Abstract
Objectives. The impact of myocardial bridge (MB) on left ventricular (LV) systolic synchrony is insufficiently understood. Design. Thirty-five subjects with isolated mid-left, anterior, descending artery (LAD) MB, preserved LV ejection fraction (LVEF > 50%), and otherwise, normal coronary angiogram were identified from 3607 patients who underwent diagnostic coronary angiography and were evaluated by tissue Doppler imaging and real-time three-dimensional echocardiography (RT3DE). Control subjects consisted of 26 age and sex-matched coronary angiographically “normal” subjects. Results. MB patients were characterized by reduced, early, diastolic strain rate in LAD-supplied apical segments (lateral and anterior), with prevalence of LV systolic dyssynchrony of 25.7% (9/35). MB patients were further classified by the medians of MB stenosis and length. For MB stenosis < 52.5%, Class I: length < 17 mm (n = 7), Class II: length ≥ 17 mm (n = 10); for stenosis ≥ 52.5%, Class III: length < 17 mm (n = 10), Class IV: length ≥ 17 mm (n = 8). Binary Logistic regression model revealed that higher MB lesion classification (odds ratio: 4.944, 95%CI 1.174–20.82, P < 0.05) and hypertension (odds ratio: 15.32, 95%CI: 1.252–187.6, P < 0.05) are statistically associated with LV systolic dyssynchrony, which was independent of LV mass. Conclusions. MB in the mid LAD is associated with myocardial dyssynchrony. Hypertensive individuals and those with more severe bridging (determined by length and stenosis) tend to have an increased incidence of dyssynchrony.
Introduction
The anatomical prevalence of myocardial bridge (MB) in general population, detected by autopsy or multi-slice computed tomography, is much higher than those confirmed by coronary angiography (Citation1). On the basis of clinical and histopathological data, coronary segments involving MB are usually spared from atherosclerosis due to a unique, local, hemodynamic microenvironment provided by surrounding myocardium (Citation2). However, coronary segment proximal to MB shows increased atherosclerosis, and is even more severe when the “culprit” MB is longer and thicker (Citation3). So far the clinical significance of MB is controversial. In general, MB is regarded as a benign process. However, the fact that there is an increasing number of case reports, in which MB has been associated with myocardial infarction, depressed LV function, arrhythmias or sudden death, strengthens the notion that MB is an important but yet an unresolved clinical issue.
LV systolic dyssynchrony is commonly seen in patients with heart failure, and is shown to indicate more severe myocardial disease and poor cardiovascular prognosis (Citation4). Recently, real-time three- dimensional echocardiography (RT3DE) has been demonstrated to provide an accurate and reproducible quantification of LV systolic dyssynchrony in various clinical conditions. Among RT3DE-derived parameters, RR interval normalized standard deviation (SD) of time to minimal systolic volume of 16 LV segments (Tmsv-16SD/RR) was proved to be a powerful predictor for LV systolic dyssynchrony (Citation5,Citation6).
Due to the dynamic compression nature of MB on coronary artery, it is reasonable to deduce that MB might have a negative effect on LV function. To our knowledge, the impact of MB on LV systolic synchrony has not been elucidated. Therefore, the objectives of the current study were: 1) to investigate the impact of MB on LV systolic synchrony using tissue Doppler imaging and RT3DE in a carefully defined population with isolated MB located in mid-left, anterior artery (LAD) segment, and with normal LV ejection fraction (LVEF) and 2) to explore potential clinical predictors associated with the development of LV dyssynchrony in this population.
