Abstract
Introduction. Transcatheter aortic valve implantation (TAVI) is established as an attractive treatment option for high-risk patients with aortic valve stenosis. One concern is the high risk of prosthetic valve regurgitation. This study aimed to examine for potential preoperative risk factors for postprocedural transcatheter heart valve regurgitation and to quantify the risk, degree, and consequences of postprocedural regurgitation. Materials and methods. 100 consecutive patients who underwent femoral (n = 22) or transapical (n = 78) TAVI were retrospectively reviewed. Echocardiographic valve regurgitation and clinical parameters were analyzed over the first year after TAVI. Results. Seventy-five percent of all patients had prosthetic valve regurgitation. It was, however, only mild or absent in 64% of patients and did not require re-intervention in any of the patients in the series. The severity of the regurgitation appeared unchanged over the one-year follow-up period. Moderate to severe regurgitation was associated with significant yet stable dilatation of the left ventricle over one year and lesser NYHA class improvement three months after TAVI. Asymmetrical native valve calcification increased the risk of paravalvular regurgitation non-significantly. Conclusion. Transcatheter heart valve regurgitation seems to be mild in the majority of cases and unchanged over a 12 months follow-up period. While affecting left ventricular dimensions in moderate or severe cases, we observed no obvious undesirable consequences of the prosthetic valve regurgitation within the first year.
Introduction
Transcatheter aortic valve implantation (TAVI) is now an established treatment option for patients with aortic valve stenosis who are considered to have a prohibitively high risk by conventional surgical aortic valve replacement (SAVR). The results of TAVI have been encouraging since its introduction in 2002. The technique is feasible and safe, and the haemodynamic and prognostic improvements are substantial (Citation1–3).
However, the technique has inherent limitations. In contrast to SAVR, calcifications from the native valve are not resected during TAVI, leaving an uneven surrounding vessel wall for the transcatheter heart valve (THV) to adapt to. Furthermore, a THV is not sutured to the aortic orifice. Thus, TAVI carries an obvious risk of paravalvular regurgitation. In addition, the prosthesis is designed without an external solid ring, and this permit deformation of the valve during insertion with potential maladaptation of the leaflets and central leakage.
The natural focus of most publications on TAVI has been mortality and morbidity, although a high risk of some regurgitation commonly is reported. However, there are few reports on a more detailed characterization of the nature and significance of THV regurgitation (Citation4,Citation5).
We sought to investigate the prevalence and consequences of THV regurgitation. Additionally we wanted to examine, whether regurgitation progressed or diminished over time, and we also attempted to identify pre- and peroperative factors with influence on the risk of THV regurgitation.
Materials and methods
Echocardiographic examinations were analysed retrospectively in 100 consecutive patients undergoing TAVI. Transthoracic (TTE) and transoesophageal (TOE) were performed with GE Vivid 7 equipment and analysed with EchoPac software, GE Healthcare US. All patients were considered to have too high risk to be offered conventional SAVR (Citation6). The patient series represents early experiences on TAVI from our institution from august 2007 to may 2010 (Citation8). Patient characteristics are listed in .
Table I. Patient characteristics.
Apart from echocardiography before TAVI, each patient had four scheduled echocardiographic examinations within the first year after valve implantation. One examination took place during the procedure, another one immediately before discharge (5–7 days after TAVI). The last two examinations were performed 3 ± 1.8 (SD) and 12 ± 1.0 (SD) months after TAVI. Inclusion in the analyses required a sufficient TTE and TOE.
