Abstract
Objective. Epicardial adipose tissue (EAT), deposited around subepicardial coronary vessels, may contribute directly to perivascular inflammation and smooth muscle cell proliferation. This study assessed the relationship between EAT and in-stent restenosis. Methods. Four hundred and seven patients had received successful coronary intervention. EAT thickness was measured by echocardiography. Angiographic follow-up was obtained between 6 months and 2 years. Restenosis was defined as target lesion revascularization (TLR). EAT thickness of patients was compared by TLR controlling for additional well-known predictors of restenosis. The TLR-free survival analysis according to EAT thickness was estimated using the Kaplan–Meier method and the differences between groups were assessed by the log-rank test. Results. Median EAT thickness was significantly increased in patients undergoing TLR compared with those without restenosis (3.7 vs. 3.0 mm, p = 0.001). EAT thickness was one of the independent factors associated with restenosis (Odds ratio = 1.19, 95% confidence interval = 1.01–1.33, p = 0.007). The TLR-free survival of patients with thick EAT was significantly worse than patients with thin EAT (log-rank p = 0.001). Conclusions. EAT thickness is related with restenosis and may provide additional information for future restenosis.
Key words::
Background
Inflammation and endothelial dysfunction have central roles in the pathophysiology of restenosis of coronary artery, in particular neointimal hyperplasia (Citation1–3). Visceral adipose tissue modulates vascular inflammation and function through adipose-derived relaxing and contracting factors, cytokines and infiltration of inflammatory cells (Citation4).
Epicardial adipose tissue (EAT), deposited around the heart particularly around subepicardial coronary vessels, may contribute directly to perivascular inflammation (Citation5–7) and smooth muscle cell proliferation (Citation7,Citation8). We previously demonstrated an association of EAT thickness determined by echocardiography with insulin resistance and inflammation (Citation9). The additional local inflammation of EAT could contribute to the increased risk of restenosis. However, there have been no data demonstrating the association of EAT with restenosis after coronary stenting.
We hypothesized that thick EAT might be a risk factor for coronary restenosis. To explore this hypothesis, we assessed the relationship of EAT and short-term angiographic outcomes after percutaneous coronary intervention (PCI).
Methods
The study population consisted of patients admitted to Ajou University Medical Center for PCI between August 2007 and July 2010. A total of 1400 patients with angiographically significant coronary artery disease (CAD) received successful PCI. Among these patients, 407 patients underwent angiographic follow-up between 6 months and 2 years after PCI. We consecutively enrolled 407 patients (mean age 59 ± 11 years old, 279 males) with angiographically significant coronary artery disease, who received successful PCI. The medical records of all patients were retrospectively reviewed. This study was approved by the Ajou University Hospital Institutional Review Board (approval number: AJIRB-MED-MDB-13-007). Upon quantitative analysis of the coronary angiograms, significant CAD was considered to be the presence of stenoses, ≥ 50% in diameter, of a major epicardial vessel. Revascularization was indicated if there was ≥ 70% diameter stenosis on coronary angiography or ≥ 50% stenosis together with a positive stress test or with ischemic symptoms. We excluded patients from the study if they had a history of prior revascularization, heart failure or acute myocardial infarction.
Two-dimensional transthoracic echocardiography was performed within 1 week, either after or before undergoing PCI. Recordings of three cycles of the two-dimensional parasternal long-axis view were obtained. Images were enlarged for better visualization and accurate measurement of EAT thickness. EAT thickness was measured on the free wall of the right ventricle (RV) in a still image at end diastole on the parasternal long-axis view. EAT thickness was measured at the point on the free wall of the RV at which the ultrasound beam was oriented perpendicularly to the aortic annulus (Citation10–13). The area above the RV was preferred to measure EAT thickness, as this area is recognized as having the thickest EAT layer. In addition, the parasternal long-axis view allows the most accurate measurement of EAT thickness with optimal cursor beam orientation. Among various echocardiographic views, the measurement of EAT thickness on the parasternal long-axis view during diastole reportedly correlates best with the total amount of EAT (Citation10). Consequently, we chose the parasternal long-axis view in the present study. The anterior echo-lucent space between the linear echo-dense parietal pericardium and the RV epicardium was considered to be EAT. We measured the thickest point of EAT in each cycle. The average value of the EAT thickness was calculated. One sample paired t test was performed to evaluate inter- and intra-observer variability in the measurement of EAT from 30 randomly selected patients.
