Abstract
Objectives. Resuscitation after cardiac arrest may lead to ischemia-reperfusion injury and infarction. We evaluated whether Sildenafil, a phosphodiesterase-5 inhibitor, has an impact on recovery after cardiac arrest in a rat cardiac transplantation model. Design. Sixty-one Fischer344 rats underwent syngeneic heterotopic cardiac transplantation after ischemia and ligation of the left anterior coronary artery of the heart to yield myocardial infarction (IRI + MI). Of these, 22 rats received subcutaneously injected Sildenafil (1 mg/kg/day) (IRI +MI + S). Twenty-three additional grafted animals with transplantation only served as controls with ischemia reperfusion injury (IRI). After 2 days, immunohistochemistry for eNOS, and RT-PCR for iNOS and Aquaporin-7 were performed after graft harvesting and histology. Results. Two days after transplantation, remote intramyocardial arteries were more preserved in IRI + MI + S as compared with IRI +MI and IRI (0.74 ± 0.14, 0.56 ± 0.23 and 0.55 ± 0.22, PSU, p < 0.05, respectively). Decreased eNOS staining confirmed the presence of developing infarction in IRI + MI and IRI + MI + S. The expression of iNOS was significantly lower during IRI + MI +S as compared with IRI + MI (0.02 ± 0.01 and 1.02 ± 0.02, FC, p < 0.05). Conclusions. Administered at the onset of reperfusion and developing infarction, Sildenafil has an impact on myocardial recovery after cardiac arrest and ischemia.
Introduction
Uncontrolled ischemia-reperfusion injury (IRI) after cardiac arrest may lead to permanent ongoing ischemia eventually developing myocardial infarction (MI). Traditionally, resuscitation aims at stabilizing the heart after cardiac arrest in order to avoid global and permanent heart damage. When feasible, the heart can be temporarily assisted by inserting a left ventricular assist device to reduce the myocardial work load. However, even though prompt revascularization is performed to stenosed culprit coronary arteries, irreversible myocardial damage may occur and prevent myocardial recovery (Citation1).
IRI and local ongoing ischemia has an early global effect on myocardial remodeling. IRI and MI may be investigated by the widely used heterotopic rat cardiac transplantation model (Citation2–4). This model provides the possibility to investigate the cardiac graft in a non-working state simulating the presence of a left ventricle assist device, while the recipient heart keeps the animal alive. Instead of endangering the recipient heart, in order to achieve MI, it is practical to ligate permanently the left anterior descending coronary artery (LAD) of the heterotopically transplanted cardiac graft. This animal model mimics the clinical concept of having an area of local and persisting MI after complete revascularization of the globally ischemic heart.
There is growing interest towards nitric oxide (NO) metabolism in terms of attenuating the consequences of IRI after resuscitation (Citation5). The ubiquitous involvement of NO in cardiac pathophysiology suggests that adjunct therapeutic manipulation aimed at interfering with NO metabolism may prove successful after myocardial damage (Citation6). Sildenafil, a potent phosphodiesterase-5 inhibitor, inhibits the breakdown of cGMP and enhances NO-driven cGMP accumulation. It has been shown that preoperatively administered Sildenafil decreases renal IRI (Citation7), enhances cardioprotection after experimental transplantation (Citation8,Citation9) and attenuates myocardial infarction area after reversible IRI (Citation6). These beneficial effects of Sildenafil are related to NO metabolism, though other possible mechanisms may also be involved (Citation10). However, previous studies have concentrated on the preconditioning effect of Sildenafil (Citation11,Citation12), and the treatment has been initiated well before the onset of induced IRI. In this study, we investigate whether Sildenafil has an impact on remote myocardial changes during IRI after MI.
Materials and methods
Rats
The study was approved by the Finnish State Provincial Office. Eighty-four inbred Fischer 344 rats (F344/NHsd, Harlan Laboratories, The Netherlands) weighing 175–200 g, underwent heterotopic cardiac transplantation. The rats were kept in Tampere University vivarium and received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1996).