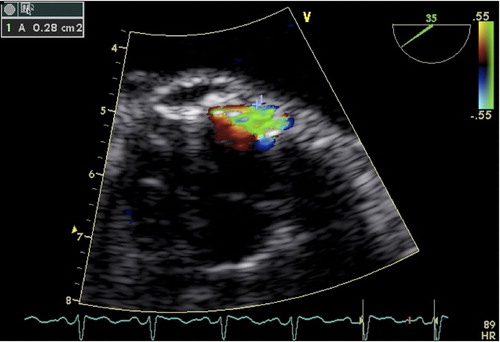
Pre-implant TOE images were analysed qualitatively for presence of particular subvalvular muscular hypertrophy, i.e. any muscular prominence bulging into the left ventricular outflow tract making the outflow hour-glass shaped. Native aortic valve calcification was considered asymmetrical if calcification was prominent in one third or less of the native valve and annulus. On post-implant images and with use of the highest possible pulse repetition frequency, the minimum area of each colour-Doppler regurgitation jet was measured by tracking cross sectional images (). The total area of regurgitation was obtained by adding the regurgitant areas, and the total regurgitation was classified as either absent, mild < 0.20 cm2, moderate 0.20–0.29 cm2, or severe 0.30 cm2 (Citation7).
Figure 1. Colour-Doppler image from a transoesophageal echocardiography examination. The area of the paravalvular leak is traced (A 0.28 cm2).
As suggested by the supplier of the valve, patients with annulus diameter of 19–22 mm had a THV size 23 inserted, and patients with annulus dimension of 23–25 mm had a 26 mm THV. We analysed the influence on THV regurgitation of the difference in mm between the annulus diameter measured on TOE and the available THV sizes of 23 and 26 mm.
The height position of the stent valve in relation to the aortic annulus was recorded by measurement of the distance between the lower edge of the stent and the annulus defined as the transition between the sinus of Valsalvae and the anterior leaflet of the mitral valve imaged in a TOE long axis view. We analysed for a potential relationship between the height position of the THV and THV regurgitation.
Left ventricular dimensions were measured by M-mode echocardiography.
Statistical analysis
Results were analysed using STATA 11.2 (Statacorp LP, Texas, USA).
Pre- and postoperative variables were compared using the paired student's t-test. Between groups comparisons were performed with the unpaired student's t-test.
Regression analyses were performed when correlating continuous variables. Data are presented as means with 95% confidence intervals in brackets.
Results
During the 1-year follow-up, 12 patients died. Eight patients died within the first 30 days after TAVI, and a total of seven patients died from cardiac reasons. Three of these died peroperatively because of heart failure and heart failure was also the cause of death on day 13 in another patient. One patient died peroperatively from resuscitation-related complications to left main coronary artery occlusion. Another patient undergoing transfemoral TAVI died from type A aortic dissection induced by the THV insertion catheter. The remaining patient who suffered a cardiac death died from myocardial infarction on the first postoperative day. Of the surviving patients, 77 were evaluated before discharge, 66 after three months and 55 after 12 months.
Prevalence of regurgitation
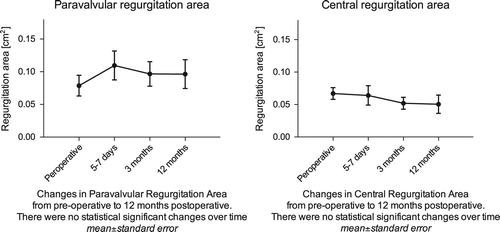
Immediately after TAVI, the proportion of patients exhibiting any THV regurgitation was 75% [CI 65–85%] with similar representation of mild, moderate, and severe valve insufficiency in the groups undergoing TAVI via the femoral and apical approach (p = 0.6). Paravalvular regurgitation was present in 51% [CI 40–63%] of cases, whereas central regurgitation was seen in 55% [CI 43–67%]. The regurgitation was mild or absent in the majority of cases ().
Table II. Incidence and severity of transcatheter aortic prosthetic regurgitation measured by transoesophageal echocardiography during TAVI immediately after insertion of the prosthetic valve.
Twelve patients had severe paravalvular regurgitation according to our classification; however, no patient was considered to require re-intervention to reduce THV regurgitation during the one-year follow-up.
Both paravalvular and central regurgitation appeared to be constant over time ().
The prevalence of regurgitation did not change from the first half of the cohort compared to the latter half (p = 0.2).
