Abstract
Objectives. Statins decrease cardiovascular events mainly by lowering cholesterol but anti-inflammatory effects also play a role. The effects of the cholesterol absorption inhibitor ezetimibe on markers of inflammation remain unclear. We performed an exploratory post-hoc analysis whether these drugs influence the pro-inflammatory markers interleukin-6 and high-sensitivity C-reactive protein in subjects with very-low cardiovascular risk. Design. Single center, randomized, parallel 3-group study in 72 healthy men without apparent cardiovascular disease (age 32 ± 9 years, BMI 25.7 ± 3.2 kg/m2). Each group of 24 subjects received a 14-day treatment with either simvastatin 40 mg, ezetimibe 10 mg, or their combination. Results. Baseline IL-6 and hsCRP concentrations in the total cohort were 0.72 ± 0.57 ng/l and 0.40 ± 0.65 mg/l, respectively, with no differences between the 3 groups. Median changes (interquartile range) in IL-6 and hsCRP concentrations were −22% (−43 to 0%) and −30% (−44 to +19%) after simvastatin, −5% (−36 to +30%) and +9% (−22 to +107%) after ezetimibe, and +15% (−15 to +86%) and +1 (−30 to +49%) after the combination. Using a generalized linear model, the multivariable adjusted overall P-values for these changes were 0.008 (IL-6) and 0.1 (hsCRP). Conclusions. Simvastatin decreases the pro-inflammatory markers IL-6 and almost significantly hsCRP while ezetimibe monotherapy or the combination with simvastatin has no effect.
Trial registration: ClinicalTrials.gov identifier: NCT00317993.
Introduction
Statins exert beneficial effects in both primary and secondary cardiovascular disease prevention based on their potent cholesterol-lowering effects. The overall benefits observed with statins appear to occur much earlier and seem to be greater than what might be expected from changes in lipid levels, suggesting effects beyond cholesterol lowering alone (Citation1). Various “pleiotropic effects” of statins have been implicated in their antiatherosclerotic effects, such as (among others) improved endothelial function and blood flow, decreased low-density lipoprotein (LDL) cholesterol oxidation, enhanced stability of atherosclerotic plaques, inhibition of vascular smooth muscle proliferation and platelet aggregation, and reduction of vascular inflammation (reviewed in (Citation1)).
Low-grade, subclinical systemic inflammation seems to be an important component of atherosclerosis development. The most common inflammatory marker used in clinical practice is the high-sensitivity C-reactive protein (hsCRP), but the pro-inflammatory cytokine interleukin-6 (IL-6) has also been implicated in the pathogenesis of atherosclerosis (Citation2). Both these inflammatory markers are known to predict the development of cardiovascular disease in otherwise healthy populations (Citation2). IL-6 is produced in a variety of tissues, including activated leukocytes, adipocytes, and endothelial cells. C-reactive protein is the principal downstream mediator of the acute phase response and is primarily derived via IL-6-dependent hepatic biosynthesis (Citation3). It has been suggested that hsCRP contributes to the development of atherosclerosis by binding to modified LDL cholesterol within coronary plaques (Citation4). Decreasing hsCRP with statins seems to be pathophysiologically beneficial and several clinical trials have indicated that statins decrease hsCRP (Citation5,Citation6).
Ezetimibe, a selective cholesterol absorption inhibitor, lowers LDL-C concentrations by approximately 20% (Citation7), either alone or in combination with statins. Whether ezetimibe has pleiotropic, anti-inflammatory effects in patients with hypercholesterolemia remains unclear. A recent pooled analysis has found that ezetimibe causes a small, non significant decrease in hsCRP while when combined with a statin causes a significant additional hsCRP reduction (in comparison to statin monotherapy) in patients with hypercholesterolemia (Citation8).
The current study is the first prospective study in healthy subjects to investigate the effects of ezetimibe monotherapy, simvastatin and their combination on both, IL-6 and hsCRP.
Patients and methods
Study design
The study design has been published before (Citation7,Citation9–11) but data presented herein represent analyses of newly performed measurements that have not been published before. In short, the present study is a single center prospective, randomized, parallel 3-group study (N = 24 subjects for each group). The participants were randomized to receive either simvastatin (40 mg/day), ezetimibe (10 mg/day), or simvastatin (40 mg/day) plus ezetimibe (10 mg/day) for a period of 2 weeks. Blood was drawn in the morning after a 12 hour fast on days 1 (before the initiation of treatment) and 15 (at the end of the 2-week treatment period). The original study has been registered at ClinicalTrials.gov NCT00317993.