Surgery
During harvesting of the donor heart, the ascending aorta of the cardiac graft was infused with antegrade infiltration of 2 ml cold (4°C) temperature physiologic saline fluid without heparinization. Eighty-four heterotopic cardiac transplantations were performed intra-abdominally by joining the graft aorta to the aorta and the graft pulmonary artery to the inferior vena cava of the recipient, as previously described (Citation3). From the recipient aorta, the transplanted heart received oxygenated blood that was introduced into the coronary arteries of the graft. Through the coronary sinus, this blood circulated into the right atrium and eventually the right ventricle, from where deoxygenated blood was repulsed to the recipient rat throughout the pulmonary vein. Therefore the nutritional flow of the myocardium consisted of oxygenated blood, and the transplanted heart was not ischemic after reperfusion upon transplantation. Since the aortic valve was competent, oxygenated blood was not allowed to fill the left ventricle, and therefore the transplanted heart simulated a non-working resting state of the left side of the graft. This heterogenous transplantation model allowed one to study IRI in-vivo without interferences of myocardial stress factors. The model thus simulated the clinical concept of acute cardiac arrest resuscitated with initiation of cardiopulmonary bypass and left ventricle assist device (Citation1). Total warm ischemia time before total graft reperfusion was 20–30 minutes after cardiac arrest. In 61 grafts, the LAD was also ligated permanently at its proximal part with a single 7-0 suture to yield MI at the apex of the heart graft. The ligation knot for LAD obstruction was placed at the bifurcation of the 1rst diagonal branch due to coronary vessel distribution, since the apex is most vulnerable to ischemic changes leading to MI. Of these grafts, 22 rats were treated with Sildenafil administered 1 mg/kg/day subcutaneously initiated after reperfusion of the graft (IRI + MI + S), hence 39 grafts with IRI underwent transplantation and LAD ligation without treatment (IRI + MI). Twenty-three grafts underwent transplantation only (IRI).
Graft patency
Graft patency was achieved by means of palpation using a score from 0 to 6; 0 indicated no pulse, 2 indicated weak pulsation, and 6 meant normal contractility and strong pulsation. The palpation score, as a direct measure of cardiac vitality and effective contractility, proved to be a reliable and convenient test for definition of the end point for graft survival, with no variability or bias in the evaluations of independent observers (Citation13).
Tissue samples
The recipient rats were followed and sacrificed upon termination of graft palpation (palpation score 0 to 2) yielding 63 hearts after 2 days of reperfusion. The basal part of the cardiac graft that did not include the infarction was procured for quantitative RT-PCR analysis to investigate for the remote myocardium. In contrast, the apex part of the graft, including MI in cardiac grafts with LAD ligation, was embedded in paraffin and 5-μm sections were cut.
Histology
For histology, the 5-μm sections were stained with Hematoxylin and Eosin. Evaluation was performed blinded to the study protocol, treatment allocation and time sequence by 2 investigators (AM and VV) and technically unclear slides were rejected. The following variables were evaluated: presence of myocardial edema, hemorrhage and ischemia. As the vacuolization of nuclei of the media layer of intramyocardial arteries reflected edema, a representative cross-sectional intramyocardial artery was chosen randomly from the left anterior ventricular wall and the left posterior ventricular wall representing remote myocardium. As the minority of the arterial wall nuclei was round-shaped representing normal nuclei, sharp-edged blue nuclei of the media cells were defined as non-preserved and manually counted together with vacuolated and edematous nuclei. Periadventitial inflammation was graded according to an arbitrary scale from 0 to 2 and expressed as point score units (PSU): 0, no inflammation; 1, presence of occasional inflammatory cells; 2, groups of inflammatory or proliferating cells.
