Abstract
Objectives. Stroke following cardiac surgery may occur either in association with surgery (early) or occur postoperatively (delayed). The hemispheric distribution of lesions may provide information about embolic routes, which was analyzed here. Design. In 10,809 patients undergoing cardiac surgery, early (n = 223) and delayed stroke (n = 116) were explored. Symptoms and computed tomography findings were evaluated to categorize hemispheric distributions. This was compared with pre- and intra-operative characteristics and survival, using logistic regression and Kaplan-Meier statistics. Results. Early stroke had preponderance for the right rather than the left hemisphere (P = 0.009), whereas delayed stroke had a uniform distribution. Several intraoperative variables predicted the development of bilateral stroke compared with its unilateral counterpart. At multivariable analysis, the use of tranexamic acid was associated with bilateral stroke (P = 0.017), but was also associated with right rather than left-hemispheric stroke (P = 0.001). Bilateral lesions dramatically impaired survival versus those with unilateral lesions (P < 0.001). There was no survival difference between left and right-hemispheric stroke. Conclusions. When stroke, after cardiac surgery, is subdivided into early and delayed forms, it becomes evident that early, but not delayed stroke, demonstrates a hemispheric side difference. The preponderance for right-hemispheric lesions may indicate embolic mechanisms routed via the brachiocephalic trunk.
Introduction
Stroke is a serious complication with respect to cardiac surgery and contributes to both mortality and morbidity (Citation1,Citation2). Previous studies have listed a number of risk factors for stroke, such as advanced age, female gender, history of stroke, and atherosclerosis (Citation1,Citation3). Mechanisms explained by hypoperfusion (Citation4) and embolization are mostly referred. Embolism, during surgery, involves gas (Citation3) and particles from the ascending aorta (Citation5). In the postoperative period, atrial fibrillation is another embolic mechanism (Citation6). The difference between stroke occurring intraoperatively (early) and that occurring in the postoperative period (delayed) has been emphasized in previous reports (Citation6–9).
The anatomical distribution of lesions at stroke may reflect underlying mechanisms. In our previous – but smaller – studies, we reported that early stroke in cardiac surgery had preponderance for the right hemisphere, evaluated from both symptoms (Citation10) and verified from computed tomography (CT) (Citation11). A hypothesis about embolic routes suggested particles being tangetially expelled into the brachiocephalic trunk rather than perpendicularly into the left common carotid artery (Citation10,Citation11). In opposition, Weinstein presented contradictory results with a dominance of left-hemispheric lesions, assumed to be caused by stream jets from the aortic cannula directing particles to the left hemisphere (Citation12). Our findings of a right-hemispheric preponderance have been supported in more recent studies (Citation1,Citation13,Citation14). In addition to unilateral lesions, up to 30% of the patients were diagnosed with bilateral lesions (Citation1,Citation11). It is here postulated that these groups of disease differ in characteristics and potential mechanisms.
The aim of the present study was to analyze the hemispheric distribution of early and delayed stroke, to explore potential embolic routes, and to be compared with patient characteristics and survival. A large cohort of patients undergoing cardiac surgery was required for this ambition, with subdivision into right versus left hemispheric and bilateral cerebral stroke.
Materials and methods
Study cohort and stroke definitions
From January 1994 to December 2004, data were collected from all adult patients operated at the Cardiothoracic Surgery Department, Heart Center, Umeå University Hospital. Patients operated more recently were not included, for two reasons. First, the database changed character with less information about stroke symptoms. Second, the present study addresses survival which becomes more precise with longer follow-up. The study protocol was approved by the local ethics committee. A small part of the analyzed cohort was included in two of our previous studies (Citation10,Citation11). Moreover, in a more recent study, restrained to coronary bypass surgery, risk factors and survival associated with early and delayed stroke were investigated (Citation8). In the present study, the distribution of hemispheric lesions is, instead, analyzed. For this purpose, a much wider patient inclusion was required rather than being restrained to coronary procedures only. All patients in this study have cardiopulmonary bypass in common. The cohort was subdivided by a procedure type. The ‘vascular’ group included aortic aneurysm or dissections operated on using circulatory arrest and exposed to deep hypothermia of 25°C or colder. The ‘cardiac-type’ group contained all other patients operated with the use of cardiopulmonary bypass, typically including aortic cross clamping. Patients undergoing off-pump coronary bypass were thus excluded. Eight patients underwent deep hypothermic arrest for reasons other than aortic procedures, and were also excluded.
