Abstract
Objectives. Tadalafil, an oral phosphodiesterase type-5 inhibitor, induces pulmonary vasorelaxation by inhibiting the breakdown of cyclic guanosine monophosphate whereas simvastatin, an oral 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitor, has been shown to reverse pulmonary hypertension (PH) and attenuate vascular remodeling in animal models of pulmonary hypertension. We investigated whether the combination of tadalafil and simvastatin, which has different mechanisms of action, is superior to either drug alone in a rat model of monocrotaline-induced PH. Methods. Male Sprague-Dawley rats were randomized to gavage with a vehicle, tadalafil (10 mg/kg/day), simvastatin (2 mg/kg/day), or tadalafi + simvastatin 21 days after the monocrotaline (60 mg/kg) injections. The hemodynamic and histological changes in the pulmonary arterioles, right heart hypertrophy, interleukin 6 (IL-6) levels and perivascular inflammation in the lungs were measured 35 days after monocrotaline exposure. Results. The combination of tadalafil and simvastatin showed significantly more improvement in the mean pulmonary hypertension pressure (mPAP) and right ventricular hypertrophy compared with each monotherapy (p < 0.05). Combination therapy had additive effects on the increases in lung IL-6 levels and the perivascular inflammation score. Conclusions. These results suggest that the combination of tadalafil and simvastatin bears promise as an approach to treat PH, especially PH associated with inflammation.
Introduction
Pulmonary hypertension (PH) is a serious disease characterized by a progressive elevation of the pulmonary arterial pressure and pulmonary arterial resistance, leading to right ventricular failure and death (Citation1,Citation2). The recent development of drug therapies, such as prostacyclin analogues, phosphodiesterase-5 (PDE-5) inhibitors, and endothelin receptor antagonists, has shown varying degrees of improvement in different categories of PH associated with limitations in hemodynamics, exercise capacity, functional class, and quality of life. However, clinical worsening over time is inevitable for the majority of patients (Citation3,Citation4).
Tadalafil, an orally administered PDE-5 inhibitor with once-daily dosing, inhibits the breakdown of cyclic guanosine monophosphate (cGMP), thereby increasing the effects of nitric oxide (NO) and resulting in vascular smooth muscle relaxation and pulmonary vasodilation (Citation5). It has been shown that tadalafil improves pulmonary hemodynamics and reduces mortality in rat models of PH (Citation6). Galiè et al. have reported that tadalafil (40 mg) is well tolerated, improves exercise capacity and quality of life measures, and reduces clinical worsening in patients with PH (Citation7).
Simvastatin, an inhibitor of HMG-CoA reductase, confers substantial cardiovascular benefits that exceed its capacity to lower serum cholesterol levels. These so-called pleiotropic, cholesterol-independent effects are believed to include antiproliferative, anti-inflammatory, antioxidant, antithrombogenic, and vascular function-restoring effects (Citation8). Simvastatin has been shown to reverse established monocrotaline induced-PH in rats by inducing apoptosis in neointimal smooth muscle cells (Citation9). Simvastatin has also been shown to reverse PH induced in rats via a combination of VEGF inhibition and chronic hypoxia by inducing endothelial cell apoptosis (Citation10). Recently, Wilkins et al. have reported that simvastatin, in addition to conventional therapy, produces a small, transient, and early reduction in RV mass and NT-proBNP levels in patients with PH (Citation11).
In this study, we hypothesized that the combination of tadalafil and simvastatin would be more effective than the use of any individual drug in rats with PH.
Methods
Animals
Sprague-Dawley rats (220–250 g) were purchased from the Institute of Zoology at the Chinese Academy of Sciences. The rats were individually housed in 5-compartment, stainless-steel cages in an animal room set at 23°C and 55% relative humidity. The rats had free access to food and water. All of the experiments were performed in accordance with the guidelines of the Animal Research Committee of the Chinese Academy of Medical Sciences.
