Abstract
Background. Renal impairment is a risk factor for poor clinical outcome in cardiac surgical patients and low circulating levels of the vitamin D hormone 1,25-dihydroxyvitamin D (1,25[OH]2D) may contribute to this risk. Methods. We investigated the association between glomerular filtration rate (GFR) and 1,25(OH)2D in 151 heart transplant recipients and 59 other cardiac surgical patients in postoperative week 1 and at postoperative month 1. GFR estimates (eGFR) were calculated from cystatin C (CysC) and serum creatinine (SCr)-based formulas. Results. With both formulas, linear models provided a better fit between eGFR and circulating 1,25(OH)2D than nonlinear models. Nonetheless, the association between 1,25(OH)2D and eGFR in the early postoperative period was stronger with the CysC-based formula (r = 0.560; P <0.001) than with the SCr-based equation (r = 0.386; P <0.001). CysC-eGFR and SCr-eGFR displayed considerably lack of agreement in the early postoperative period, especially in heart transplant recipients. Conclusions. There is a relatively close association between CysC-eGFR and circulating 1,25(OH)2D in cardiac surgical patients. Data underline the importance of preserved kidney function in cardiac surgery for adequate circulating 1,25(OH)2D levels. The SCr-based formula is probably too imprecise for estimating GFR in the early postoperative period correctly.
Introduction
Cardiac surgery can result in a transient impairment in renal function (Citation1,Citation2). The pathophysiology of the renal injury is of multifactorial origin. The main mechanisms responsible include low cardiac output, hypoperfusion and renal ischemia, loss of the pulsatile flow during extracorporeal circulation, hypothermia, and embolism or renovascular microthrombi and the generalized inflammatory response induced by cardiac surgery (Citation3). Renal impairment is a risk factor for poor clinical outcome in cardiac surgery (Citation1).
Low circulating levels of the vitamin D hormone 1,25(OH)2D have also been recognized as an independent risk factor for poor clinical outcome in cardiac surgery (Citation4,Citation5). There is evidence that low circulating 1,25(OH)2D levels may contribute to the adverse effects of renal impairment in cardiovascular disease (Citation6). It is well-known that a decline in the glomerular filtration rate (GFR) to less than 30 ml/min/1.73 m2 can cause low circulating 1,25(OH)2D levels. This is due to inadequate renal reserve of the enzyme 1α-hydroxylase in patients with chronic kidney disease (CKD) stages 4 and 5 (Citation7). However, upregulated 24-hydroxylase may also be of importance (Citation8). Data concerning the association between 1,25(OH)2D and GFR in patients with GFR values >30 ml/min/1.73 m2 are less clear. Notably, synthesis of 1,25(OH)2D is also suppressed by the phoshaturic hormone fibroblast growth factor-23 (Citation9) and in case of inadequate circulating levels of its precursor 25-hydroxyvitamin D (25[OH]D) (Citation10), whereas parathyroid hormone (PTH) stimulates 1,25(OH)2D synthesis (Citation11).
In cardiac surgery, data about changes in GFR and the effects of GFR changes on circulating 1,25(OH)2D levels in the early postoperative period are scarce. Gaps in current knowledge include the effect of the type of surgery, the formula used for estimating GFR and the postoperative time point of the assessment of GFR and 1,25(OH)2D values. Therefore, we aimed to investigate the association between estimated GFR (eGFR) and circulating 1,25(OH)2D in two different cohorts of cardiac surgical patients by the use of a Cystatin-C (CysC)-based formula (Citation12) and the serum creatinine (SCr)-based Modification of Diet in Renal Disease (MDRD) formula (Citation13) for estimating GFR.
Here, we present results demonstrating that there is considerably lack of agreement between the SCr-based formula and the CysC-based formula of estimating GFR in the early postoperative period. In addition, we provide data that circulating 1,25(OH)2D is stronger correlated with kidney function, if GFR is estimated by Cys-based formula instead of SCR-based formula. Results may be helpful for a better assessment and understanding of renal impairment and for the development of new treatment strategies in the early postoperative period in cardiac surgery.