Material and methods
Study participants
From Jun 2008 to Dec 2009 and Mar 2012 to Jun 2012, a total of 3607 patients suspected of coronary heart disease (CHD) had undergone diagnostic coronary angiography in our center. Among them, 137 cases with bridged coronary segments were identified, and 50 cases (36.5%) were associated with angiographically confirmed atherosclerotic lesions (fixed stenosis > 50%). Among the 137 identified MBs, 91.2% (125/137) located in LAD (117 lesions in mid-LAD, 8 lesions in distal-LAD). We enrolled patients with the following criteria: 1) LVEF > 50%; 2) isolated MB involving mid-LAD segment and otherwise normal coronary angiogram. The exclusion criteria were: 1) existence of abnormality in coronary angiogram with fixed stenosis of > 50%; 2) atrial fibrillation; 3) hypertrophic cardiomyopathy; 4) valvular heart disease; 5) bundle branch block; 6) cancer and hematological disorders; 7) other conditions that resulted in suboptimal quality during RT3DE evaluation. Finally, 35 patients who met the above criteria were enrolled for echocardiographic analysis. Control group consisted of 26 age and sex-matched subjects suspected of CHD but with normal coronary angiogram.
The degree of bridged segment narrowing during systole was quantified as following: (systolic diameter of the artery directly distal to bridge segment minus systolic diameter of the bridged segment)/ systolic diameter of the artery directly proximal to bridged segment × 100%. The systolic narrowing of the coronary segment was classified according to Nobel grading, as severe (> 75%, score = 3), moderate (50%–75%, score = 2) and mild (< 50%, score = 1). To explore the interaction between MB stenosis and length, we classified MB patients into four groups by the medians of MB stenosis (52.5%) and length (17 mm): for patients with stenosis < 52.5%, Class I: length < 17 mm (n = 7), Class II: length ≥ 17 mm (n = 10); for stenosis ≥ 52.5%, Class III: length < 17 mm (n = 10), Class IV: length ≥ 17 mm (n = 8). The research protocol was reviewed and approved by the ethical committee of the Pingjin Hospital. All participants provided informed consent, and the study was conducted according to the principles of the Declaration of Helsinki and subsequent amendments.
Echocardiography and data analysis
All participants underwent standard transthoracic M-mode, 2D pulsed Doppler, tissue Doppler imaging, and RT3DE analysis, which were performed by Philips iE33 system (Phillips, Andover, MA, USA). LV mass was calculated using 2D linear formula recommended by American Society of Echocardiography (Citation7). Standardized LV mass was calculated by dividing it to body surface area, which was according to Du Bois formula: body surface area = body weight (Kg)0.425 × height (cm)0.725 × 0.007184.
For tissue Doppler imaging, a S5-1 phased array transducer (2.0–3.5 MHz) was used with optimized pulse-repetition frequency, sector size, color saturation and depth. At least three consecutive beats were recorded and analyzed off-line by Q-Lab 3DQ Advanced (version 6.0, Philips, Andover, MA, USA). We calculated longitudinal tissue velocity, strain and strain rate (corresponding to early diastolic, late diastolic and systolic phase) of standard 17 LV segments (six basal, six middle, and five apical segments) according to American Heart Association and American Society of Echocardiography segmentation schema (Citation8). Among the 17 LV segments, 7 segments [apical-lateral (Apical-L), basal-anterior (Basal-A), mid-anterior (Mid-A), apical-anterior (Apical-A), basal-anteroseptal (Basal-AS), mid- anteroseptal (Mid-AS) and apical-septal (Apical-S)] are considered as LAD specific, based on recommendation by American Heart Association and recent PET-CT-based analysis (Citation8,Citation9).