Experimental design
The rats were administered a subcutaneous injection of saline (control, n = 8) or monocrotaline (Sigma-Aldrich USA, 60 mg/kg, n = 32). The monocrotaline (MCT)-injected rats were randomized into 4 groups and orally treated by gavage with placebo, simvastatin (Merck Sharp & Dohme, Inc.), tadalafil (Eli Lilly Company, USA) or simvastatin + tadalfil after 21–35 days.
The efficacy of tadalafil and simvastatin was assessed in dose-response curves for mPAP. Tadalafil (1, 2, 5, 10 mg/kg per day, n= 6 in each group) and simvastatin (0.5, 1, 2, 5 mg/kg per day, n= 6 in each group) were orally administered from 21 to 35 days after monocrotaline (60 mg/kg) was injected. mPAP was measured as described below in “measurement of pulmonary arterial pressure.” The doses of tadalafil (10 mg/kg per day) and simvastatin (2 mg/kg per day) were chosen for this study, because they were maximally effective doses at the plateau of the dose-response curves and equally effective on decreasing mPAP (14.3 ± 4.5 mm Hg and 12.8 ± 3.7 mm Hg, respectively).
Measurement of pulmonary arterial pressure and right ventricular hypertrophy
Hemodynamic studies were performed on day 35. The rats were anesthetized with intraperitoneal pentobarbital (30 mg/kg) and placed onto a heating pad to maintain their body temperature at 37–38°C throughout the study. The arterial blood pressure and heart rate were monitored with a polyethylene catheter inserted into the right carotid artery. A polyvinyl catheter was introduced into the right jugular vein and pushed through the right ventricle and into the pulmonary artery. The shape of the pressure trace curve confirmed the catheter's position. After catheter placement, we waited 15 min to reach a steady state with respect to the systemic and pulmonary pressures before beginning the recordings. These hemodynamic parameters were measured with a pressure transducer connected to a physiological polygraph (ML785, AD Instruments Pty Ltd). All of the data were recorded on a computer, and the results were analyzed offline. Finally, cardiac arrest was induced by injecting 2 mmol of potassium chloride through the catheter. The ventricles and lungs were excised, dissected free, and weighed. The right ventricle (RV) was separated from the left ventricle (LV) and septum (S) and weighed separately. The ratio of the RV weight to the LV + S weight (RV/LV + S) was calculated as an index of ventricular hypertrophy (RVHI).
Morphometric analysis of pulmonary arteries and the perivascular inflammation score
After pressure measurement, the left lung was inflated to a standard airway pressure (25 cm H2O) with fixative (4% w/v paraformaldehyde in normal saline solution) via the trachea. The pulmonary vessels were perfused with fixative (62.5 cm H2O) and immersed in 10% buffered formalin for at least 72 hours before being embedded in paraffin (Citation12). Sections (5 μm) were cut at two levels in the paraffin block and stained with standard hematoxylin-eosin. For each lung section, pulmonary vascular remodeling was assessed using a CMTAS-2100B image analysis system (Beijing University of Aeronautic and Astronautics). Thirty consecutive vessels (between 50 and 150 μm) were analyzed in each section. Vessel remodeling was assessed by analyzing the wall area or wall diameter according to the following formula: percent wall area= [(areavessel−arealumen)/areavessel]× 100 and percent wall diameter= [(diametervessel−diameterlumen)/diametervessel]× 100.
Lung sections were examined by a pathologist who was blinded to the identities of the samples. Inflammation was graded 0, 1, 2, 3, or 4 based on the intensity and extent of perivascular inflammatory cell infiltrates.
Measurement of IL-6 levels using an enzyme-linked immunoassay
Right lung tissue (100 mg) was thawed slightly on ice, washed twice with PBS, and homogenized rapidly in 1 ml RIPA buffer containing protease inhibitors before centrifugation at 12,000 g for 10 min at 4°C to obtain the supernatant. The IL-6 level in the lung homogenate was measured using a sandwich enzyme-linked immunospecific assay according to the manufacturer's instructions (Boster, Wuhan, China). The absorbance was measured at 450 nm using a universal microplate assay. The results were normalized to the protein concentration as determined by the BioRad protein assay.