The proportion of patients who according to the analysis had moderate or severe THV regurgitation was 36% at discharge, 35% after three months, and 28% after 12 months (p > 0.3 in comparison with discharge and 3-months proportions).
Clinical implications of regurgitation
Of the 12 patients who died during the follow-up period, none had severe regurgitation. Altogether, we found no correlation between regurgitation and death during the first year after TAVI.
Of the entire population, 75% improved their NYHA classification at 30 day follow-up after the procedure. Among patients with none or mild regurgitation, 80% improved their NYHA class after the procedure, compared to 60% in patients with moderate or severe regurgitation (p < 0.05 one-tailed).
Preoperatively there were no significant differences between these two regurgitation groups regarding NYHA class (p = 0.6) or left ventricular end-diastolic dimension (p = 0.1).
After 12 months, the dataset on NYHA classification was not sufficiently recorded.
The patients’ diuretic dosages were analysed before and after the procedure. These dosages were categorised as either constant, decreasing or increasing 3 months after the TAVI procedure. In 70% of the patients the diuretic dosages were reduced and there was no correlation between the severity of regurgitation and the patients’ needs for diuretic treatment postoperatively, R2 = 0.01.
Features with possible influence on regurgitation
A summary of these analyses is seen in .
The presence of asymmetrical calcification of the native valve non-significantly increased the extent of paravalvular leakage from 0.05 [0.02–0.07] cm2 in patients with symmetrical calcification to 0.1 [0.03–0.17] cm2 in patients who had asymmetrical calcifications (p = 0.12). The mean central leakage was the same in the two groups (0.06 cm2), p = 0.94. The total regurgitant areas are listed in .
Table III. Mean total regurgitation area + SD, with or without pre-existing asymmetrical calcification of the native aortic valve and subvalvular septal muscular hypertrophy.
No correlation between the height of the THV position and paravalvular or central leakage was found, R2 = 0.04 and R2 = 0.008, respectively.
The difference between the available THV sizes and the annulus diameters measured by TOE did not correlate with the degree of paravalvular or central leakage, R2 < 0.001 and R2 = 0.004, respectively, and we found no relationship between asymmetrical subvalvular hypertrophy and THV regurgitation ().
Left ventricular responses to stent valve regurgitation
When comparing groups by degree of regurgitation, the initial peroperative classification was used (although the regurgitant areas did not change significantly over time []).
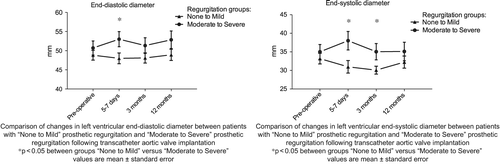
Patients with moderate to severe regurgitation (> 0.20 cm2) immediately after TAVI had significantly larger left ventricular end-diastolic dimension than patients with none to mild regurgitation, 53 [49–57] mm vs. 48 [45–51] mm at 5–7 days postoperatively (p = 0.04). Left ventricular end-systolic dimension was also significantly increased in patients with moderate to severe regurgitation compared to patients with none to mild regurgitation 38 [33–43] mm vs. 31 [28–34] mm after 5–7 days (p = 0.02), and 35 [31–40] mm vs. 30 [28–32] mm after 3 months, (p = 0.03) ().
All patients were weighed prior to the procedure. Pre-procedure weight was sought before echocardiography at discharge, in order to avoid any effect of the degree of hydration on the left ventricular volumes.
Discussion
Our results showed a high prevalence of THV regurgitation of 75% (paravalvular and/or central). The regurgitation was most frequently mild to moderate and it did neither change nor prove to be clinically serious over the one-year follow-up period. Regardless of regurgitation severity most patients improved their NYHA class along with a decreased need for diuretic treatment in 70% of the patients. Thus, based on the clinical evaluation of our cohort, it seemed that THV regurgitation in general was easier tolerated than severe native aortic valve stenosis.