A postoperative evaluation is required for stroke diagnosis. Among patients dying within 24 hours following surgery this evaluation was unsecure. These patients were excluded from this analysis (n = 118), among which a few stroke events may have occurred. Patients with short episodes of neurological symptoms (i.e., transient ischemic attacks) were also rejected (n = 28). These incidences tended to be, only briefly, summarized in patient records. Some patients had neurological deficits explained by other etiologies (i.e., preoperative symptoms, peripheral mechanism, or global ischemia due to heart-lung resuscitation) and were also excluded (n = 64). Fourteen non-stroke patients of foreign citizenship were excluded from the cohort because their survival data were unknown. In total, the study included 10,809 patients.
Clinical variables were prospectively collected from the time of referral to hospital discharge, by surgeons, anesthesiologists, intensivists, perfusionists, and nurses. A total of 43 variables were here extracted for analysis and are listed in . The database also contained 17 unique variables describing neurological complications (not listed in ). Based on these variables, patients with any type of neurological symptoms were extracted (n = 720). Patient records were reviewed in detail according to a protocol to confirm the diagnosis and to separate the patients into early and delayed stroke groups. Stroke definitions were according to routine guidelines (Citation15,Citation16) and encountered any new focal or global neurologic deficit lasting for more than 24 hours. Patients who did not meet the stroke criteria were grouped together with control subjects. Stroke criteria were fulfilled in 339 patients. The control group contained 10,470 patients. Early stroke was defined as symptoms observed at extubation (n = 223), whereas delayed stroke followed upon an identified symptom-free interval after extubation (n = 116). For 82% of the stroke patients, a computer tomography was performed, and a neurologist was consulted in 50% of the events. Stroke appearing after discharge was not considered in this study.
Table I. Patient demographics and mode of variable categorization.
The hemispheric distribution of stroke lesions was recorded according to symptoms and CT- findings. These were subdivided into left hemispheric (n = 106), right hemispheric (n = 127), and bilateral (n = 35) findings. For some patients with a clinically verified stroke, an exact hemispheric location could not be concluded (n = 71). Among these patients, isolated cerebellar (n = 5) or brainstem (n = 2) lesions were discovered, whereas the remaining patients were anatomically non-conclusive. Hence, the present analysis focused on cerebral stroke only, with a verified hemispheric subdivision. The size of lesions was not measured, nor was their sub-anatomical location considered.
Database definitions
The tested variables are shown in . During the period of inclusion the database remained consistent. Preoperative variables had their definitions according to contemporary risk scores; Parsonet, Higgins, and Euroscore. Left ventricular function was assessed by either ventriculography and/or echocardiography, graded as; good, reduced, or poor. This subdivision approximately corresponded to ejection fractions of: > 50%, 30–50%, and < 30%, respectively. The variables ‘tranexamic acid’ and ‘aprotinin’ referred to drugs administered in the operating room.
Data on all-cause mortality were collected from the Swedish population registry, encountering the unique 10-digit national identification number. Survival had no missing data, with knowledge about the exact day of death. The follow-up was closed on May 6th, 2010.
Statistics
It was presumed that the surgical and clinical management during the 11-year inclusion period differed. Therefore, the variable ‘period of surgery’ was introduced to account for possible variation over time (). All continuous variables were multi-level categorized to explore non-linear characteristics (). The categorized variables were checked against their numerical counterpart in logistic regressions. Deviant results were analyzed. Obvious phenomena of co-linearity between variables were avoided, a typical example being surgery time which includes time during CPB. Instead, CPB time was subtracted from surgery time to create a new independent variable. Correlation matrices were reviewed and considered during the process. Missing categorical data were compensated by introducing an inert category which was tested in the analysis. Missing numeric variables were handled by casewise deletion. For most variables the occurrence of missing data was modest, and these actions had no obvious effects on presented results.