For RT3DE evaluation, pyramidal full volume images of LV were acquired with a matrix-array transducer (X3-1, 1.9/3.8 MHz), and analyzed off-line by Q-Lab 3DQ Advanced. “Casts” of the LV endocardium were automatically obtained from which global LV volumes versus time curves were derived. From these curves, the end-diastolic volume (EDV), and end-systolic volume (ESV), stroke volume (SV), and LVEF were then calculated. From 17 regional segment curves, the regional ejection time, defined as the Tmsv was automatically calculated. To assess LV systolic dyssynchrony, the SD of the regional volume time curves was obtained using 16 segments (Tmsv-16SD, excluding the apical segment), 12 segments (Tmsv-12SD, 6 basal segments plus 6 middle segments), and 6 segments (Tmsv-6SD, 6 basal segments) in each patients. Systolic dyssynchrony index (SDI) could be calculated as the SD of these Tmsv values of a single heart beat (Tmsv/RR) as percentage of Tmsv relative to the averaged RR interval. In addition to the Tmsv indices, the maximal difference of Tmsv was calculated as well, generating the following additional indices of dyssynchrony: the maximal difference or range of Tmsv among 16 segments, among the 6 basal and 6 middle segments; and among the 6 basal segments. Finally, the difference of Tmsv between the basal septal and basal lateral segments and the difference of Tmsv between the basal septal and basal posterior segments were calculated as well (Citation10–12). To determine inter- and intra-observer variability for RT3DE evaluation, 20 patients were randomly selected, who were evaluated by two independent, blinded investigators and by the same observer within 5 days. The inter- and intra-observer variability for Tmsv-16SD was 6.2% and 5.3%, respectively, and LVEF was 6.7 and 5.0%, respectively.
Statistical analysis
The Shapiro–Wilk test was used to assess normality of quantitative variables. Continuous variables with normal distributions are presented as mean ± SD or median and quartiles, unless otherwise specified. For comparisons between two groups, unpaired Student's t test, or Mann–Whitney U test was used. For multiple group comparisons, one way analysis of variance (one way ANOVA, followed by Tukey's multiple comparisons) or Kruskal–Wallis test (followed by Dunns’ multiple comparisons) was used. Correlations between variables were calculated using Pearson's (continuous variables) or Spearman (ordered categorical variables) coefficient. Categorical data were compared with the use of Fisher's exact test. Predictors of LV systolic dyssynchrony in patients with MB were assessed using univariate followed by multivariate logistic regression analyses. Receiver operator characteristic (ROC) curves were plotted to assess the accuracy of clinical parameters for identifying LV systolic dyssynchrony, and to identify the optimal cutoff points, which was defined as the point with the shortest distance from point (0,1) (top left corner). The statistical analysis was performed using SPSS version 15.0 (SPSS, Chicago, IL). A two-tailed P value < 0.05 was considered statistical significance.
Results
General clinical characteristics of participants
As shown in , gender distribution (female, 12/26 vs. 17/35), mean age (year, 51.5 ± 6.9 vs. 52.8 ± 8.0), and history of hypertension (10/26 vs. 15/35) are comparable between control group and MB group. When compared with controls, trans-mitral A peak velocity is significantly greater and E/A ratio is smaller in the MB group.
Table I. General characteristics of participants.
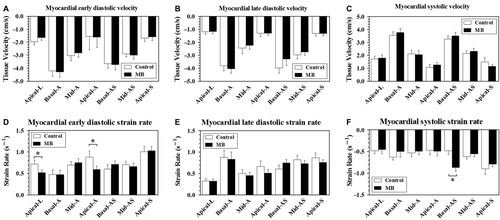
shows key parameters derived from tissue Doppler imaging of LAD supplied 7 LV segments. There are no differences in terms of diastolic (both early and late) and systolic tissue velocity (). In LV supplied apical segments, especially apical lateral and apical anterior segments, early diastolic strain rate was significant reduced (), whereas no statistical differences of strain value of regional segments were detected (data not shown).Interestingly, basal anteroseptal segment, with proximal location to mid-LAD MB lesions, exhibited increased systolic strain rate ().
Figure 1. Tissue Doppler Imaging of left anterior descending artery (LAD) supplied left ventricular segments (17 segments model) in patients with and without mid-LAD myocardial bridge. The bar above each column indicates standard error of mean. A to C shows tissue velocity of 7 LAD-specific segments, and D to F show the corresponding strain rate values. MB, myocardial bridge; Apical-L, apical lateral; Basal-A, basal anterior; Mid-A, mid anterior; Apical-A, apical anterior; Basal-AS, basal anteroseptal; Mid-AS, mid anteroseptal; Apical-S, apical septal. *P < 0.05.