Statistical analysis
All of the data are expressed as means ± SEMs unless otherwise indicated. The parameters in the five groups were compared using one-way analysis of variance followed by the Newman–Keuls test. A value of p < 0.05 was considered to be statistically significant.
Results
The effect of combination therapy on body weight, the mean systemic arterial pressure and the heart rate
The body weights in the PH group were significantly lower than those in the control group 3 weeks after MCT injection. Weekly weighing of the animals revealed that, after 2 weeks of single-drug or combination therapy, the rats in the treatment groups have a slightly but significantly increased body weight compared with the rats in the PH group (). There were no significant differences in the mean systemic arterial pressures and heart rates across the experimental groups ().
Figure 1. The effect of combination therapy on body weight. C, control group; PH, PH group; T, tadalafil group; S, simvastatin group; TS, combination group. The data are presented as means ± SEMs. #p < 0.01, the PH group vs. the control group; *p < 0.01, the therapy groups vs. the control group; †p < 0.05 the therapy groups vs. the PH group.
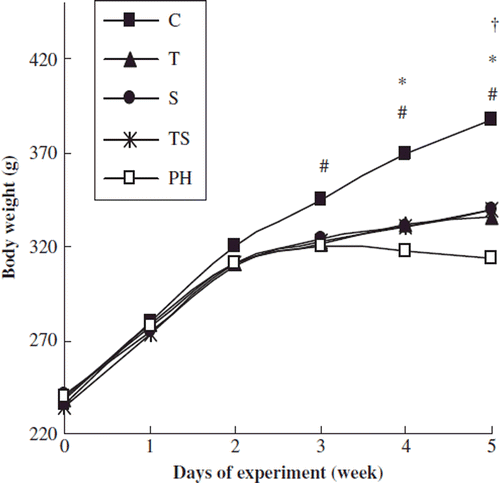
Table I. The effect of combination therapy on the mean systemic arterial pressure and heart rate.
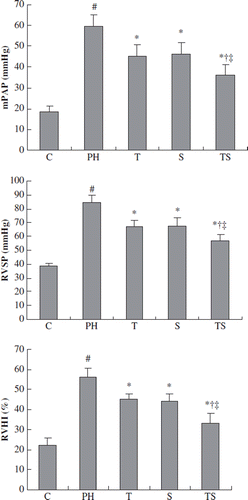
The effect of combination therapy on pulmonary hemodynamics
In the PH rats 35 days after MCT injection, the mPAP and RVSP were markedly elevated (58.3 ± 5.1 mm Hg and 84.4 ± 5.5 mm Hg, respectively). The tadalafil-and simvastatin-treated rats showed statistically significant reductions in their mPAP and RVSP compared with the PH group (p < 0.01). The combination group showed a further reduction in its mPAP and RVSP that was significantly different from the monotherapy groups (p < 0.05, ).
Figure 2. A comparison of the pulmonary hemodynamics and right ventricular hypertrophy across the experimental groups (mean ± SD). mPAP, mean pulmonary arterial pressure; RVSP, right ventricular systolic pressure; RVHI, the ratio of the right ventricular weight to the left ventricular + septal weight. # p < 0.001 compared with the control group; *p < 0.01 compared with the PH group; †p < 0.05 compared with the tadalafil group, ‡p < 0.05 compared with the simvastatin group.