Subjects
The study protocol was approved by the Ethics Committee of the University of Cologne, and all subjects gave written informed consent. Seventy-two male volunteers were recruited by word of mouth and through advertisements in the Cologne area and on campus. Inclusion criteria were age between 18 and 60 years, body mass index (BMI) between 18.5 and 30 kg/m2, LDL-C concentrations < 250 mg/dl and normal blood pressure (< 140/90 mmHg). Subjects who had received lipid-lowering drugs within 12 weeks prior to study entry, those with a history of excessive alcohol intake, liver disease, renal dysfunction (estimated glomerular filtration rate < 60 ml/min), rheumatologic disease, coronary heart disease, diabetes or other endocrine disorders, eating disorders, history of recent substantial (< 190 mg/dl, triglycerides 10%) weight change, history of obesity (BMI = 35 kg/m2) or taking medications known to affect lipoprotein metabolism or the immune system were excluded from the study. All patients were advised to keep their usual dietary habits throughout the trial.
Assays
Blood was drawn in the morning after a 12-hour fast. Lipoproteins were analyzed on the day of blood collection in the core laboratory of the Cologne University Medical Center. Insulin levels were measured by RIA (Diagnostic System Laboratories, Inc., Webster, TX; sensitivity, 1.3 μU/ml; inter- and intra-assay coefficients of variation, 4.7–12.2% and 4.5–8.3%, respectively). Insulin resistance was estimated at baseline using the homeostasis model assessment (HOMA) index―the product of fasting glucose (mmol/l) and insulin (μU/ml) divided by the constant 22.5. Serum high-sensitivity C-reactive protein was determined using the Quantikine Human C-reactive protein immunoassay (R&D Systems, Minneapolis, MN). Interleukin-6 was determined using the human IL-6 Platinum ELISA (eBioscience Diagnostics, San Diego, CA). Serum adiponectin, leptin, and resistin levels were measured using radioimmuno assays (Linco Research, St. Charles, MO, and BioVendor, Brno, Czech Republic) and HMW adiponectin was measured using an ELISA (ALPCO Diagnostics, Salem, NH) as previously described (Citation11).
Data analysis
Statistical analyses were performed using Stata 11 (StataCorp LP, College Station, TX). Descriptive statistics are presented as counts (percentage proportions), means ± SD or medians and interquartile ranges, where appropriate. Initial comparisons between baseline and treatment values were performed using Student's paired t-test in case of normally distributed values and using the non-parametric Wilcoxon signed rank test in case of skewed distributions. Comparisons between groups were performed using analysis of variance and in case of significance the post-hoc test of Bonferroni and Dunn. Categorical variables were analyzed by Chi-square statistics. All analyses were performed two-sided. P-values of < 0.05 were considered statistically significant. Triglyceride, hsCRP, and IL-6 values were log transformed before statistical analysis. Treatment-induced changes are given as median percent differences and interquartile range.
The main outcome parameters in the study were percent change in hsCRP, IL-6 and the ratio of hsCRP to IL-6 from baseline. Spearman's rank correlation coefficients were calculated to estimate associations between baseline values. These calculations were used to generate covariates for multivariable analyses. We performed adjusted analyses using as covariates (i) parameters of glucose metabolism (fasting glucose, fasting insulin, HOMA index), since there is evidence for a possible role of inflammation in the development of diabetes, and (ii) thyroid hormone levels due to the known association between inflammation and thyroid dysfunction. Moreover, since obesity is known as a risk factor for cardiovascular disease and adipose tissue represents an important site for the production of pro-inflammatory (leptin, resistin, IL-6) and anti-inflammatory (adiponectin) adipose tissue-factors and acute phase response proteins (CRP), we (iii) also analyzed the association of these factors with the main outcome parameters. A generalized linear model (GLM) was calculated to adjust for multiple covariates. We used Stata's backward-selection estimation for modeling.
Results
The mean age of the subjects was 32 ± 9 years (range 20–60 yrs), mean body weight 85 ± 12 kg (range 64–115 kg), and mean BMI was 25.7 ± 3.2 kg/m2 (range 19.5–32.8 kg/m2). The baseline subject characteristics (shown in ) were not different between the groups. The effects of the lipid-lowering drugs were as expected and have been reported before (Citation10). In short, simvastatin decreased LDL-C by 41 ± 12%, ezetimibe by 22 ± 10% and the combination of the 2 drugs by 60 ± 10%.