The magnitude of dyssynchrony defined by Tmsv-16SD and RR interval adjusted Tmsv-16SD (Tmsv-16SD/RR) was significantly greater in MB than normal controls (). To further define LV systolic dyssynchrony, we used a cutoff value of Tmsv-16SD/RR of 1.91% to define the presence of LV systolic dyssynchrony, which was derived from + 2SD above the mean value of 26 controls (1.09% + 0.82%). By this definition, 1 (3.85%) in control group, and 9 (25.7%) in MB group presented with LV systolic dyssynchrony ().
Figure 2. Relationships between myocardial bridge angiographic features [stenosis (A) and length (B)], their interactions (C) and left ventricular systolic synchrony. The dash line started from Y axis indicates the cutoff value of 1.91% derived from control group to define the presence of LV systolic dyssynchrony. Solid line in each scatter plot indicating the median. Tmsv-16SD/RR, the time-to-minimum systolic volume corrected by RR interval. SDI, systolic dyssynchrony index. *P < 0.05, **P < 0.01, ***P < 0.001.
![Figure 2. Relationships between myocardial bridge angiographic features [stenosis (A) and length (B)], their interactions (C) and left ventricular systolic synchrony. The dash line started from Y axis indicates the cutoff value of 1.91% derived from control group to define the presence of LV systolic dyssynchrony. Solid line in each scatter plot indicating the median. Tmsv-16SD/RR, the time-to-minimum systolic volume corrected by RR interval. SDI, systolic dyssynchrony index. *P < 0.05, **P < 0.01, ***P < 0.001.](/cms/asset/7728e3c8-877e-4a7d-b051-119630f69874/icdv_a_736635_f0002_b.gif)
Relationship between MB angiographic features (stenosis and length) and LV mechanical function
In the MB group, the median of percent stenosis is 52.5% (minimum 20% to maximum 90%). The median of segment length is 17.0 mm (minimum 10.4 mm to maximum 28.4 mm). As shown in msv-16SD/RR markedly and significantly increased from control group (shown as “No MB”) to group with percent stenosis ≥ 52.5%, being equal to 1.09% ± 0.41%, 1.24% ± 0.37% and 2.29% ± 1.44% (P < 0.01, by one way ANOVA), respectively. Similar increasing trend was observed in , which shows the effect of MB length dichotomy on LV synchrony. In addition, there seems to be an association between MB lesion classification and degree of LV dyssynchrony ().
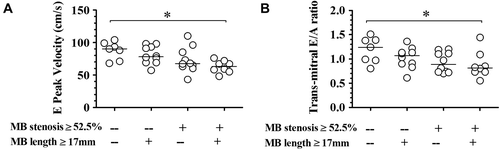
The relationship between MB lesion classification and 2D-echocardiography-derived parameters is shown in . By Spearman rank correlation analysis, MB lesion classification is negatively correlated with parameters concerning LV diastolic function (, for E peak velocity, r = − 0.563, P < 0.001; for E/A ratio, r = − 0.436, P < 0.01).
Figure 3. Interaction of myocardial bridge percent stenosis and length on trans-mitral E wave peak velocity (A) and E/A ratio (B). Solid line in each scatter plot indicating the median. MB, myocardial bridge. *P < 0.05.
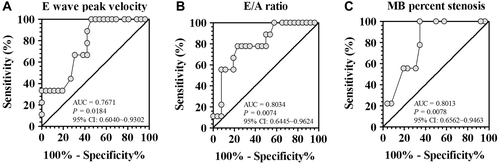
By using ROC curve analysis E wave peak velocity (), E/A ratio and MB percent stenosis showed statistically significant and acceptable discrimination (refers to the value of area under curve between 0.7 and 0.8 (Citation13)) between patients with and without LV systolic dyssynchrony. For E wave peak velocity, the optimal cutoff value is 76.5 cm/s, with sensitivity of 100% and specificity of 53.85%. For E/A ratio, the optimal cutoff value is 0.84, with sensitivity of 77.78% and specificity of 76.92%. For MB percent stenosis, the optimal cutoff value is 52.5%, with sensitivity of 100% and specificity of 65.38%.