Deviation from equal distribution between left and right hemispheric stroke was tested using the binominal test. Variables with association to stroke were extracted by univariate logistic regression to present their odds ratio (OR) and 95% confidence intervals (CI). The distribution of hemispheric lesions was analyzed by multivariable logistic regressions, applied manually in a backward conditional mode. The Wald value is given for predictive influence. The obtained models were tested on the patient cohort and the calculated probabilities were reviewed by receiver-operating characteristics (ROC). Kaplan–Meier estimates and log-rank testing were performed for mortality-rate comparisons. The statistical analyses were performed using SPSS 18.0 software (SPSS Inc, Chicago, IL). A P value ≤ 0.05 was considered significant.
Results
Overall stroke
Patient characteristics are described in . The overall stroke rate for all patients was 3.1%, subdivided into 2.9% and 19.2% for cardiac type and vascular procedures, respectively. Early stroke accounted for 2.1% of the entire cohort and delayed stroke for 1.1%. Delayed stroke occurred at a median of 3.5 days after surgery. The radiological findings were near, exclusively, ischemic. Only three stroke patients (1.1%) presented CT with signs of hemorrhage. Two of these patients, both with early stroke, were found to have small hemorrhages in the cerebellum and whereas their neurologic symptoms were more likely explained by concurrent ischemic lesions which were not yet visualized by the CT scan. The third patient had a delayed intracerebral hemorrhage 18 days after surgery which occurred during treatment with extracorporeal membrane oxygenation.
Hemispheric distribution
For early stroke there was a hemispheric side difference, with preponderance for right-sided lesions (P = 0.009, ). This was confirmed significant for cardiac-type operations. A similar trend was observed for the vascular group but the number of unilateral events were few and hence with low statistical power. Delayed stroke did not present a hemispheric side difference, and this pattern differed from early stroke (P = 0.011 and P = 0.019, for all patients and cardiac-type procedures, respectively). If early and delayed stroke had been grouped together no significant side difference was confirmed, with a P value of 0.190 for all procedures and P = 0.196 for cardiac-type procedures only. For vascular procedures the number of patients was few, which hampered the statistical comparison between early and delayed stroke.
Table II. Hemispheric distribution of stroke.
Within the group of cardiac-type procedures early stroke was mainly unilateral (). For vascular procedures, bilateral findings were more frequent compared to the cardiac-type group (P = 0.039). Moreover, the distribution between unilateral and bilateral lesions differed between early and delayed stroke. Bilateral lesions were fewer in the delayed-stroke group which was observed for all patients and for cardiac-type procedures (P = 0.001 and P = 0.006, respectively).
Characteristics associated with bilateral versus unilateral lesions
The difference between bilateral and unilateral lesions is explored in . A number of variables were found related to the occurrence of bilateral hemispheric stroke, versus its unilateral counterpart. Vascular procedures were confirmed to be associated with bilateral lesions. Similarly, impaired aortic quality and variables suggesting complex and long-duration procedures indicated the same effect. In this analysis, the administration of tranexamic acid unexpectedly evolved in relation to bilateral lesions and presented a strong significance level. At multivariable analysis tranexamic acid remained independently associated, together with some other variables listed in . With only categorized variables used at multivariate mode, the requirement of inotropic support and the use of tranexamic acid in the operating room predicted the occurrence of bilateral lesions. When continuous variables were handled numerically in the analysis, rather than categorized, indicators of volume load and bleeding were added to the list. Noteworthy, the variable ‘vascular procedure’ was not significantly related to bilateral lesions at multivariable testing. Moreover, an inverse relationship was found between infused volume and operative bleeding (). The implication suggests that bilateral lesions occur when the bleeding is high and the infused volume is low. The logistic model correctly predicted 84.8% of the events, associated with a Nagelkerke-R2 value of 0.272. The ROC analysis gave an area-under-curve of 0.76 (CI: 0.66–0.86, ).