Materials and methods
Patients and study procedures
This investigation is based on data from two previously published prospective cohort studies. These studies are described in detail elsewhere (Citation4,Citation5). In brief, we assessed 1,25(OH)2D and other biochemical parameters of mineral metabolism in 59 cardiac surgical patients, who underwent coronary artery bypass grafting and/or valve replacement and in 151 heart transplant recipients. None of the patients received vitamin D or active vitamin D metabolites. In both study groups, the analysis is based on blood samples obtained during the first postoperative week (designated t1) and at the end of the first postoperative month (designated t2). All blood samples were centrifuged immediately after being drawn and serum aliquots were subsequently frozen at ‐80°C until analysis. We assessed the following biochemical parameters in both study cohorts: 1,25(OH)2D, calcium (Ca), magnesium (Mg), 25(OH)D, and PTH. In the cohort of cardiac transplant recipients, CysC and SCr values were also available from 114 patients at postoperative month 12 (designated t3). In the other 59 cardiac surgical patients, additional CysC, SCr, and 1,25(OH)2D values were available preoperatively (designated t0). All procedures were in accordance with the Helsinki Declaration of 1975, as revised in 1983. The two studies were approved by the local ethics committee and all patients gave written informed consent to the study procedures.
Immunosuppressive therapy of transplanted patients
Immunosuppressive therapy of transplanted patients was initially performed with cyclosporine A (CSA), azathioprine, and methyprednisone. At discharge, 39.5% of the study cohort still received CSA, 63.2% tacrolimus, 34.2% azathioprine, 43.4% mycophenolate mofetil, 84.2% methylprednisone, whereas 10.6% received other immunosuppressive agents as maintenance therapy. The corresponding values at the 1-year follow-up were 22.3% for cyclosporine A, 75.5% for tacrolimus, 7.9% for azathioprine, 56.5% for mycofenolate mofetil, 88.4% for methylprednisone, and 14.8% for others. The CSA target range for our patients was 110–190 μg/L within the first six postoperative months and 60–80 μg/L thereafter.
Biochemical analyses
We analyzed circulating 1,25(OH)2D using a competitive enzyme-linked immunosorbent assay after solid-phase extraction (Immundiagnostik, Bensheim, Germany). Cross-reactivity is 100% for 1,25(OH)2D3 and <0.01% for vitamin D2 and D3, 25(OH)D2, 25(OH)D3 and alfacalcidol. Circulating 25(OH)D was measured with a RIA test kit (heart transplant recipients) and with the autoanalyzer Liaison (other cardiac surgical patients). Both test kits were provided by DiaSorin, Stillwater, MN, USA. The tests provide comparable results and show a performance very similar to liquid chromatography tandem mass spectrometry, which is considered the gold standard (Citation14). We measured Ca, Mg, PTH, C-reactive protein (CRP), SCr, and CysC using the Architect autoanalyzer (Abbott, Wiesbaden, Germany). Intra- and inter-assay coefficients of variation for the 1,25(OH)2D measurement are below 7.0% and below 9.0%, respectively. Imprecision of the CysC measurement is <5%.
Estimation of glomerular filtration rate
We used the following CysC-based formula for estimating GFR (Citation12): eGFR (ml/min/1.73m2) = 71 * (CysC)−1.28. For calculating SCr-based eGFR, we used the MDRD equation (Citation13): 186 *SCr−1,154 * age−0,203 * (0.743 in females).
Statistics
We expressed continuous variables as mean and standard deviation (SD). For group comparisons of age and body mass index (BMI), we used the unpaired t-test. For comparison of biochemical parameters and eGFR values, we used a two-factor, repeated-measures analysis of variance (ANOVA) with time and study group as the within-subjects factors. Some biochemical parameters (PTH, CRP) were not normally distributed. Before being analyzed, these data were, therefore, transformed using the natural logarithm. We applied the Bland-Altman test (Citation15) to assess agreement between the two methods of estimating GFR. We used Pearson's correlation coefficient and nonlinear regression analysis to assess interrelationships between 1,25(OH)2D values and eGFR. We performed stepwise multiple regression analysis to assess independent predictors of circulating 1,25(OH)2D. A p-value <0.05 was considered as statistically significant. We used the statistical software package PASW, version 18 (Chicago, Illinois, USA) for the analyses.