Angiographic follow-up was obtained between 6 months and 2 years after PCI. Regardless of ischemic symptoms, angiography was performed. Restenosis was defined as target lesion revascularization (TLR). The TLR was defined as clinically indicated percutaneous or surgical revascularization of the index lesion during follow-up. Revascularization was decided as indicated if there was ≥ 70% target lesion diameter stenosis on angiography or ≥ 50% target lesion stenosis together with a positive stress test or with ischemic symptoms. The target lesion was considered to be the area covered by the previous stent site plus a 5-mm margin proximal and distal to the stent edges.
All patients were divided into a no TLR group (n = 307) and a TLR group (n = 100). EAT thickness was compared in the groups by echocardiography, in addition to the well-known predictors (Citation14) for restenosis in the TLR group to those in the no TLR group. These predictors were age, gender, body–mass index (BMI), the presence of hypertension, diabetes mellitus and dyslipidemia, smoking, multivessel disease and multiple stenting.
By constructing a receiver operating characteristic curve, we derived a cut-off value of EAT thickness for TLR. Based on this cut-off value, all patients were reclassified into a thick EAT group and a thin EAT group. Event (TLR)-free survival analysis for patients in these groups was performed using the Kaplan–Meier method, and the differences between groups were assessed by the log-rank test.
SPSS 13.0 statistical software package (SPSS, Chicago, Illinois, USA) was used for all calculations. Data are shown as the mean ± standard deviation for continuous variables and as numbers and percentages for categorical variables. Comparisons were conducted by unpaired Student's t test. Owing to its skewed distribution (Citation9), EAT thickness was shown as the median value and comparison of EAT thickness according to TLR was performed using the Wilcoxon rank-sum test. Multiple logistic regression analysis was performed to assess independent factors associated with restenosis. Null hypotheses of no difference were rejected if p values were < 0.05.
Results
Median and mean EAT of the 407 patients (mean age 59 ± 11 years, 279 males) were 3.1 mm and 3.3 ± 1.9 mm, respectively. The absolute values of the mean paired differences were 0.020 ± 0.396, p = 0.773 and 0.003 ± 0.469, p = 0.969 for inter- and intra-observer variability of the EAT measurement, respectively, indicating good reproducibility. The mean follow-up interval was 12 ± 5 months. Clinical characteristics by TLR are summarized in . Twenty-five percent of patients were in the TLR group. All the patients in the TLR group received re-PCI. No patient developed infarction or needed coronary artery bypass graft. The TLR group consisted of 100 patients (62 males) with a mean age of 59 ± 10 years. The no TLR group consisted of 307 patients (217 males) with a mean age of 58 ± 11 years. All patients except three received drug-eluting stents. Three patients who received bare metal stents were included in the no TLR group. Median EAT thickness was significantly increased in patients undergoing TLR compared with those without restenosis (3.7 vs. 3.0 mm, p = 0.001, ). Compared with the no TLR group, subjects in the TLR group had significantly higher rates of hypertension, diabetes mellitus, multivessel disease and multiple stenting (p = 0.025, 0.011, 0.001 and < 0.001, respectively). In the TLR group, the rate of current smoking tended to be higher than in the no TLR group (p = 0.098). There was no statistical difference in medication use between the groups.
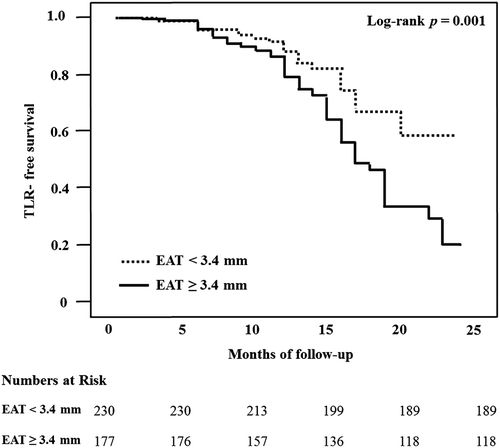
Figure 1. Comparison of the EAT thickness by clinical outcome. EAT, epicardial adipose tissue; TLR, target lesion revascularization.
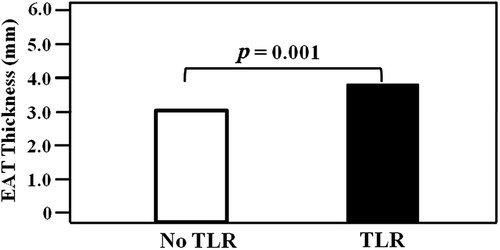
Table I. Baseline characteristics by TLR.