Left ventricular dimensions revealed significant systolic dilatation in patients with moderate and severe regurgitation compared to patients with none and mild regurgitation, at least one week and three months after TAVI (). Thus, after all some haemodynamic consequence of moderate and severe regurgitation was indicated although significantly progressing ventricular dilatation over the follow-up did not take place. Postoperative early improvement of NYHA class was also more common in patients with absent or mild regurgitation than in patients with moderate to severe regurgitation.
According to our data it did not seem possible to predict a particular high risk of significant THV regurgitation from preoperative TOE evaluation of the calcifications in the aortic orifice nor from presence of a subvalvular septal muscular bulge (). Within the ranges recommended by the supplier, the discrepancy between valve size and annulus dimension was also with no detectable influence of THV regurgitation. Finally, the height position of the THV within the target region for delivery of the valve did not seem to be of importance for regurgitation. We considered the target region for the position of the lower edge of the stent to be within the range of the lower edge of the Sinus of Valsalvae and the proximal 5 mm of the anterior mitral leaflet. The occurrence of THV regurgitation also seemed to be independent of the THV insertion route (femoral vs. apical) and our data did not indicate any learning curve influence on THV regurgitation.
Our results indicate that there is no particular need for systematic serial echocardiography in patients who exhibit none or only mild THV regurgitation peroperatively, immediately after valve insertion. As depicted in , the regurgitation can be regarded virtually unchanged after the initial TOE. The finding of unchanged regurgitation after TAVI is in concordance with other reports (Citation2,Citation4,Citation9,Citation10). In patients with moderate to severe regurgitation, scheduled follow-up by physical examination and echocardiography seems to be reasonable and unexpected worsening of the clinical status, should obviously lead to a new examination in any patient.
Even if THV regurgitation did not prove to be clinically significant over one year in our study, the presence of regurgitation may increase the risk of both endocarditis and haemolysis and later clinical worsening, particular in those with moderate to severe aortic regurgitation can certainly not be excluded (Citation11). Our patients were not systematically examined for haemolysis but none of the patients developed anaemia over time. Thus, in our patients with THV regurgitation haemolysis was not a clinical problem. Still, the promising TAVI technique could probably benefit further from development of valve prostheses designed to reduce THV regurgitation. Prosthetic regurgitation following conventional aortic valve surgery has been proven to be much less frequent, with reported rates of less than 2% (Citation11).
The patients in this series were evaluated using echocardiography, and the annulus size was measured preoperatively by means of TOE. However, recent studies by means of CT have documented the aortic annulus to be ellipsoid rather than circular (Citation12). TOE only provides the smaller antero-posterior dimension of the ellipsoid annulus and may thus underestimate the true size of the annulus.
Study limitations
We classified the degree of THV insufficiency from our own TOE method. However, according to Leon and others, the precise grading of prosthetic valve regurgitation is controversial (Citation9,Citation13–15). Although the combination of many individual echocardiographic parameters are recommended to create a general impression of the degree of aortic regurgitation it may seem difficult to classify the regurgitation from a multiple of criteria including left ventricular dimensions, jet width, jet density, jet deceleration rate etc. and none of these parameters can hardly stand alone (Citation13) Central and paravalvular leaks do commonly appear in one and the same patient and our technique at least has the advantage of making it possible to add the various leaks.
Furthermore, we used the classification into mild (< 0.20 cm2), moderate (0.20–0.29 cm2) and severe (> 0.30 cm2) as suggested by the American Society of Echocardiography and the European Association of Echocardiography (Citation7).
Yet, it must be admitted, that the cross section of a jet on colour-Doppler TOE not necessarily reflects the true leak. It should also be mentioned that some anterior jets might have been hidden for the TOE probe by the shadowing stent in the THV. Nevertheless, our classification of the degree of THV regurgitation is supported by the findings, of continuously less affected left ventricular dimensions and better improved 3-months postprocedural NYHA class in patients with none or mild regurgitation than in patients with moderate to severe regurgitation.
It is an obvious limitation that TTEs and TOEs adequate for analysis were not available in all surviving patients at all follow-up examinations. However, this merely reflects the difficulties in obtaining high-fidelity serial recordings in weak elderly patients. Yet, the proportions of moderate to severe THV valve regurgitation and none or mild regurgitation remained the same during the follow-up.