Results
The cohort of heart transplant recipients was younger (age: 53.0 ± 13.5 years) and lighter (BMI: 23.7 ± 3.5 kg/m2) than the cohort of other cardiac surgical patients (age: 68.0 ± 10.5 years; BMI: 27.9 ± 4.1 kg/m2; P-values <0.001). Moreover, vitamin D status and mineral metabolism was more deranged and eGFR values were lower in the early postoperative period in heart transplant recipients than in other cardiac surgical patients (). In both study groups, CysC-eGFR resulted in lower eGFR values than the SCr-based method at t1 and t2. The differences at t1 and t2 were more pronounced in heart transplant recipients than in the other cardiac surgical patients (mean differences in heart transplant recipients at t1 and t2: 83.6% and 57.6%, respectively; mean differences in other cardiac transplant recipients: 23.5% and 24.4%, respectively). At t3, CysC-eGFR and SCr-eGFR values in heart transplant recipients were 49.9 ± 22.6 ml/min/1.73 m2 and 55.3 ± 23.4 ml/min/1.73 m2, respectively, (mean difference: 10.8%). In the other cardiac surgical patients, CysC-eGFR and SCr-eGFR values were 72.8 ± 20.8 ml/min/1.73 m2 and 87.2 ± 27.1 ml/min/1.73 m2, respectively, at t0 (mean difference: 19.8%).
Table I. Biochemical characteristics and eGFR values of the study groups.
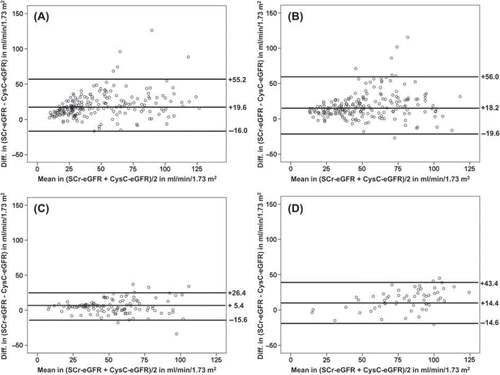
and B illustrate the Bland-Altman plots of the differences between the two methods for estimating GFR against their mean (= (CysC-eGFR + SCr-eGFR)/2)) at t1 and t2. The plots display considerable lack of agreement between CysC-eGFR and SCr-eGFR, with discrepancies of up to 126 ml/min/1.73 m2 at t1 and up to 115 ml/min/1.73 m2 at t2. At t1 and t2, mean eGFR values were 19.6 ml/min/1.73 m2 and 18.2 ml/min/1.73 m2, respectively, higher with the SCr-based formula compared to the CysC-based formula. Results did not improve when instead of the MDRD formula other SCr-based formulas for estimating eGFR such as the Cockroft-Gault or the CKD-EPI formula were used (data not shown). CysC-eGFR and SCr-eGFR values displayed much better agreement in the cohort of heart transplant recipients at t3 () and also in the cohort of other cardiac surgical patients at t0 ().
Figure 1. Bland-Altman plots showing the bias between the SCr-eGFR and the mean of the two eGFR methods at t1 (A), t2 (B), t3 (C) and t0 (D); Figure 1C is restricted to heart transplant recipients only and Figure 1D is restricted to other cardiac surgical patients only; the lines indicate the mean +/2 2 standard deviations. CysC, cystatin C; SCr, serum creatinine; eGFR, estimated glomerular filtration rate.
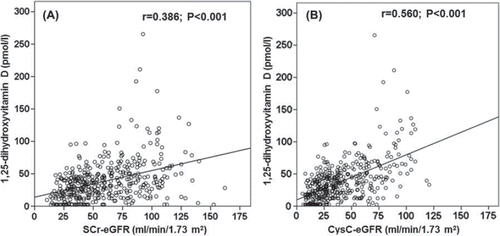
and B illustrate the correlation of 1,25(OH)2D with the SCr-based and the CysC-based eGFR over all samples collected at t1 and t2. With both formulas, the linear model provided a better fit than nonlinear models (data not shown). However, the association between 1,25(OH)2D and eGFR was much weaker with the SCr-based formula than with the CysC-based equation. The association decreased slightly when patients with a CysC-based eGFR below 30 ml/min/1.73 m2 were excluded (CysC-based data: r = 0.460,P <0.001; SCr-based data: r = 0.290, P <0.001). Likewise, the association decreased slightly when patients with a CysC-based eGFR greater than 75 ml/min/1.73 m2 were excluded (CysC-based data: r = 0.430, P <0.001; SCr-based data: r = 0.232, P <0.001). Even when the data were analyzed separately for the two study cohorts at t1 and t2, the association of 1,25(OH)2D remained consistently better with the CysC-eGFR formula compared to the SCr-eGFR formula (). In the cohort of cardiac surgical patients, the association of 1,25(OH)2D with CysC-eGFR and SCr-eGFR was low at t0 ().