Figure 4. Receiver operator characteristic curve analysis of trans-mitral E wave peak velocity (A), E/A ratio (B) and myocardial bridge stenosis (C) for the diagnostic value of left ventricular dyssynchrony detected by real-time three dimensional echocardiography. AUC, area under curve; CI, confidence interval; MB, myocardial bridge.
Using Person or Spearmen correlation analysis, neither bridge stenosis nor length correlated with RT3DE parameters [3D-end diastolic volume (EDV), 3D-end systolic volume (ESV), 3D-LV ejection fraction (EF) and 3D-stroke volume (SV)]. However, when we selected cases in MB group with percent stenosis ≥ 50%, MB length is positively correlated with 3D-ESV (Spearman r = 0.469, P < 0.05), 3D-EDV (Spearman r = 0.460, P < 0.05), and 3D-SV (Spearman r = 0.397, P = 0.068), indicating a threshold stenosis value for bridge length to take effect.
To explore potential clinical features associated with the development of LV systolic dyssynchrony, logistic regression analysis was carried out. Predictors of LV systolic dyssynchrony in MB group with statistical significance in univariate analysis were hypertension, MB lesion classification, and E peak velocity and E/A ratio (). By multivariate analysis (), MB lesion classification (odds ratio: 4.944, 95% CI 1.174–20.82, P < 0.05) and the existence of hypertension (odds ratio: 15.32, 95% CI: 1.252–187.6, P < 0.05) are two independent predictors for LV systolic dyssynchrony.
Table II. Univariate logistic regression analyses.
Table III. Multivariate logistic regression analyses.
Discussion
The present work, for the first time, demonstrated that in a carefully defined population with isolated, mid-LAD MB and normal LVEF, the prevalence of LV systolic dyssynchrony is 25.7% (9/35). In addition, patients who presented with LV systolic dyssynchrony all have critical systolic compression (≥ 52.5%). Moreover, the stenosis and length of MB presented a synergistic effect on the development of LV systolic dyssynchrony. Their interaction, in combination with the existence of hypertension, is an independent determinant for LV systolic dyssynchrony in patients with isolated mid-LAD MB and normal LVEF.
Previous work has shown that in patients with ≥ 50% systolic compression, MB is associated with abnormalities in diastolic coronary blood flow reserve (Citation14), vasoreactivity (Citation15), coronary perfusion defect (Citation16,Citation17), and related clinical symptoms (Citation18). Therefore, it is conceivable that MB with critical, systolic narrowing contributes to the development of LV systolic dyssynchrony. We show here that MB patients with LV systolic dyssynchrony are also associated with decreased, early, diastolic strain rate in LAD-supplied apical segments, and alterations in global diastolic function (decreased transmitral E and E/A). These evidence further strengthen the previous hypothesis that systolic inhomogeneity could lead to prolonged, inhomogeneous, and presumably incomplete relaxation, which may in turn contribute to reduced maximal velocity of filling and delay in the peak velocity of filling, thus impairing rapid filling of the LV during early diastole (Citation19).
Interestingly, we found that systolic strain rate of basal-anteroseptal segment is enhanced in patients with MB. As we only chose patients with mid-LAD bridging, we speculated that enhanced systolic strain rate in this region, which is supplied by proximal LAD (also proximal to bridged lesion), is probably due to increased perfusion pressure and enhanced systolic performance, because previous work showed that intracoronary pressure proximal to bridge lesion is higher than normal segment (Citation20).