It is also a limitation that our data on NYHA class after 12 months were insufficient. However, the measurements of left ventricular end-diastolic dimension supported the impression of a stable condition over the first postprocedural year, even in patients with moderate to severe THV regurgitation. Further studies are needed in order to investigate the potential long-term clinical effects, as recent studies have reported increased late mortality related to the severity of THV regurgitation (Citation11). It is also possible that other imaging modalities, for example MRI scans, may enable a better quantification of THV regurgitation and identification of patients with a high risk after TAVI.
Conclusion
Our results suggest that aortic prosthetic regurgitation after transcatheter aortic valve implantation is common and does not significantly change over time. While affecting left ventricular dimensions in cases with moderate and severe regurgitation, there seem to be no marked further consequences of THV regurgitation within one year of follow-up.
Our results support that scheduled serial examinations for THV regurgitation are unnecessary in a patient leaving the operating room with none or mild THV regurgitation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
CJ Jakobsen has worked as proctor for Edwards lifesciences from march 2009 to march 2010. L. Thuesen has worked as full time physician proctor for Edwards Lifesciences, January 2012 till 2013. H.R. Andersen has a patent and consult agreement with Edwards Lifesciences.
References
- Walther T, Schuler G, Borger MA, Kempfert J, Seeburger J, Ruckert Y, . Transapical aortic valve implantation in 100 consecutive patients: comparison to propensity-matched conventional aortic valve replacement. Eur Heart J. 2010;31: 1398–403.
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, . Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363: 1398–403.
- Clavel MA, Webb JG, Pibarot P, Altwegg L, Dumont E, Thompson C, . Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol. 2009; 53: 1398–403.
- Rajani R, Kakad M, Khawaja MZ, Lee L, James R, Saha M, . Paravalvular regurgitation one year after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010; 75: 1398–403.
- Webb JG, Altwegg L, Boone RH, Cheung A, Ye J, Lichtenstein S, . Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation. 2009;119: 1398–403.
- Vahanian A, Alfieri OR, Al-Attar N, Antunes MJ, Bax J, Cormier B, . Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2008; 34: 1398–403.
- Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, . Recommendations for evaluation of the severity of native valvular regurgitation with two- dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16: 1398–403.
- Nielsen HH, Thuesen L, Egeblad H, Poulsen SH, Klaaborg KE, Jakobsen CJ, . Single center experience with transcatheter aortic valve implantation using the Edwards SAPIEN Valve. Scand Cardiovasc J. 2011;45: 261–6.
- Rallidis LS, Moyssakis IE, Ikonomidis I, Nihoyannopoulos P. Natural history of early aortic paraprosthetic regurgitation: a five-year follow-up. Am Heart J. 1999;138: 1398–403.
- Gurvitch R, Wood DA, Tay EL, Leipsic J, Ye J, Lichtenstein SV, . Transcatheter aortic valve implantation: durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation. 2010;122: 1319–27.
- Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, . Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366: 1398–403.
- Schoenhagen P, Kapadia SR, Halliburton SS, Svensson LG, Tuzcu EM. Computed tomography evaluation for transcatheter aortic valve implantation (TAVI): imaging of the aortic root and iliac arteries. J Cardiovasc Comput Tomogr. 2011; 5: 1398–403.
- Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, . Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography‘s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22:975–1014; quiz 82–4.
- Kupferwasser II, Mohr-Kahaly S, Erbel R, Nolting A, Dohmen G, Oelert H, . Improved assessment of pathological regurgitation in patients with prosthetic heart valves by multiplane transesophageal echocardiography. Echocardiography. 1997;14: 1398–403.
- Mohr-Kahaly S, Kupferwasser I, Erbel R, Wittlich N, Iversen S, Oelert H, . Value and limitations of transesophageal echocardiography in the evaluation of aortic prostheses. J Am Soc Echocardiogr. 1993;6: 1398–403.