Table II. Correlation coefficients of 1,25(OH)2D with CysC-eGFR and SCr-eGFR in the two study groups at different time points.
We then performed multiple regression analyses to assess independent predictors of circulating 1,25(OH)2D. The following independent variables were included into the analysis: age, BMI, Ca, Mg, CRP, CysC-eGFR, PTH and 25(OH)D. The analysis was performed over all samples collected at t1 and t2. Only the three parameters CysC-eGFR, PTH, and 25(OH)D were independent predictors of circulating 1,25(OH)2D. The multiple R2 was 0.369 and the equation was as follows: 1,25(OH)2D (pmol/l) = 0.702 * CysC-eGFR + 0.250* 25(OH)D + 0.085* PTH – 3.44. When CysC-eGFR was replaced by SCr-eGFR, the association was much weaker. The multiple R2 was 0.234. The equation was as follows: 1,25(OH)2D (pmol/l) = 0.428* Cr-eGFR + 0.338* 25(OH)D +0.076* PTH 21.87.
Discussion
This investigation in cardiac surgical patients provides three major results: First, unlike the results of the preoperative and late postoperative period, CysC-eGFR and SCr-eGFR display considerably lack of agreement in the early postoperative period, especially in heart transplant recipients. Second, in the early postoperative period the CysC-based formula results in a closer association between 1,25(OH)2D and eGFR compared to the SCr-based formula of estimating GFR. Third, the association between circulating 1,25(OH)2D and eGFR is closer in the early postoperative period than in the preoperative period, even if the SCr-based formula for estimating GFR is used. In total, our results indicate a relatively close association between 1,25(OH)2D and CysC-eGFR in the early postoperative period in cardiac surgical patients.
Generally, CysC-based and SCr-based formulas demonstrate good agreement (Citation16). However, SCr-eGFR may be too insensitive to assess the early postoperative changes in renal function in cardiac surgical patients correctly, probably because SCr is more affected by age, gender, and body composition than CysC (Citation17). In cardiac surgical patients, SCr may be less accurate because this population suffers from cachexia and immobilization and has a reduced muscular mass, notably due to corticosteroids (Citation18), factors that can influence creatinine metabolism independent of renal function. There is indeed some evidence that CysC-based formulas are superior to SCr-based formulas in determining GFR in cardiac surgical patients in the early postoperative period (Citation19). In our study, the discrepancy between the two formulas was most obvious in heart transplant recipients. Notably, in the early postoperative period immunosuppressive therapy was based on high CSA doses, which may have resulted in tubular hypersecretion of creatinine (Citation18). The assumption that the SCR-based formula may result in falsely elevated eGFR values is also in line with the clinical experience that a significant percentage of heart transplant recipients need hemofiltration in the early postoperative period.
The relative close association between circulating 1,25(OH)2D and renal function is in line with the hypothesis that some acute effects on clinical outcome in patients with reduced kidney function may be mediated by low 1,25(OH)2D levels. Experimental studies in animals lacking 1,25(OH)2D action support this assumption: These animals are prone to autoimmune diseases, cardiac hypertrophy and increased thrombogenicity (Citation20). They also have ectopic calcification and a short life span (Citation21). In humans, there is increasing evidence for a close association between renal impairment and cardiovascular morbidity and mortality (Citation6). It has been assumed that insufficient circulating levels of 1,25(OH)2D contribute to cardiorenal syndromes (Citation6). More importantly, 1,25(OH)2D is independently associated with clinical outcome in cardiac surgery (Citation4,Citation5). It is a limitation of our study that FGF-23 values were not measured. FGF-23 does not only suppress 1,25(OH)2D synthesis (Citation9), but high FGF-23 levels are also associated with poor clinical outcome (Citation22).
Surprisingly, in the cohort of cardiac surgical patients the association between 1,25(OH)2D and eGFR was much closer in the early postoperative period compared to the preoperative period, despite similar and relatively high absolute eGFR-values at both time points. These findings need confirmation in future studies. However, if this relatively strong association can be confirmed and 1,25(OH)2D does indeed contribute to clinical outcome in cardiac surgery, then it may be possible to develop new treatment strategies for the early postoperative period. As we could demonstrate by multiple regression analysis, circulating 1,25(OH)2D levels depended on both 25(OH)D availability and renal function, indicating that preoperative vitamin D administration may to some extent be able to prevent a marked decline in postoperative 1,25(OH)2D levels. It is noteworthy that in CKD patients low 25(OH)D levels are associated with increased mortality (Citation23,Citation24). In patients with postoperative renal impairment, administration of activated vitamin D (e.g. 1α-vitamin D or 1,25[OH]2D) may probably also be able to compensate for the suppressed renal 1,25(OH)2D synthesis. Although therapy with the vitamin D analog paricalcitol did not alter left ventricular mass or function in CKD patients (Citation16), paricalcitol treatment resulted in significantly fewer hospitalizations for cardiovascular events. However, it is of concern that paricalcitol treatment was also responsible for significantly more cases of hypercalcemia.