Figure 1. ROC curves describing the results of multivariable logistic regressions applied on the patient cohort. See text for further details and interpretations.
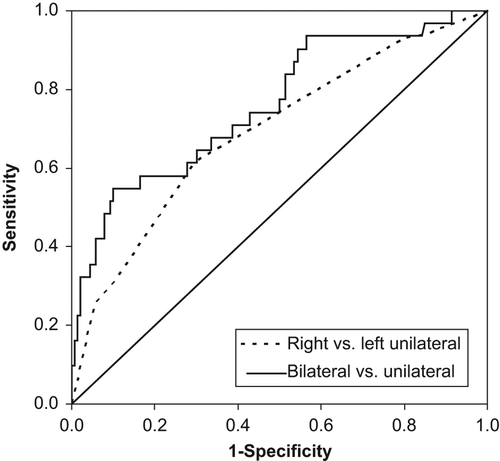
Table III. Variables associated with hemispheric differences for early stroke at univariate testing.
Table IV. Hemispheric differences for early stroke at multivariable testing.
Characteristics associated with right versus left unilateral lesions
The potential difference between right- and left-hemispheric lesions was analyzed. At univariate level, an increased volume load at surgery was associated with right-hemispheric lesions versus its contralateral side (). Administration of tranexamic acid indicated the same effect. Multivariable testing supported the independent role of tranexamic acid in this context but not the influence from infused volume. Instead, the role of volume requirements at surgery was taken over statistically by the variable complicated weaning from CPB. The CPB weaning problem coincided with left-hemispheric lesions (). The predictive power of this model was moderate, with a Nagelkerke R2 of 0.153 to correctly predict 65.0% of the events. At ROC presentation the area-under-curve was 0.69 (CI: 0.60–0.78, ). Poor aortic quality showed a weak trend in the same direction, to address an embolic route to the right hemisphere rather than to the left side, although this was not statistically supported (P = 0.121).
Tranexamic acid and Aprotinin in relation to early and delayed stroke
The variables tranexamic acid and aprotinin were not evaluated in our previous report (Citation8). These variables were here added into the analysis, of which tranexamic acid unexpectedly influenced the statistical results regarding hemispheric subdivisions. In these aspects they deserve attention in terms of their general relationship to early and delayed stroke. In the cohort here analyzed, administration of tranexamic acid was found associated with early stroke (OR 2.03, CI 1.49–2.76, P < 0.001) but not delayed stroke (OR 1.08, CI 0.67–1.74, P = 0.762). Aprotinin showed a similar effect, for early (OR 1.93, CI 1.21–3.08, P = 0.006) but not delayed stroke (OR 1.06, CI 0.46–2.43, P = 0.886), respectively. Their multivariable relationship to stroke was not tested, being outside the scope of the present study.
Survival analysis
Both early and delayed stroke were associated with impaired long-term survival compared to control subjects (P < 0.001). Hemispheric side differences were seen for early but not for delayed stroke. Therefore, survival analyses were restrained to that of early stroke. shows survival curves for left hemispheric, right hemispheric, and bilateral stroke in contrast to control subjects. Patients with bilateral lesions presented a dramatically higher mortality compared with those having unilateral stroke (P < 0.001). This difference occurred early after surgery. On the contrary, for those patients with bilateral stroke who survived their first postoperative year no difference was verified against unilateral stroke (P = 0.630), or against control subjects (P = 0.414). These comparisons must be considered with care because of the few patients with bilateral stroke.
Figure 2. Kaplan-Meier survival curves for hemispheric groups of early stroke patients compared to control subjects.