Table I. Baseline characteristics of the randomized patients.*
shows Spearman's rank correlation coefficients between baseline parameters. These coefficients were calculated in order to identify covariates that potentially influence the effects of treatment on the main outcome parameters. hsCRP was significantly positively correlated with IL-6, neutrophil count, age, BMI, body fat and lean body mass, and leptin negatively with HMW adiponectin. IL-6 was significantly positively correlated with neutrophil count, BMI, and lean body mass. The ratio of hsCRP to IL-6 was positively correlated with BMI, body fat, and leptin and negatively with HMW adiponectin. Multiple correlations were observed among the various covariates as seen in .
Table II. Spearman's rank correlation coefficients between baseline parameters.
shows the individual data of the main outcome parameters. shows the results of the effects of treatment on the main outcome parameters. The overall effects were significant for changes in IL-6 (P = 0.008) and borderline significant for hsCRP (P = 0.1) and for the ratio of hsCRP to IL-6 (P = 0.12). Simvastatin decreased IL-6 by a median of 21.8%, hsCRP by 30.1% and increased the ratio by 13.4%. Ezetimibe decreased IL-6 by 5.3% and increased hsCRP by 9.4% and the ratio by 19.8%. The combination of the 2 drugs increased IL-6 by 14.9%, hsCRP by 0.6% and the ratio by 4.2%. The respective P-values of the non-parametric tests can be seen in . Baseline hsCRP and IL-6 values had no influence on the changes under treatment (data not shown).
Figure 1. Baseline serum levels of interleukin-6 (upper panels) and high-sensitivity C-reactive protein (lower panels) in healthy male subjects (N = 24 per group) before and after treatment with (A) simvastatin 40 mg/day, (B) ezetimibe 10 mg/day or (C) simvastatin 40 mg/day + ezetimibe 10 mg/day.
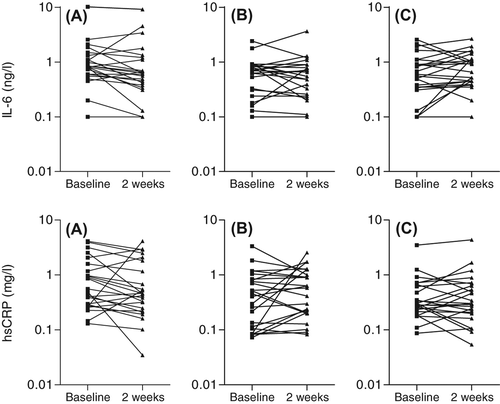
Table III. Effects of treatment on main outcome parameters. Data are medians (interquartile range).
Backward-selection estimation using age, BMI, body fat, lean body mass, neutrophil count, HOMA index, leptin, high-molecular weight (HMW) adiponectin, resistin, creatinine, thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), free thyroxine (fT4), and the respective baseline values of IL-6, hsCRP, and the ratio of hsCRP to IL-6 identified the following significant influences on the results: The change in IL-6 was significantly influenced by treatment (P = 0.008), and slightly by fT3 (P = 0.039), fT4 (P = 0.033), and BMI (P = 0.047). The change in hsCRP was borderline significantly influenced by treatment and fT3 (P = 0.004), and the change in the ratio of hsCRP to IL-6 tended to be significantly influenced by treatment (P = 0.12), fT3 (P < 0.0001) and baseline resistin (P < 0.005).
Discussion
The present study is the first one to investigate whether simvastatin, ezetimibe or their combination have anti-inflammatory effects in subjects with very low baseline cardiovascular risk. We chose to examine hsCRP as a prototypic marker of inflammation and IL-6 due to its regulatory role on CRP synthesis (Citation3). In the parent randomized trial, we have examined the effects of these lipid-lowering drugs on pro- and anti-inflammatory adipokines (leptin, adiponectin, high molecular weight adiponectin and resistin) and we observed no significant effects (Citation11).
Our study has three main findings. First, that simvastatin has potent anti-inflammatory effects in healthy subjects with very low baseline values of inflammation markers; second, that ezetimibe alone does not affect hsCRP and IL-6 levels, and finally that the combination has no anti-inflammatory effects under these conditions. In multivariable analyses, these effects were only influenced by thyroid hormones and body mass index.
