat isolated arterioles. Br J Pharmacol. 2006;148:696–702.
- Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, . Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1903–12.
- Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, . Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Study investigators. Circulation. 2000;102:2222–7.
- Kivikko M, Lehtonen L, Colucci WS. Sustained hemodynamic effects of intravenous levosimendan. Circulation. 2003;107:81–6
- Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, . Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360:196–202.
- Moiseyev VS, Põder P, Andrejevs N, Ruda MY, Golikov AP, Lazebnik LB, . Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J. 2002;23:1422–32.
- Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, . Levosimendan vs Dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA. 2007;297:1883–91.
- de Lissovoy G, Fraeman K, Teerlink JR, Mullahy J, Salon J, Sterz R, . Hospital costs for treatment of acute heart failure: economic analysis of the REVIVE II study. Eur J Health Econ. 2010;11:185–93.
- From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, . Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006; 119:591–9.
- Fisman EZ, Tenenbaum A. A cardiologic approach to non-insulin antidiabetic pharmacotherapy in patients with heart disease. Cardiovasc Diabetol. 2009;8:38: doi:10.1186/1475-2840-8-38
- Andersson C, Olesen JB, Hansen PR, Weeke P, Norgaard ML, Jørgensen CH, . Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia. 2010;53:2546–53.
- Sandell EP, Hayha M, Antila S, Heikkinen P, Ottoila P, Lehtonen LA, Pentikainen PJ. Pharmacokinetics of levosimendan in healthy volunteers and patients with congestive heart failure. J Cardiovasc Pharmacol. 1995;26:S57–62.
- Kivikko M, Antila S, Eha J, Lehtonen L, Pentikäinen PJ. Pharmacokinetics of levosimendan and its metabolites during and after a 24-hour continuous infusion in patients with severe heart failure. Int J Clin Pharmacol Ther. 2002;40:465–71.
- Lilleberg J, Laine M, Palkama T, Kivikko M, Pohjanjousi P, Kupari M. Duration of the haemodynamic action of a 24-h infusion of levosimendan in patients with congestive heart failure. Eur J Heart Fail. 2007;9:75–82.
- Bergh CH, Andersson B, Dahlström U, Forfang K, Kivikko M, Sarapohja T, . Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on beta-blockers. Eur J Heart Fail. 2010;12: 404–10.
- Szilágyi S, Pollesello P, Levijoki J, Kaheinen P, Haikala H, Edes I, Papp Z. The effects of levosimendan and OR-1896 on isolated hearts, myocyte-sized preparations and phosphodiesterase enzymes of the guinea pig. Eur J Pharmacol. 2004;486:67–74.
- Landoni G, Biondi-Zoccai G, Greco M, Greco T, Bignami E, Morelli A, . Effects of levosimendan on mortality and hospitalization. A meta-analysis of randomized controlled studies. Crit Care Med. 2012;40:634–46.
- Aguilar RB. Evaluating treatment algorithms for the management of patients with type 2 diabetes mellitus: a perspective on the definition of treatment success. Clin Ther. 2011;33:408–24.