In clinical practice, MB is frequently associated with a high prevalence of co-existing coronary atherosclerotic lesions and, and some locations of MB (distal LAD, right coronary and left circumferential artery) may have minimal hemodynamic consequences on LV function. For example, in a retrospective study in China involving 37105 patients receiving selective coronary angiography, MB prevalence is 2.7% (1002/37105), whereas the co-existence of MB and CHD could be up to 42.2% (423/1002) (Citation21). In addition, multiple sites of MB may have occurred more frequently in patients with hypertrophic obstructive cardiomyopathy (Citation22). Therefore, in the present work, to explore the exact contribution of MB on LV systolic synchrony, a vigorous inclusion/exclusion criterion was used. Moreover, our results indicated that RT3DE could identify LV systolic dyssynchrony in 25.7% patients with isolated MB and preserved LVEF in resting state, and this finding is particularly useful for routine clinical practice for risk stratification in patients suffering from bridged coronary artery.
An important finding of our results is that the percent stenosis and length of MB wound have synergistic effect on LV mechanical function. It is conceivable that these two morphological features of MB would work in concert to aggravate dynamic compression-induced myocardial ischemia. This phenomenon is particularly true when percent stenosis of MB exceeds 52.5% (the median of MB group), at which threshold the length of MB presented an obvious effect on LV end-systolic, end-diastolic, and stroke volumes. A second determinant of dyssynchrony is the existence of hypertension, which is in line with a recent study showing that LV systolic dyssynchrony frequently coexisted with diastolic abnormalities in hypertensive patients with a normal LVEF (Citation23). Recent studies have provided evidence indicating that LV dyssynchrony in hypertensive patients with preserved LVEF is a dynamic process and could be responsive to blood pressure-lowering drug therapy (Citation24). It should be noted that previous work provides evidence indicating that increased LV mass is related to greater extent of dyssynchrony (Citation25). Although we did not find a relationship between LV mass and dyssynchrony, the relative contribution of LV mass to LV dyssynchrony in MB patients remains to be established in larger cohort. Anyway, these results support the hypothesis that blood pressure control could be an important therapy for ameliorating LV dyssynchrony in patients with MB, and might have a long-term clinical benefit.
Our study has the following limitations. First, the ultimate sample size is relatively small because of a rigorous inclusion/exclusion criterion, which inevitably limited the precision of our estimates of odds ratio, as well as the statistical power of the present work and its generalizability. Second, the observational nature of the present cross- sectional study did not allow us to examine the cause and effect relationship between MB and LV dyssynchrony. Third, follow-up data, concerning the relationship between LV dyssynchrony and cardiovascular outcomes in patients with MB, are lacking.
In conclusion, using tissue Doppler imaging and RT3DE, we demonstrate that MB in the mid LAD is associated with myocardial dyssynchrony. Hypertensive individuals and those with more severe bridging (determined by length and stenosis) tend to have an increased incidence of dyssynchrony.
Notice of Correction
The version of this article published online ahead of print on 1 November 2012 contained an error in the Declaration of interest section. The correct Declaration of interest has been included in this version.
Declaration of interest: This work was supported by an intramural research program (WY200901) from Logistics University of Chinese People's Armed Police Forces, Tianjin Municipal Science and Technology Committee (09ZCZDSF04200 and 11JCYBJC12000) and Natural Science Foundation of China (81170238 and 81070121).
References
- Mohlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002;106:2616–22.
- Chatzizisis YS, Giannoglou GD. Myocardial bridges spared from atherosclerosis: overview of the underlying mechanisms. Can J Cardiol. 2009;25:219–222.
- Ishikawa Y, Akasaka Y, Suzuki K, Fujiwara M, Ogawa T, Yamazaki K, . Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation. 2009;120: 376–83.
- Antoni ML, Boden H, Hoogslag GE, Ewe SH, Auger D, Holman ER, . Prevalence of dyssynchrony and relation with long-term outcome in patients after acute myocardial infarction. Am J Cardiol. 2011;108:1689–96.
- Samir R, Tawfik M, El Missiri AM, El Shahid G, Maaty MA, El Sayed M. Assessment of left ventricular mechanical dyssynchrony using real time three-dimensional echocardiography: a comparative study to Doppler tissue imaging. Echocardiography. 2012;29:173–81.
- Caselli S, Di Paolo FM, Pisicchio C, Di Pietro R, Quattrini FM, Di Giacinto B, . Three-dimensional echocardiographic characterization of left ventricular remodeling in Olympic athletes. Am J Cardiol. 2011;108:141–7.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002; 105:539–42.