Immunohistochemistry for eNOS and iNOS
Twenty hearts were randomly selected 2 days after transplantation for immunohistochemistry. Paraffin-embedded slides were deparaffinized with 3 changes of xylene, and rehydrated in a series of graded ethanol, and rinsed well under running distilled water. Slides were placed in a preheated retrieval buffer, 0.1 mmol EDTA, pH 8.0, for 30 minutes, then cooled in the buffer for 5 minutes, followed by a 5-minute rinse under running distilled water. After heat-induced epitope retrieval, slides were placed on an autostainer (DAKO Corp, Carpinteria, California, USA). Sections were incubated with 3% hydrogen peroxide in ethanol for 5 minutes to inactivate the endogenous peroxides, incubated either in 1:100 eNOS (eNOS, DAKO Corp) or iNOS (iNOS, DAKO Corp) for 30 minutes, followed by rinsing with Tris-buffered saline solution with Tween 20 (TBST) wash buffer. Secondary incubation was with DUAL-labeled polymerhorseradish peroxidase (K4061; DAKO Corp) for 15 minutes. The slides were rinsed with TBST wash buffer. Sections were then incubated in 3,3-diaminobenzidine (K3467, DAKO Corp) for 5 minutes, counterstained with modified Schmidt hematoxylin for 5 minutes, and rinsed for 3 minutes in tap water to blue sections, dehydrated with graded alcohols, and cleared in 3 changes of xylene before mounting. Stainings for eNOS and iNOS were scored according to an arbitrary scale from 0 to 3 and expressed as PSU, where 0 means, no staining visualized; 1, individual positively stained nuclei; 2, groups of positively stained nuclei; 3, intensive and global positively stained area. Two hearts were rejected due to technical failure.
Quantitative RT-PCR analysis
The frozen tissue of the base of the heart was homogenized and RNA was extracted using a rotor- stator homogenizer and NucleoSpin® RNA II kit (Machery-Nagel GmbH & Co, D ü ren, Germany) according to the manufacturer's instructions. 50 ng of total RNA was reverse-transcribed into cDNA in reaction volume of 20 μl. The quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed with standard protocols on Abi Prism 7300 instrument (Applied Biosystems, CA, USA). The PCR reaction was performed with TaqMan® Gene Expression assays for aquaporin-7 (ID Rn00569727_m1) and GAPDH (ID Rn01462662_g1) (both from Applied Biosystems) according to the manufacturer's instructions with TaqMan® Universal PCR Master Mix. All samples were performed as three replicates.
The expression levels of Aquaporin-7, iNOS and GAPDH as an internal control/house keeping gene were evaluated. Ct values were determined for every reaction and the relative quantification was calculated using the 2−∆∆Ct method (Citation14). Briefly, the data were normalized to the expression of house keeping gene GAPDH, and values of Control samples were used as a calibrator. The media and standard error of mean for the Aquaporin-7 and iNOS expression levels were calculated between IRI + MI and IRI + MI + S groups to demonstrate the effect of Sildenafil on remote myocardium during MI.
Statistical analysis
Data were presented as the mean and standard error of the mean. Kruskall-Wallis non-parametric statistics was included for comparison between independent groups. Two-way ANOVA was utilized to analyze among groups. Nonparametric data between 2 groups were analyzed with Mann-Whitney U test. Statistical analyses were performed with commercial statistical software (SPSS 19.0, SPSS Inc, Chicago, IL).
Results
Heart graft patency
A third of the hearts (33%) with IRI + MI and only 18% with IRI + MI + S had a palpation score less than 2 out of 6 at 2 days of reperfusion, whereas 82% of the hearts with IRI and IRI + MI + S remained patent for 2 days in comparison with only 67% hearts with IRI + MI (p < 0.05).
Histology
There were no hemorrhagic or major ischemic myocardial differences between the hearts with IRI + MI + S and IRI + MI at 2 days after operation (1.90 ± 0.25 vs 1.80 ± 0.22 and 2.20 ± 0.19 vs 1.85 ± 0.16, PSU, respectively. Global myocardial edema and inflammation tended to decrease in IRI + MI + S as compared with IRI + MI (2.00 ± 0.27 vs 2.45 ± 0.21 and 0.60 ± 0.17 vs 1.00 ± 0.12, PSU, respectively), but no significant changes were observed among groups. After 2 days, the relative number of recovered remote intramyocardial artery wall nuclei, that is the number of clear smooth-edged media cell nuclei divided by the total number of media cell nuclei including vacuolated and sharp-edged dark media cell nuclei, was significantly increased in IRI +MI + S as compared with IRI + MI (72 ± 3.60 vs 56 ± 4.46, PSU, respectively, p < 0.05, and ).
Figure 1. Representative histology of a remote intramyocardial artery of a normal heart (A), a graft with ischemia-reperfusion injury only (IRI; B), a graft with ischemia-reperfusion injury and myocardial infarction (IRI + MI; C), and a graft with ischemia-reperfusion injury and myocardial infarction treated with Sildenafil (IRI + MI + S; D) 2 days after reperfusion simulating resuscitation. X40. Note edema of intramyocardial artery shown as vacuolization of vessel wall in C (small arrows).