In multivariate analysis of the clinical parameters, smoking, multivessel disease, multiple stenting and EAT thickness were strongly related to TLR (). In addition to the well-known predictors for restenosis, such as smoking, multivessel disease and multiple stenting, EAT thickness was an independent factor associated with restenosis in this study population (Odds ratio = 1.19, 95% confidence interval = 1.01–1.33, p = 0.007).
Table II. Multiple logistic regression analysis of the clinical predictors for restenosis.
For predicting TLR, the cut-off value of EAT thickness was 3.4 mm with a sensitivity and specificity of 60% and 61%, respectively (AUC = 0.618, 95% confidence interval = 0.55–0.68, p < 0.001). When the patients were reclassified based on the derived cut-off value of EAT thickness for TLR, the thick EAT group (EAT ≥ 3.4 mm) consisted of 177 patients (107 males) with a mean age of 60 ± 10 years. The thin EAT group (EAT < 3.4 mm) consisted of 230 patients (172 males) with a mean age of 57 ± 11 years. The thick EAT group was significantly older than the thin EAT group (p < 0.001). There were more females in the thick EAT group (p = 0.003). The thick EAT group had significantly higher BMI than the thin EAT group (25.1 ± 3.3 kg/m2 vs. 24.4 ± 3.1 kg/m2, p = 0.022). The TLR occurred in 33% of patients with thick EAT and in 18% of those with thin EAT. The TLR-free survival of patients with thick EAT was significantly worse than patients with thin EAT (log-rank p = 0.001) ().
Discussion
The present study demonstrated the close relationship between thickness of EAT measured by echocardiography and restenosis in patients with significant CAD after successful PCI.
Several reports demonstrated a central role of inflammation in the process of neointimal hyperplasia (Citation1,Citation2). Inflammation plays a pivotal role linking early vascular injury to the eventual consequence of neointimal growth and lumen compromise (Citation1). Elevated circulating levels of inflammatory markers correlated with propensity for restenosis through recruitment of lymphocytes and monocytes into the inflamed vessel wall resulting in the induction of intimal hyperplasia (Citation15). Another important mechanism of restenosis might be endothelial dysfunction (Citation3). Endothelial dysfunction primarily reflects decreased availability of nitric oxide (NO), a critical endothelium-derived vasoactive factor with vasodilatory and anti-atherosclerotic properties (Citation16). As NO has been demonstrated to decrease migration and proliferation of vascular smooth muscle cells to attenuate binding of inflammatory cells to the vascular wall, inhibit thrombosis by reducing platelet adhesion and aggregation, and maintain vascular relaxation (Citation17,Citation18), reduced synthesis of NO might result in early restenosis after PCI.
Several biomolecular studies in humans have shown that EAT is metabolically active and an important source of inflammatory mediators, such as tumor necrosis factor-α, interleukin 1, interleukin 6 and nerve growth factor (Citation6,Citation7,Citation19,Citation20). Inflammatory mediators originating outside the coronary artery are also capable of inducing compositional changes in the inner layer of intima (Citation6,Citation21). The paracrine and vasocrine secretions of inflammatory adipokines by EAT contributes to the metabolic and inflammatory milieu (Citation6,Citation7). The presence of inflammatory mediators in EAT surrounding coronary arteries could lead to amplification of vascular inflammation (Citation6,Citation7,Citation22). The anatomical characteristics of EAT may produce a local proatherosclerotic effect on the underlying coronary arteries (Citation6,Citation7,Citation23). Through additional local tissue inflammation, the presence of metabolically active adipose stores that surround epicardial coronary arteries could contribute toward the increased risk of restenosis in patients with significant CAD after successful PCI.
Central obesity is associated with endothelial dysfunction by the production of adipokines and pro-inflammatory cytokines, which induce oxidative stress leading to a reduced NO availability (Citation23). Through a protein kinase C-β dependent, site-specific phosphorylation of endothelial NO synthase, EAT attenuates coronary endothelial NO production (Citation24,Citation25). By propagating inflammation to underlying coronary arteries, EAT also alters the balance between vascular NO, endothelin-1 and superoxide production, promoting endothelial dysfunction (Citation7,Citation26). Thick EAT thickness by echocardiography has been associated with endothelial dysfunction assessed as flow-mediated dilatation at the brachial artery (Citation27). Even in patients without obstructive coronary artery disease, a close relationship between thick EAT thickness by echocardiography and coronary microvascular dysfunction, demonstrated by reduced coronary blood flow reserve, was evident (Citation28).