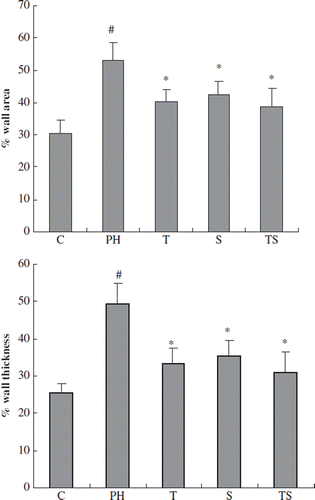
The effect of combination therapy on pulmonary artery remodeling
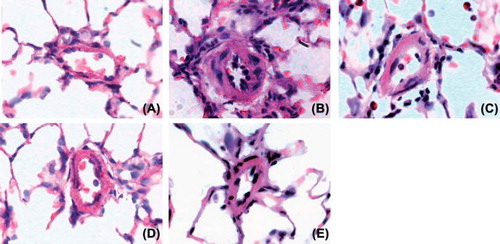
Representative photomicrographs show that pulmonary vessel wall hypertrophy was attenuated in the single-drug and combination groups compared with the PH group (). A quantitative analysis of the peripheral pulmonary arteries demonstrated that the increase in the % wall thickness and the % wall area after MCT injection was significantly attenuated in the tadalafil, simvastatin and combination therapy groups. However, combination therapy was no more effective than single therapy with tadalafil or simvastatin ().
Figure 3. Photomicrographs of the pulmonary arteries in the control group (A), PH group (B), simvastatin group (C), tadalafil group (D) and combination group (E) (× 400; HE staining). A histological examination of the lung revealed a marked increase in pulmonary arterial wall thickening in the PH group. This increase in thickening was attenuated in the simvastatin, tadalafil and combination groups.
The effect of combination therapy on lung IL-6 levels and perivascular inflammation
In the lungs of the rats with MCT-induced PH, primarily mononuclear cells infiltrated into the perivascular areas. As determined by histological scoring, simvastatin or tadalafil significantly reduced the perivascular score compared with the PH group. Combination treatment additionally reduced the perivascular score compared with the monotherapy groups ().
Figure 5. The effect of combination therapy on lung IL-6 levels and the perivascular score. C, control group; P, PH group; T, tadalafil group; S, simvastatin group; TS, combination group. The data are presented as means ± SEMs. #p < 0.001 vs. the control group; *p < 0.05 vs. the PH group; †p < 0.05 vs. the tadalafil group; ‡p < 0.05 vs. the simvastatin group.
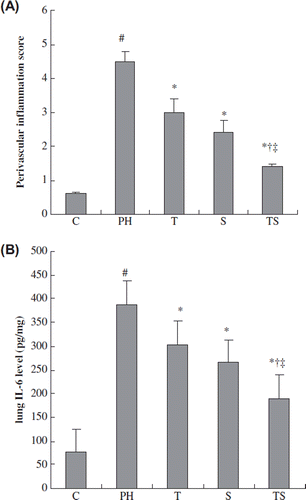
Lung IL-6 levels were significantly lower in the simvastatin and tadalafil groups compared with the PH group. Combination therapy significantly decreased lung IL-6 levels compared with either monotherapy ().
Discussion
The most important observations of this study are as follows: (Citation1) the combination of tadalafil and simvastatin inhibited the increase in the mPAP and right ventricular hypertrophy to a greater degree than either drug alone in the rats with MCT-induced PH; (Citation2) tadalafil, simvastatin and combination therapy attenuated the medial wall thickening of the pulmonary artery to the same extent; (Citation3) combination therapy induced additive effects on the decreases in perivascular inflammatory cell infiltration and IL-6 levels.
The model of pulmonary hypertension in MCT-injected rats reproduced the vascular remodeling, proliferation of pulmonary arterial smooth muscle cells, upregulation of cytokines, and perivascular inflammatory cell infiltration that occurs in human PH. After a single subcutaneous injection, MCT causes a pulmonary vascular endothelial cell injury that is followed by a perivascular inflammatory response, leading to the progressive muscularization of the small arterioles and an elevation in the pulmonary arterial pressure. Previous studies have shown that the pulmonary arterial pressure begins to increase 2 weeks after MCT (60 mg/kg) injection and continues to increase in a time-dependent manner (Citation13). To investigate the combined effects of simvastatin and tadalafil on established PH, we chose a PH rat model studied 21 days after MCT injection, in which the pulmonary arterial pressure markedly increases and the pulmonary vessels exhibit typical muscular hypertrophy.