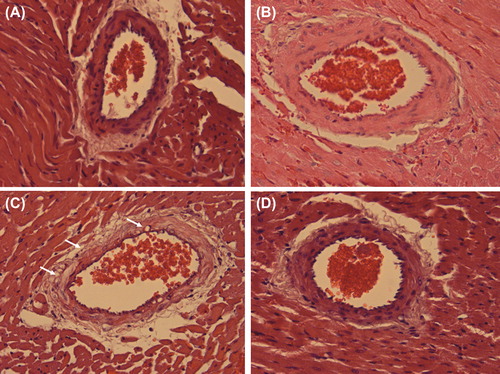
Figure 2. Relative number of recovered remote intramyocardial artery wall nuclei in grafts with ischemia-reperfusion injury only (IRI, circles), grafts with ischemia-reperfusion injury and myocardial infarction (IRI + MI, boxes), and grafts with ischemia-reperfusion injury and myocardial infarction treated with Sildenafil (IRI + MI + S, triangles). As compared with IRI and IRI + MI, IRI + MI + S have increased relative number of recovered remote intramyocardial artery nuclei 2 days after reperfusion. *p < 0.05, Kruskal-Wallis. Horizontal bars indicate median.

Immunohistochemistry for eNOS and iNOS
Statistically, decreased staining of eNOS was observed in hearts with IRI + MI + S and IRI + MI as compared with IRI after 2 days (0.75 ± 0.29 and 0.60 ± 0.40 vs 2.50 ± 0.28, PSU, respectively, p < 0.05, ). There was a tendency for decrease staining in iNOS in hearts with IRI + MI + S as compared with IRI + MI, though statistical significance was not reached (1.87 ± 0.33 vs 2.50 ± 0.25, PSU, respectively, ).
Figure 3. Representative immunohistochemistry for eNOS of a remote intramyocardial artery of a normal heart (A), a graft with ischemia-reperfusion injury only (IRI; B), a graft with ischemia-reperfusion injury and myocardial infarction (IRI + MI; C), and a graft with ischemia-reperfusion injury and myocardial infarction treated with Sildenafil (IRI + MI + S; D) 2 days after reperfusion simulating resuscitation. X40. Note intensive positive endothelial staining (arrows) in a remote intramyocardial artery of a graft with ischemia-reperfusion injury only (IRI; B) as compared with grafts with infarction without (IRI + MI; C) or with Sildenafil (IRI + MI + S; D).
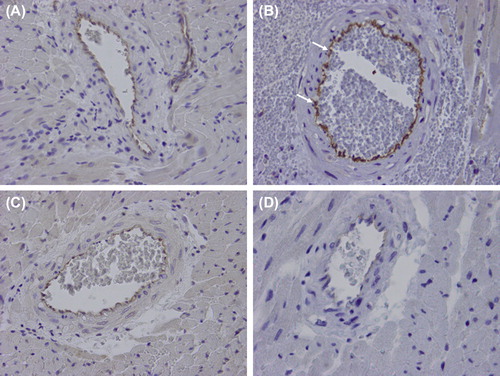
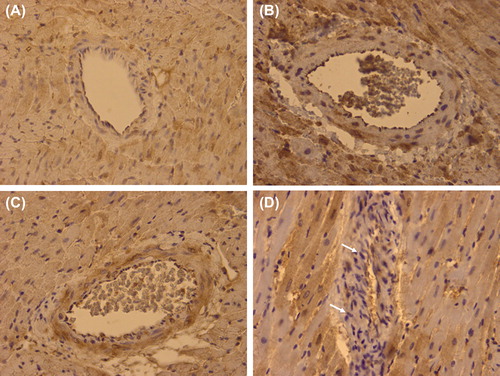
Figure 4. Representative immunohistochemistry for iNOS of a remote intramyocardial artery of a normal heart (A), a graft with ischemia-reperfusion injury only (IRI; B), a graft with ischemia-reperfusion injury and myocardial infarction (IRI + MI; C), and a graft with ischemia-reperfusion injury and myocardial infarction treated with Sildenafil (IRI + MI + S; D) 2 days after reperfusion simulating resuscitation. X40. Note tendency for decreased positive staining (arrows) in a remote intramyocardial artery of a graft with ischemia-reperfusion injury and myocardial infarction treated with Sildenafil (IRI + MI + S) as compared with grafts without (IRI; B) or with infarction (IRI + MI; C).
iNOS and Aquaporin-7 expressions
iNOS expression was significantly lower in IRI + MI + S as compared with IRI + MI after 2 days (0.02 ± 0.01 vs 1.01 ± 0.23, FC, respectively, p < 0.05), whereas no major difference was observed in Aquaporin-7 expression (0.47 ± 0.05 vs 0.78 ± 0.23, FC, respectively).