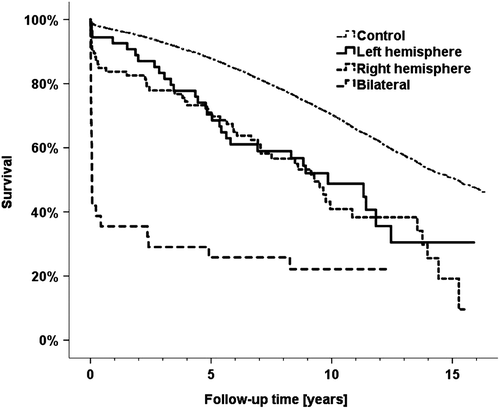
A survival difference was not verified between left- and right-hemispheric stroke (P = 0.627). Nevertheless, it is tempting to address an apparently higher acute-phase mortality for the right-hemispheric group compared with its left counterpart (16.2% versus 7.4%, 1-year mortality, P = 0.128, ). For comparison only, a significant difference was indeed observed when early and delayed stroke were grouped together, referring to the stroke disease rather than to its temporal subdivision. With these presumptions, right-hemispheric stroke had a confirmed higher mortality during the first postoperative year (P = 0.042).
Discussion
The understanding of stroke mechanisms in cardiac surgery remains a challenge. In overview, stroke may either reflect a global hypoperfusion or be due to embolic mechanisms. Stroke may be the effect of a single macro-particle or result from the impact of numerous microemboli. In these perspectives, the hemispheric distribution of lesions becomes of interest, and directs the attention on possible mechanisms of stroke.
From our previous experience, we strongly emphasize that early and delayed stroke represent two separate entities (Citation8). Intuitively understood, early stroke mainly relates to intraoperative mechanisms whereas delayed stroke reflects the postoperative situation. The two stroke groups are known to be characterized by separate risk factors (Citation8). The difference between two stroke groups is further elucidated in the present study in that early but not delayed stroke showed preponderance for right-hemispheric lesions. The present study finds support in our previous observations based on smaller groups of patients (Citation10,Citation11). A hemispheric side difference has also been suggested elsewhere (Citation1,Citation9, Citation13,Citation14). Of interest in the present study, no hemispheric side difference was statistically verified when early and delayed stroke were grouped together. The observation illustrates the importance of this subdivision with the hope to better understand the underlying cause to each of the two groups of stroke.
The right-to-left hemispheric difference was confirmed for patients exposed to cardiac-type procedures. A similar trend was seen for vascular procedures although the number of observations was few and not statistically supported. Also, in the latter group the occurrence of bilateral stroke increased to nearly one third of the events. Overall, the stroke rate among the vascular patients was far higher than after cardiac-type procedures. Presumably, the two procedure groups have many stroke mechanisms in common. This assumption was here strengthened as the procedure group was not an independent risk factor to explain bilateral lesions. The vascular group has influence from its underlying vessel disease (dissections and aneurysms), which was not compensated for in the analyses. These patients were also exposed to circulatory arrest. Contemporary techniques were used at the time of operation, including retrograde perfusion for some of the patients. Antegrade cerebral perfusion at circulatory arrest had not been adopted during the observational period.
The mechanistic distinction between bilateral and unilateral stroke is not fully understood. Hemodynamic mechanisms associated with cerebral hypoperfusion may contribute to bilateral lesions. This was partly supported in the present study. Numerous variables at univariate level showed association to bilateral lesions, including the surgery group of vascular-type procedures. At multivariable analysis an interesting pattern developed. The procedure type was then rejected among independent variables, in favor of bleeding, volume characteristics, and drug administration. In theory, bleeding with insufficient volume compensation, combined with the use of inotropic drugs, had influence on the occurrence of bilateral stroke. These results may indicate hypovolemia, hypotension, and, hence, cerebral hypoperfusion. The administration of tranexamic acid was independently associated with bilateral lesions. Tranexamic acid may here represent a surrogate variable for something unknown. However, its statistical independence from surgical bleeding is intriguing. Also, tranexamic acid contributed strongly within the multivariable model.