- Pereztol-Valdes O, Candell-Riera J, Santana-Boado C, Angel J, Aguade-Bruix S, Castell-Conesa J, . Correspondence between left ventricular 17 myocardial segments and coronary arteries. Eur Heart J. 2005;26:2637–43.
- Kapetanakis S, Kearney MT, Siva A, Gall N, Cooklin M, Monaghan MJ. Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation. 2005;112: 992–1000.
- Zhang Q, Yu CM, Fung JW, Zhang Y, Chan YS, Chan HC, . Assessment of the effect of cardiac resynchronization therapy on intraventricular mechanical synchronicity by regional volumetric changes. Am J Cardiol. 2005;95:126–9.
- Takeuchi M, Jacobs A, Sugeng L, Nishikage T, Nakai H, Weinert L, . Assessment of left ventricular dyssynchrony with real-time 3-dimensional echocardiography: comparison with Doppler tissue imaging. J Am Soc Echocardiogr. 2007; 20:1321–9.
- LaValley MP. Logistic regression. Circulation. 2008;117: 2395–99.
- Escaned J, Cortes J, Flores A, Goicolea J, Alfonso F, Hernandez R, . Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol. 2003;42: 226–33.
- Herrmann J, Higano ST, Lenon RJ, Rihal CS, Lerman A. Myocardial bridging is associated with alteration in coronary vasoreactivity. Eur Heart J. 2004;25:2134–42.
- Gawor R, Kusmierek J, Plachcinska A, Bienkiewicz M, Drozdz J, Piotrowski G, Chizynski K. Myocardial perfusion GSPECT imaging in patients with myocardial bridging. J Nucl Cardiol. 2011;18:1059–65.
- Tang K, Wang L, Shi R, Zheng X, Li T, Zhao X, Lu R. The role of myocardial perfusion imaging in evaluating patients with myocardial bridging. J Nucl Cardiol. 2011; 18:117–22.
- Mookadam F, Green J, Holmes D, Moustafa SE, Rihal C. Clinical relevance of myocardial bridging severity: single center experience. Eur J Clin Invest. 2009;39:110–15.
- Perrone-Filardi P, Bacharach SL, Dilsizian V, Bonow RO. Effects of regional systolic asynchrony on left ventricular global diastolic function in patients with coronary artery disease. J Am Coll Cardiol. 1992;19:739–44.
- Ge J, Erbel R, Gorge G, Haude M, Meyer J. High wall shear stress proximal to myocardial bridging and atherosclerosis: intracoronary ultrasound and pressure measurements. Br Heart J. 1995;73:462–5.
- Li JJ, Shang ZL, Yao M, Li J, Yang YJ, Chen JL, . Angiographic prevalence of myocardial bridging in a defined very large number of Chinese patients with chest pain. Chin Med J (Engl). 2008;121:405–8.
- Basso C, Thiene G, Mackey-Bojack S, Frigo AC, Corrado D, Maron BJ. Myocardial bridging, a frequent component of the hypertrophic cardiomyopathy phenotype, lacks systematic association with sudden cardiac death. Eur Heart J. 2009;30:1627–34.
- Yu CM, Zhang Q, Yip GW, Lee PW, Kum LC, Lam YY, Fung JW. Diastolic and systolic asynchrony in patients with diastolic heart failure: a common but ignored condition. J Am Coll Cardiol. 2007;49:97–105.
- Lee AP, Song JK, Yip GW, Zhang Q, Zhu TG, Li C, . Importance of dynamic dyssynchrony in the occurrence of hypertensive heart failure with normal ejection fraction. Eur Heart J. 2010;31:2642–9.
- Rosen BD, Fernandes VR, Nasir K, Helle-Valle T, Jerosch-Herold M, Bluemke DA, . Age, increased left ventricular mass, and lower regional myocardial perfusion are related to greater extent of myocardial dyssynchrony in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:859–66.