Discussion
It is important to acknowledge the limited possibility in treating the ischemic myocardium. An infarction represents an irreversible process, which itself is no longer amenable to treatment. This effect is well-known in clinics, where revascularization of an acutely obstructed target coronary artery causing MI is insufficient to prevent global heart dysfunction leading to acute stunning. The heart needs immediate rest such as infusion of cardioplegia and insertion of a left ventricular assist device that decreases cardiac overload. These maneuvers, however, do not abolish the ongoing effect of MI that induces remote histopathological changes.
The presence of a developing local infarction, often the cause of acute cardiac dysfunction during cardiac arrest, has also been advocated as enhancing the poor cardiac outcome after resuscitation (Citation1). After cardiac arrest, resuscitation aims at preventing the heart from further damage due to MI (Citation1). The coexistence of myocardial edema and inflammation remote to the infarction area may be reversible (Citation15). Salvation and protection of the viable myocardium are of uttermost importance, particularly as infarction has the remote ongoing myocardial effects (Citation15). This study demonstrates that Sildenafil treatment initiated after IRI and MI can prolong cardiac graft patency in a rat transplantation model simulating resuscitation. This finding is confirmed by a preserved palpation score (2 or more out of 6) in IRI + MI + S compared with IRI + MI.
Experimental data suggest that preoperative Sildenafil augments the nitric oxide reserves of the heart, which in turn, enhances the cardiac capacity to survive after IRI (Citation16). According to in-vitro preconditioning studies, Sildenafil increases myocardial iNOS (Citation16). We attempted to simulate the clinical setting, were treatment is initiated after onset of resuscitation, that is after reperfusion from cardiac arrest and warm ischemia together with induction of myocardial infarction. In this study, Sildenafil has an impact even when treatment was initiated after induction of warm ischemia and infarction. Infarction itself was irreversible, since LAD was permanently ligated, therefore eNOS was down-regulated in hearts with MI (Citation3). The remote intramyocardial changes, in contrast, were reversible with Sildenafil (,).
Controversial data exist on whether eNOS and/or iNOS are elevated after myocardial ischemia (Citation6), but most interpret iNOS elevation as a surrogate for myocardial damage (Citation6). On the other hand, some state that eNOS is increased after induction of myocardial protection. As a consensus, eNOS decrease may reflect the state of heart ischemia, whereas iNOS increase reflects response to IRI (Citation15,Citation17). It was beyond our scope to demonstrate the specific mechanisms underlying the effectiveness of Sildenafil per se in terms of NO metabolism, and therefore we concentrated on defining the outcome of remote myocardium susceptible to reversible ischemia. We selected evaluation of iNOS expression of the remote myocardium to indicate the severity of myocardial damage after IRI and MI. After 2 days, Sildenafil decreased remote myocardial iNOS expression after IRI and MI, suggesting for salvation of the remote myocardium.
We may thus speculate that in our model iNOS expression, obtained from the basal heart tissue far away from the infarction area, may mirror the state of the remote myocardium itself instead of revealing the mechanism of action of the medication. Decreased iNOS expression may also suggest for a temporary loss of NO reserves of the heart with IR and MI (Citation18), and long-term myocardial protection may be due to delayed iNOS increase. Sildenafil may also include other mechanisms as speculated in a renal study (Citation10). We therefore wanted to confirm the permanent effect of LAD ligation on remote myocardium by evaluating Aquaporin-7, a mediator of water balance associated with glycerol metabolism considered as a surrogate of MI (Citation2,Citation19). Thus, Aquaporin-7 expression was observed in both untreated and Sildenafil-treated hearts with MI due to LAD ligation at the apex of the heart.