In our previous reports, tranexamic acid has not been reviewed in terms of stroke. Administration of tranexamic acid was here tested in the analyses, wrongly presuming that the variable had no influence on the hemispheric distribution. For overview, tranexamic acid was therefore tested against stroke, regardless of its hemispheric subdivisions. The variable was then found significantly contributing to early but not delayed stroke. It is expected that the use of tranexamic acid hides numerous confounding mechanisms, which were not explored further. In the literature tranexamic acid has been suggested to cause convulsive seizures (Citation17,Citation18), whereas stroke is not reported. In the present study, tranexamic acid strongly and independently indicated right-hemispheric lesions of early stroke. One possible interpretation suggests embolism via the brachiocephalic trunk.
Stroke in the general population has a uniform hemispheric distribution. However, Foerch et al. (Citation19) reported an overweight for left-hemispheric lesions compared to the right side, comparing 56% and 44%. Nevertheless, it was speculated that these findings were influenced by an easier recognition of symptoms evolving from the left dominant hemisphere (Citation19). If applied to our results with right- hemispheric overweight for early stroke, additional lesions on this non-dominant side might have escaped detection in our registry. The side difference might therefore have been underestimated. Delayed stroke after cardiac surgery appeared more similar to that of the general stroke population, with a close to uniform hemispheric distribution. Presumably, the underlying mechanisms of delayed stroke also resemble that of the general population. Bilateral lesions account for less than 1% in acute ischemic stroke of the general population (Citation20), and similarly, in the present study bilateral lesions were uncommon in delayed stroke after cardiac surgery.
It was intriguingly observed that stroke in conjunction with cardiac surgery was near exclusively ischemic. This was despite full heparinization during CPB and use of anticoagulants in the postoperative period. Only three patients had signs of hemorrhage, and all these events were questionable in their clinical relevance. The ischemic nature of stroke after cardiac surgery should therefore be kept in mind. For delayed stroke, a potential benefit is suggested from rapidly initiated anticoagulation therapy rather than awaiting a CT scan to be performed for the exclusion of hemorrhage.
A survival difference between right- and left-hemispheric strokes was not supported in this study. This was despite an apparently higher acute mortality from right-hemispheric lesions. Patients with bilateral stroke showed a depressive survival with an acute-phase mortality of nearly 60%. However, the few patients who survived the acute phase indicated a normalized remaining life expectancy.
This study is limited from its descriptive modality and from the heterogeneous population of cardiac surgery patients. Although data were prospectively entered, details around the stroke events were retrospectively collected. Also, our data referred to the period of hospitalization only. Fortunately, stroke after cardiac surgery is a rare complication. Despite a fairly, large, analyzed cohort, the statistical power was limited. Moreover, stroke was subdivided into its early and delayed forms, into cerebral and non-cerebral lesions, and into hemispheric subgroups. In these perspectives, the statistical potentials become further limited. Also, questions about procedure-specific stroke details were left without answers, other than from the subdivision into “cardiac-type” and “vascular-type” procedures. The unexpected observation regarding tranexamic acid requires a more detailed review than here reported. Only a randomized study can provide the necessary answers in these perspectives.
In conclusion, stroke after cardiac surgery is near exclusively ischemic. Early cerebral stroke shows an obvious preponderance for right- hemispheric lesions. Delayed stroke has a uniform hemispheric distribution, similar to that known for stroke events in the general population. Subdivision into early and delayed stroke is essential for exposing this difference. The side difference directs the attention on embolic mechanisms rather than general hypoperfusion, with an embolic route via the brachiocephalic trunk. Administration of tranexamic acid influenced the hemispheric distribution of lesions, although the clinical relevance of this finding remains both unexplained and speculative. Early stroke with bilateral lesions is more common after vascular than after cardiac-type procedures.
Declaration of interest: The authors report no conflicts of interest. The study had no commercial funding. The authors alone are responsible for the content and writing of the paper.
References
- Filsoufi F, Rahmanian PB, Castillo JG, Bronster D, Adams DH. Incidence, topography, predictors and long-term survival after stroke in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2008;85:862–71.
- Wolman RL, Nussmeier NA, Aggarwal A, Kanchuger MS, Roach GW, Newman MF, et al. Cerebral injury after cardiac surgery: identification of a group at extraordinary risk. Multicenter Study of Perioperative Ischemia Research Group (McSPI) and the Ischemia Research Education Foundation (IREF) Investigators. Stroke. 1999;30:514–22.