Interestingly, the correlation coefficient between 1,25(OH)2D and CysC-eGFR decreased only slighty when eGFR values <30 ml/min/1.73 m2 were excluded, indicating that the association between both parameters remains relatively linear above an eGFR value of 30 ml/min/1.73 m2. This assumption is in line with recent findings demonstrating that eGFR is significantly associated with circulating 1,25(OH)2D in men without CKD (Citation25).
In summary, our data indicate a relatively close association between CysC-eGFR and 1,25(OH)2D in the early postoperative period. The SCr-based formula is probably too imprecise for estimating GFR in cardiac surgical patients in the early postoperative period correctly.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200.
- Hornum M, Andersen M, Gustafsson F, Oturai P, Sander K, Mortensen SA, Feldt-Rasmussen B. Rapid decline in glomerular filtration rate during the first weeks following heart transplantation. Transplant Proc. 2011;43:1904–7.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32.
- Zittermann A, Schleithoff SS, Götting C, Fuchs U, Kuhn J, Kleesiek K, . Calcitriol deficiency and 1-year mortality in cardiac transplant recipients. Transplantation. 2009;87:118–24.
- Börgermann J, Lazouski K, Kuhn J, Dreier J, Schmidt M, Gilis-Januszewski T, . 1,25-dihydroxyvitamin D fluctuations in cardiac surgery are related to age and clinical outcome. Crit Care Med2012; May 11 [epub ehead of print]
- Ronco C, Cozzolino M. Mineral metabolism abnormalities and vitamin D receptor activation in cardiorenal syndromes. Heart Fail Rev. 2012;17:211–20.
- Chonchol M, Kendrick J, Targher G. Extra-skeletal effects of vitamin D deficiency in chronic kidney disease. Ann Med. 2011;43:273–82.
- Helvig CF, Cuerrier D, Hosfield CM, Ireland B, Kharebov AZ, Kim JW, . Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 2010;78:463–772.
- Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth fator-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91: 3144–9.
- Zittermann A, Frisch S, Berthold HK, Götting C, Kuhn J, Kleesiek K, . Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular risk markers. Am J Clin Nutr. 2009;89:1321–7.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357:266–81.
- Abbott Diagnostics. www.abbottdiagnostics.at/images/content/pdfs/CystatinCInfobroschuere.pdf. Assessed: September, 13th, 2011.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70.
- Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-Art vitamin D assays: a comparison of automated immunoassays with liquid chromatography- tandem mass spectrometry methods. Clin Chem. 2012;58: 531–42.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
- Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, . Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307:674–84.
- Selvin E, Köttgen A, Coresh J. Kidney function estimated from serum creatinine and Cystatin-C and peripheral arterial disease in NHANES 1999–2002. Eur Heart J. 2009;30:1918–25.
- Tomlanovich S, Golbetz H, Perlroth M, Stinson E, Myers BD. Limitations of creatinine in quantifying the severity of cyclosporine-induced chronic nephropathy. Am J Kidney Dis. 1986;8:332–7.
- Brondén B, Eyjolfsson A, Blomquist S, Dardashti A, Ederoth P, Bjursten H. Evaluation of Cystatin-C with iohexol clearance in cardiac surgery. Acta Anaesthesiol Scand. 2011; 55:196–202.
- Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, . Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76.
- Tuohimaa P. Vitamin D and aging. J Steroid Biochem Mol Biol. 2009;114:78–84.
- Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, . Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92.
- Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58:374–82.
- Pilz S, Tomaschitz A, Friedl C, Amrein K, Drechsler C, Ritz E, . Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant. 2011;26:3603–9.
- Karhapää P, Pihlajamäki J, Pörsti I, Kastarinen M, Mustonen J, Niemelä O, . Glomerular filtration rate and parathyroid hormone are associated with 1,25-dihydroxyvitamin D in men without chronic kidney disease. J Intern Med. 2012;271:573–80.