Simvastatin 40 mg significantly decreased hsCRP in healthy individuals by ˜30%, similar to the decrease induced in patients at high cardiovascular risk such as patients with dyslipidemia, diabetes mellitus, or coronary heart disease (Citation5,Citation6,Citation12). We also found that in healthy individuals with very low baseline levels of inflammatory markers, simvastatin significantly decreased IL-6 levels, by ˜20%. Our results are in agreement with previous reports in patients with the metabolic syndrome (Citation13), hypercholesterolemia (Citation14), and CAD (Citation15) and in normocholesterolemic patients with sickle cell disease (Citation16).
There were no significant effects of ezetimibe monotherapy on hsCRP. Our results are in agreement to those of Kater et al. in prediabetic subjects (Citation17) and of Krysiak and Okopien in patients with isolated hypercholesterolemia after 12 weeks of treatment (Citation12). However, two studies in Japanese patients with dyslipidemia found a significant decrease in hsCRP with ezetimibe after 4–8 weeks of treatment (Citation18,Citation19). We observed no significant effects of ezetimibe monotherapy on IL-6. Our results are in agreement with two other studies in patients with hypercholesterolemia and pre-diabetes, respectively (Citation12,Citation17).
The question whether the addition of ezetimibe to statin therapy further improves inflammatory markers is still unclear. There was no decrease in hsCRP and IL-6 in the group taking simvastatin combined with ezetimibe in the present study. In accordance with our findings, two studies comparing the effects on inflammatory markers of atorvastatin monotherapy to those of an atorvastatin/ezetimibe combination causing similar LDL cholesterol reductions, found that, while atorvastatin monotherapy decreased inflammatory markers in blood, the combination with ezetimibe did not in patients with hypercholesterolemia (Citation14) and CAD (Citation20), respectively. Our findings are also in agreement to those of Rudowsky et al. (Citation21), who showed that, while simvastatin monotherapy reduces proinflammatory transcription factor NF-kB-binding activity and hsCRP levels, the combination of simvastatin with ezetimibe, resulting in similar LDL-C reduction, does not affect these inflammatory markers. Moreover, recently a potential association between inflammation markers and proatherogenic small, dense LDL (sdLDL) particles has been described (Citation22). In this context, we have previously shown that ezetimibe may increase sdLDL subfractions in healthy men (Citation9).
Some studies in patients with hypercholesterolemia, however, have shown that ezetimibe added to a statin, e.g. atorvastatin 10–80 mg (Citation23), further decreases hsCRP. On the other hand, Pesaro et al. (Citation24) found that co-administration of simvastatin and ezetimibe in patients with CAD had neither an effect on hsCRP nor on IL-6. Dawson et al. (Citation25) showed in patients with abdominal aortic aneurysm no difference between simvastatin monotherapy and simvastatin plus ezetimibe in the plasma levels of IL-6 and hsCRP. Krysiak and Okopien (Citation12) found that in patients with isolated hypercholesterolemia simvastatin and ezetimibe significantly decrease both, hsCRP and IL-6 either alone or in combination.
The hsCRP values in our population were very low at baseline, as expected in healthy young individuals, and the putative clinical benefit of decreasing hsCRP beyond very low levels remains unclear. However, it should be pointed out that there is a continuous association between hsCRP levels and the risk of cardiovascular disease, even at values below 2 mg/l (Citation26). Furthermore, it has recently been shown that even subjects with CRP < 1.25 mg/l benefit from simvastatin treatment (Citation27). Moreover, hsCRP concentrations have been associated with CVD risk, even in healthy adolescents with very low hsCRP levels (< 1 mg/l) (Citation28).
The exact molecular mechanisms mediating the anti-inflammatory effects of statins have not been elucidated yet. Inhibition of prenylation of small-GTP-binding proteins, inhibition of nuclear factor kappa-B as well as modulation of the peroxisome proliferator-activated receptors have all been proposed as possible mechanisms (Citation1). A mechanism for potential anti- inflammatory effects of ezetimibe was never established, neither in vitro nor in vivo. It has been proposed that it may decrease cytokine release due to its action on NPC1L1 transporters located at the membrane of monocytes and macrophages (Citation12). However, monocyte and macrophage expression of NPC1L1 is only about 0.3 ± 0.5% of that found in enterocytes (Citation29), therefore the likelihood that this mechanism is clinically relevant is rather small. As expected, the only covariates in the current study modulating the main associations were adiposity, as shown by associations with body mass index, percent body fat and various adipokines, and thyroid hormones.