In summary, Sildenafil, administered at the onset of IRI and MI, maintained patent remote intramyocardial arteries while altering remote iNOS expression profile of the cardiac graft. Though still speculative at this point, Sildenafil may be a possible adjunct during resuscitation of the ischemic heart.
Acknowledgements
We thank prof Teuvo Tammela for providing Sildenafil. Dr Mennander is the recipient of the Ingegeerd and Viking O Bjork Cardiothoracic award.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study has been supported by The Research Foundation of Tampere University Hospital, Tampere Tuberculosis Foundation, The Finnish Heart Association and The Finnish Cultural Association.
References
- Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, . Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008; 118:2452–83.
- Liu Z, Vuohelainen V, Tarkka M, Tenhunen J, Lappalainen RS, Narkilahti S, . Glutamate release predicts ongoing myocardial ischemia of rat hearts. Scand J Clin Lab Invest. 2010; 70:217–24.
- Vuohelainen V, Raitoharju E, Levula M, Lehtimaki T, Pelto-Huikko M, Honkanen T, . Myocardial infarction induces early increased remote ADAM8 expression of rat hearts after cardiac arrest. Scand J Clin Lab Invest. 2011; 71:553–62.
- Suzuki K, Murtuza B, Smolenski RT, Suzuki N, Yacoub MH. Development of an in vivo ischemia-reperfusion model in heterotopically transplanted rat hearts. Transplantation. 2002;73:1398–402.
- Wu D, Bassuk J, Arias J, Kurlansky P, Lozano H, Lamas G, . Different roles of nitric oxide synthase isoforms in cardiopulmonary resuscitation in pigs. Resuscitation. 2007;73: 144–53.
- Berges A, Van Nassauw L, Timmermans JP, Vrints C. Role of nitric oxide during coronary endothelial dysfunction after myocardial infarction. Eur J Pharmacol. 2005;516:60–70.
- Choi DE, Jeong JY, Lim BJ, Chung S, Chang YK, Lee SJ, . Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol. 2009;297: F362–70.
- Loganathan S, Radovits T, Hirschberg K, Korkmaz S, Barnucz E, Karck M, . Effects of selective phosphodiesterase-5-inhibition on myocardial contractility and reperfusion injury after heart transplantation. Transplantation. 2008;86:1414–8.
- Botha P, MacGowan GA, Dark JH. Sildenafil citrate augments myocardial protection in heart transplantation. Transplantation. 2010;89:169–77.
- Elrod JW, Greer JJ, Lefer DJ. Sildenafil-mediated acute cardioprotection is independent of the NO/cGMP pathway. Am J Physiol Heart Circ Physiol. 2007;292:H342–7.
- Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase- dependent pathway in mouse heart. Circ Res. 2003;92:595–7.
- Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–10.
- Ricci D, Mennander AA, Miyagi N, Rao VP, Tazelaar HD, Classic K, . Prolonged cardiac allograft survival using iodine 131 after human sodium iodide symporter gene transfer in a rat model. Transplant Proc. 2010;42:1888–94.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆Ct method. Methods. 2001;25:402–8.
- Zhang J, Knapton A, Lipshultz SE, Weaver JL, Herman EH. Isoproterenol-induced cardiotoxicity in Sprague-Dawley rats: correlation of reversible and irreversible myocardial injury with release of cardiac Troponin T and roles of iNOS in myocardial injury. Toxicol Pathol. 2008;36:277–88.
- Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–55.
- Felaco M, Grilli A, Gorbunov N, Di Napoli P, De Lutiis MA, Di Giulio C, . Endothelial NOS expression and ischemia-reperfusion in isolated working rat heart from hypoxic and hyperoxic conditions. Biochim Biophys Acta. 2000;1524:203–11.
- Desrois M, Durrans A, Caus T, Lan C, Clarke K, Cozzone PJ, . Modulation of the NO pathway during short or prolonged blood reperfusion following ischaemia in a heterotopic rat heart transplantation model. Transplant Proc. 2004;36:1280–2.
- Hibuse T, Maeda N, Nakatsuji H, Tochino Y, Fujita K, Kihara S, . The heart requires glycerol as an energy substrate through aquaporin 7, a glycerol facilitator. Cardiovasc Res. 2009;83:34–41.