- Borger MA, Ivanov J, Weisel RD, Rao V, Peniston CM. Stroke during coronary bypass surgery: principal role of cerebral macroemboli. Eur J Cardiothorac Surg. 2001;19: 627–32.
- Gottesman RF, Sherman PM, Grega MA, Yousem DM, Borowicz LM Jr.,Selnes OA, et al. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke. 2006;37:2306–11.
- Likosky DS, Marrin CA, Caplan LR, Baribeau YR, Morton JR, Weintraub RM, et al. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Stroke. 2003;34:2830–4.
- Hogue CW Jr.,Murphy SF, Schechtman KB, Dávila-Román VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation. 1999;100:642–7.
- Peel GK, Stamou SC, Dullum MK, Hill PC, Jablonski KA, Bafi AS, et al. Chronologic distribution of stroke after minimally invasive versus conventional coronary artery bypass. J Am Coll Cardiol. 2004;43:752–6.
- Hedberg M, Boivie P, Engström KG. Early and delayed stroke after coronary surgery — an analysis of risk factors and the impact on short- and long-term survival. Eur J Cardiothorac Surg. 2011;40:379–87.
- Steuer J, Ivert T. Neurological complications after open heart surgery; risk factors identified in a retrospective study. Läkartidningen 1998;95:4348–53.
- Boivie P, Edström C, Engström KG. Side differences in cerebrovascular accidents after cardiac surgery: a statistical analysis of neurologic symptoms and possible implications for anatomic mechanisms of aortic particle embolization. J Thorac Cardiovasc Surg. 2005;129:591–8.
- Hedberg M, Boivie P, Edström C, Engström KG. Cerebrovascular accidents after cardiac surgery: an analysis of CT scans in relation to clinical symptoms. Scand Cardiovasc J. 2005;39:299–305.
- Weinstein GS. Left hemispheric strokes in coronary surgery: implications for end-hole aortic cannulas. Ann Thorac Surg. 2001;71:128–32.
- Filsoufi F, Rahmanian PB, Castillo JG, Bronster D, Adams DH. Incidence, imaging analysis, and early and late outcomes of stroke after cardiac valve operation. Am J Cardiol. 2008;101:1472–8.
- Korn-Lubetzki I, Oren A, Asher E, Dano M, Bitran D, Fink D, Steiner-Birmanns B. Strokes after cardiac surgery: mostly right hemispheric ischemic with mild residual damage. J Neurol. 2007;254:1708–13.
- Edmunds LH Jr.,Clark RE, Cohn LH, Grunkemeier GL, Miller DC, Weisel RD. Guidelines for reporting morbidity and mortality after cardiac valvular operations. Ad Hoc liaison committee for standardizing definitions of prosthetic heart valve morbidity of The American Association for Thoracic Surgery and The Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 1996;112:708–11.
- Thorvaldsen P, Asplund K, Kuulasmaa K, Rajakangas AM, Schroll M. Stroke incidence, case fatality, and mortality in the WHO MONICA project. World Health Organization Monitoring Trends and Determinants in Cardiovascular Disease. Stroke. 1995;26:361–7.
- Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110:350–3.
- Manji RA, Grocott HP, Leake J, Ariano RE, Manji JS, Menkis AH, Jacobsohn E. Seizures following cardiac surgery: the impact of tranexamic acid and other risk factors. Can J Anaesth. 2012;59:6–13.
- Foerch C, Misselwitz B, Sitzer M, Berger K, Steinmetz H, Neumann-Haefelin T; Arbeitsgruppe Schlaganfall Hessen. Difference in recognition of right and left hemispheric stroke. Lancet. 2005;366:392–3.
- Cucchiara B, Kasner SE, Wolk DA, Lyden PD, Knappertz VA, Ashwood T, et al. Lack of hemispheric dominance for consciousness in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2003;74:889–92.