Strengths and limitations
Our study has a number of limitations. A 2-week treatment period may have been too short to detect significant changes with ezetimibe, although the anti-inflammatory effects of statins occur already within this time (also shown by Plenge et al. (Citation5)). Other limitations include the fact that no a priori power calculations were made for changes in hsCRP because the primary outcome parameter of the study in the parent trial was change in LDL cholesterol, its post-hoc design and the relatively small sample size. Moreover, the clinical relevance of our findings remains to be established.
Strengths of our study are its randomized design and robust statistical methodology, the blinded measurements of all end-point parameters, the excellent treatment adherence and the use of a “drug-naïve” population, devoid of co-medications or co-morbidities which could potentially alter the results.
Conclusions
In conclusion, our results suggest that simvastatin monotherapy exerts a stronger anti-inflammatory effect compared to a combination of simvastatin with ezetimibe or to ezetimibe monotherapy. Considering that the anti-inflammatory effects of statins have been shown to be important mediators of their clinical benefits (Citation30), off-setting them may attenuate the beneficial effects of statin treatment. Since endpoint studies with ezetimibe are still pending, these findings suggest that treatment with simvastatin alone may be superior to combination therapy with ezetimibe. The findings have thus translational importance for the clinical practice of lipid-lowering therapy. The results should be considered hypothesis-generating and require confirmation in adequately powered larger, prospective studies to verify and extend on these findings.
Acknowledgements
We would like to thank Nadine Spenrath, Doris Vollmar, Cornelia Zwimpfer, and John Chamberlain for their excellent technical assistance.
Declaration of interest: W.K. has served on advisory boards of MSD Sharp & Dohme/Essex and has received consultation honoraria from Pfizer, Bayer and Astra-Zeneca. I.G.B. has received research grants from Bayer Health Care and honoraria and travel expenses from Genzyme, MSD Sharp & Dohme, Novartis and Otsuka. None of the other authors have relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was utilized in the production of this manuscript. The authors alone are responsible for the content and writing of the paper.
Funding: The parent trial (Citation10) was supported in part by an investigator-initiated grant from MSD Sharp & Dohme (Munich, Germany) and the Wilhelm-Doerenkamp Foundation (Cologne, Germany). The present study was sponsored by the Wilhelm-Doerenkamp Foundation (Cologne, Germany). The sponsors had neither an influence on design and conduct of the study, collection and interpretation of the data nor on the preparation of the manuscript.
References
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000; 342:836–43.
- Weinhold B, Ruther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem J. 1997;327:425–9.
- Zhang YX, Cliff WJ, Schoefl GI, Higgins G. Coronary C-reactive protein distribution: its relation to development of atherosclerosis. Atherosclerosis. 1999;145:375–9.
- Plenge JK, Hernandez TL, Weil KM, Poirier P, Grunwald GK, Marcovina SM, Eckel RH. Simvastatin lowers C-reactive protein within 14 days: an effect independent of low-density lipoprotein cholesterol reduction. Circulation. 2002;106:1447–52.
- Strandberg TE, Vanhanen H, Tikkanen MJ. Associations between change in C-reactive protein and serum lipids during statin treatment. Ann Med. 2000;32:579–83.
- Berthold HK, Naini A, Di Mauro S, Hallikainen M, Gylling H, Krone W, Gouni-Berthold I. Effect of ezetimibe and/or simvastatin on coenzyme Q10 levels in plasma: a randomised trial. Drug Saf. 2006;29:703–12.
- Pearson TA, Ballantyne CM, Veltri E, Shah A, Bird S, Lin J, . Pooled analyses of effects on C-reactive protein and low density lipoprotein cholesterol in placebo-controlled trials of ezetimibe monotherapy or ezetimibe added to baseline statin therapy. Am J Cardiol. 2009;103:369–74.
- Berneis K, Rizzo M, Berthold HK, Spinas GA, Krone W, Gouni-Berthold I. Ezetimibe alone or in combination with simvastatin increases small dense low-density lipoproteins in healthy men: a randomized trial. Eur Heart J. 2010;31: 1633–9.
- Gouni-Berthold I, Berthold HK, Gylling H, Hallikainen M, Giannakidou E, Stier S, . Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis. 2008;198: 198–207.