Tadalafil inhibits the breakdown of cyclic guanosine monophosphate (cGMP) and, thus, increases the effects of nitric oxide (NO), resulting in pulmonary vasodilation (Citation5,Citation6). Simvastatin improves endothelium-dependent relaxation by upregulating endothelial nitric oxide synthase expression and activity (Citation14). In this study, both simvastatin and tadalafil significantly decreased the mPAP and RV/(LV + S) of the rats with MCT-induced PH, which is consistent with earlier studies. Drugs that produce similar effects via different mechanisms could theoretically contribute to additive or synergistic effects when combined. In fact, the combination of tadalafil and simvastatin significantly attenuated the increases in the mPAP and right ventricular hypertrophy compared with each monotherapy. Thus, these findings suggest that the combination of tadalafil and simvastatin may be an alternative approach for severe pulmonary hypertension that is refractory to single-drug therapy.
Pulmonary vascular remodeling is the dominant factor that contributes to the progressive and persistent elevation in the pulmonary vascular resistance that determines the mortality of patients with PH. Consistent with previous studies (Citation15–19), our study also showed that simvastatin or tadalafil significantly decreased the percentage of pulmonary arterioles hypertrophy in rats with MCT-induced PH. However, we did not observe a greater beneficial effect on pulmonary vascular remodeling when adding simvastatin to tadalafil. One possibility is that the key pathogenetic processes in pulmonary vascular remodeling are not targeted by the current drugs. Additionally, a short-duration or “ceiling” effect of the combination therapy could have theoretically contributed to the negative result. Therefore, further studies using various drugs doses and different lengths of follow-up are needed to assess the anti-remodeling efficacy of combination therapy.
Interestingly, the combination therapy significantly decreased lung IL-6 levels and perivascular inflammatory cell infiltration compared with treatment with tadalafil or simvastatin alone. A role for inflammation and, more specifically, IL-6 in the pathogenesis or progression of PH has been suggested in recent studies (Citation20,Citation21). Multiple studies using serum from patients with PH have demonstrated increased serum IL-6 levels (Citation22). Excessive IL-6 has been shown to be released by cultured pulmonary artery cells from patients with PH (Citation23). Further evidence implicating the inflammatory response in the genesis of PH has been provided by an animal model in which PH was induced by IL-6 administration (Citation24). IL-6-overexpressing transgenic mice exhibit PH, vascular remodeling and an exaggerated response to hypoxia. In these mice, IL-6-induced vascular remodeling is accompanied by the activation of pro-proliferative growth factors and anti-apoptotic factors, such as Bcl2 and survivin (Citation25). Furthermore, IL-6-knockout mice exhibit less inflammatory cell recruitment, PH, right ventricular hypertrophy and pulmonary vascular remodeling in response to hypoxia (Citation26). Although further studies are needed, the anti-inflammatory effects of tadalafil and simvastatin indicate that the combined use of the two drugs may be an alternative approach for PH associated with inflammation.
There are a number of limitations to this study. Firstly, this study focused on the anti-inflammation effects of the combining tadalafil with simvastatin, other synergistic effects of the combined therapy, such as enhancing NO-cGMP signaling pathway, may also play a role in ameliorating monocrotaline-induced PH. Secondly, it would be more appropriate using immunohistochemical staining of monocytes/macrophages to evaluate the intensity of inflammatory infiltration around vessels. Thirdly, a survival benefit and pharmacokinetic interaction of the combination tadalafil plus simvastatin need to be investigated in future studies.
In conclusion, the combination of tadalafil and simvastatin attenuated the mPAP, right ventricular hypertrophy and vascular remodeling in rats with MCT-induced PH. These effects, particularly the effects on improving pulmonary hemodynamics and ameliorating lung inflammation, are more pronounced when the drugs are combined than when they are administered alone. These results indicate that combination therapy is a promising approach for treating PH, especially PH associated with inflammation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–117.
- Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet. 2003;361:1533–1544.
- European Society of Cardiology and the European Respiratory Society. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2009;30:2493–510.
- Provencher S, Sitbon O, Humbert M, Cabrol S, Jais X, Simonneau G. Long-term outcome with first-line bosentan therapy in idiopathic pulmonary arterial hypertension. Eur Heart J. 2006;27:589–595.
- Falk JA, Philip KJ, Schwarz ER. The emergence of oral tadalafil as a once-daily treatment for pulmonary arterial hypertension. Vasc Health Risk Manag. 2010;6:273–280.
- Sawamura F, Kato M, Fujita K, Nakazawa T, Beardsworth A. Tadalafil, a long-acting inhibitor of PDE5, improves pulmonary hemodynamics and survival rate of monocrotaline-induced pulmonary artery hypertension in rats. J Pharmacol Sci. 2009;111:235–243.
- Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z; . Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group, Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903.
- Wierzbicki AS, Poston R, Ferro A. The lipid and non-lipid effects of statins. Pharmacol Ther. 2003;99:95–112.
- Nishimura T, Faul JL, Berry GJ, Vaszar LT, Qiu D, Pearl RG, Kao PN. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med. 2002;166:1403–1408.
- Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Cool C, Wood K, Tuder RM, . Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L668–676.
- Wilkins MR, Ali O, Bradlow W, Wharton J, Taegtmeyer A, Rhodes CJ . Simvastatin as a treatment for pulmonary hypertension trial. AM J Respir Crit Care. 2010;181: 1106–1113.
- Howell K, Preston RJ, McLoughlin P. Chronic hypoxia causes angiogenesis in addition to remodelling in the adult rat pulmonary circulation. J Physiol. 2003;547:133–145.
- Dumitrascu R, Koebrich S, Dony E, Weissmann N, Savai R, Pullamsetti SS, . Characterization of a murine model of monocrotaline pyrrole-induced acute lung injury. BMC Pulm Med. 2008;8:25.
- Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–31729.
- Yen CH, Leu S, Lin YC, Kao YH, Chang LT, Chua S, . Sildenafil limits monocrotaline-induced pulmonary hypertension in rats through suppression of pulmonary vascular remodeling. Cardiovasc Pharmacol. 2010;55:574–584.
- Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–3235.
- Li B, Yang L, Shen J, Wang C, Jiang Z. The antiproliferative effect of sildenafil on pulmonary artery smooth muscle cells is mediated via upregulation of mitogen-activated protein kinase phosphatase-1 and degradation of extracellular signal-regulated kinase 1/2 phosphorylation. Anesth Analg. 2007;105:1034–1041.
- Hsu HH, Ko WJ, Hsu JY, Chen JS, Lee YC, Lai IR, . Simvastatin ameliorates established pulmonary hypertension through a heme oxygease-1 dependent pathway in rats. Respir Res. 2009;10:32.
- Wright JL, Zhou S, Preobrazhenska O, Marshall C, Sin DD, Laher I, . Statin reverses smoke-induced pulmonary hypertension and prevents emphysema but not airway remodeling. Am J Respir Crit Care Med. 2011;183:50–58.
- Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285.
- Chaouat A, Savale L, Chouaid C, Tu L, Sztrymf B, Canuet M, . Role for interleukin-6 in COPD-related pulmonary hypertension. Chest. 2009;136:678–687.
- Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, . Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151: 1628–1631.
- Yoshio T, Masuyama JI, Kohda N, Hirata D, Sato H, Iwamoto M, . Association of interleukin 6 release from endothelial cells and pulmonary hypertension in SLE. J Rheumatol. 1997;24:489–495.
- Golembeski SM, West J, Tada Y, Fagan KA. Interleukin-6 causes mild pulmonary hypertension and augments hypoxia-induced pulmonary hypertension in mice. Chest. 2005;128:572S–573S.
- Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 over expression induces pulmonary hypertension. Circ Res. 2009;104:236–244.
- Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, Eddahibi S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res. 2009;10:6.