- Gouni-Berthold I, Berthold HK, Chamberland JP, Krone W, Mantzoros CS. Short-term treatment with ezetimibe, simvastatin or their combination does not alter circulating adiponectin, resistin or leptin levels in healthy men. Clin Endocrinol (Oxf). 2008;68:536–41.
- Krysiak R, Okopien B. The effect of ezetimibe and simvastatin on monocyte cytokine release in patients with isolated hypercholesterolemia. J Cardiovasc Pharmacol. 2011;57:505–12.
- Devaraj S, Chan E, Jialal I. Direct demonstration of an antiinflammatory effect of simvastatin in subjects with the metabolic syndrome. J Clin Endocrinol Metab. 2006;91: 4489–96.
- Lee SH, Kang SM, Park S, Jang Y, Chung N, Choi D. The effects of statin monotherapy and low-dose statin/ezetimibe on lipoprotein-associated phospholipase A2. Clin Cardiol. 2011;34:108–12.
- Undas A, Machnik A, Potaczek DP, Wypasek E, Zmudka K, Tracz W. Ezetimibe combined with simvastatin compared with simvastatin alone results in a greater suppression of oxidative stress and enhanced fibrinolysis in patients after acute coronary events. J Cardiovasc Pharmacol. 2011;58: 167–72.
- Hoppe C, Kuypers F, Larkin S, Hagar W, Vichinsky E, Styles L. A pilot study of the short-term use of simvastatin in sickle cell disease: effects on markers of vascular dysfunction. Br J Haematol. 2011;153:655–63.
- Kater AL, Batista MC, Ferreira SR. Synergistic effect of simvastatin and ezetimibe on lipid and pro-inflammatory profiles in pre-diabetic subjects. Diabetol Metab Syndr. 2010;2:34.
- Nozue T, Michishita I, Mizuguchi I. Effects of ezetimibe on remnant-like particle cholesterol, lipoprotein (a), and oxidized low-density lipoprotein in patients with dyslipidemia. J Atheroscler Thromb. 2010;17:37–44.
- Yagi S, Akaike M, Aihara K, Iwase T, Ishikawa K, Yoshida S, . Ezetimibe ameliorates metabolic disorders and microalbuminuria in patients with hypercholesterolemia. J Atheroscler Thromb. 2010;17:173–80.
- Piorkowski M, Fischer S, Stellbaum C, Jaster M, Martus P, Morguet AJ, . Treatment with ezetimibe plus low-dose atorvastatin compared with higher-dose atorvastatin alone: is sufficient cholesterol-lowering enough to inhibit platelets?J Am Coll Cardiol. 2007;49:1035–42.
- Rudofsky G, Reismann P, Groener JB, Djuric Z, Fleming T, Metzner C, . Identical LDL-cholesterol lowering but non-identical effects on NF-kappaB activity: high dose simvastatin vs combination therapy with ezetimibe. Atherosclerosis. 2012;223:190–6.
- Berneis K, Rizzo M, Evans J, Rini GB, Spinas GA, Goedecke JH. Interleukin-18 levels are associated with low-density lipoproteins size. Eur J Clin Invest. 2010;40:54–5.
- Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, . Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107:2409–15.
- Pesaro AE, Serrano CV Jr., Fernandes JL, Cavalcanti AB, Campos AH, Martins HS, . Pleiotropic effects of ezetimibe/simvastatin vs. high dose simvastatin. Int J Cardiol. 2011;158:400–404.
- Dawson JA, Choke E, Loftus IM, Cockerill GW, Thompson MM. A randomised placebo-controlled double-blind trial to evaluate lipid-lowering pharmacotherapy on proteolysis and inflammation in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2011;41:28–35.
- Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40.
- Heart Protection Study Collaborative Group, Jonathan E, Derrick B, Emma L, Sarah P, John D., Jane A, Rory C. C-reactive protein concentration and the vascular benefits of statin therapy: an analysis of 20,536 patients in the Heart Protection Study. Lancet. 2011;377:469–76.
- Wijnstok NJ, Twisk JW, Young IS, Woodside JV, McFarlane C, McEneny J, . Inflammation markers are associated with cardiovascular diseases risk in adolescents: the Young Hearts project 2000. J Adolesc Health. 2010;47:346–51.
- Seedorf U, Engel T, Lueken A, Bode G, Lorkowski S, Assmann G. Cholesterol absorption inhibitor Ezetimibe blocks uptake of oxidized LDL in human macrophages. Biochem Biophys Res Commun. 2004;320:1337–41.
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, . Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–82.