Several studies identified independent clinical predictors of restenosis after coronary stenting including female gender, diabetes, hypertension, smoking, BMI, multivessel disease, and multiple stenting (Citation14). Similarly, we observed a close relationship between smoking, multivessel disease and multiple stenting with restenosis in this study population. We demonstrated that EAT thickness by echocardiography has the potential to be an additional predictor for restenosis after successful PCI. Thick EAT might affect the clinical outcome in patients with coronary stenting by coronary vascular inflammation and endothelial dysfunction. As echocardiography is frequently performed in patients with high cardiovascular risk or chest pain, EAT thickness measured by echocardiography may be readily available at no extra cost and provide additional information for predicting the clinical outcome.
There are several limitations to the present study. First, EAT thickness by echocardiography does not exactly represent the amount of total EAT. Even though echocardiography is not the optimal method for quantification of EAT, our previous study showed that EAT thickness measured by echocardiography correlates well with the total amount of EAT (Citation10). EAT thickness by echocardiography also correlates well with total EAT measured by magnetic resonance imaging (Citation29). In addition to the present study, the majority of population-based clinical studies have reported excellent intra- and inter-observer agreement for the measurement of EAT thickness by echocardiography on the parasternal long-axis view (Citation9–12,Citation29). Measurement of EAT thickness by echocardiography might be reliable and relatively accurate. Second, the present study population had a relatively high restenosis rate compared with conventionally accepted restenosis rates. As the present study was retrospective, not all patients who received successful PCI during a certain period were enrolled. Although 1400 patients received successful PCI for significant CAD, 993 patients did not undergo angiographic follow-up in the present study. These patients had no significant ischemic symptoms. As angiographic follow-up was usually more frequently done in patients with ischemic symptoms, the present study population might have a higher restenosis rate. Third, the results of the present study could not be applied to the general population. The derived cut-off value of EAT thickness was 3.4 mm for predicting TLR. The present study included Koreans. A normal upper-limit value for EAT thickness by echocardiography has not been established. As ethnic differences could influence the distribution of EAT (Citation30), the cut-off value of EAT thickness for predicting TLR might be different according to the ethnicity. Further studies might be needed for clinical application.
In conclusion, the EAT thickness is related with restenosis in patients who underwent successful PCI. The EAT thickness might provide additional information for future restenosis after coronary stenting.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–76.
- Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002;25:2974–80.
- Piatti P, Di Mario C, Monti LD, Fragasso G, Sgura F, Caumo A, et al. Association of insulin resistance, hyperleptinemia, and impaired nitric oxide release with in-stent restenosis in patients undergoing coronary stenting. Circulation. 2003;108:2074–81.
- Zhang H, Zhang C. Adipose “talks” to distant organs to regulate insulin sensitivity and vascular function. Obesity (Silver Spring). 2010;18:2071–6.
- Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–13.
- Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–7.
- Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 2011;34:S371–9.
- Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol 2005;289:H1807–13.
- Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94:e7.
- Hwang JW, Choi UJ, Ahn SG, Lim HS, Kang SJ, Choi BJ, et al. Echocardiographic plains reflecting total amount of epicardial adipose tissue as risk factor of coronary artery disease. J Cardiovasc Ultrasound. 2008;16:17–22.
- Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–10.
- Iacobellis G, Willens HJ, Barbaro G, Sharma AM. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity (Silver Spring). 2008;16:887–92.
- Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–9.
- Kastrati A, Schömig A, Elezi S, Schühlen H, Dirschinger J, Hadamitzky M, et al. Predictive factors of restenosis after coronary stent placement. J Am Coll Cardiol. 1997;15: 1428–36.
- Cipollone F, Marini M, Fazia M, Pini B, Iezzi A, Reale M, et al. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2001;21:327–34.
- Woodman RJ, Chew GT, Watts GF. Mechanisms, significance and treatment of vascular dysfunction in type 2 diabetes mellitus: focus on lipid-regulating therapy. Drugs. 2005; 65:31–74.
- Yao SK, Ober JC, Krishnaswami A, Ferguson JJ, Anderson HV, Golino P, et al. Endogenous nitric oxide protects against platelet aggregation and cyclic flow variations in stenosed and endothelium-injured arteries. Circulation. 1992;86: 1302–9.
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–5.
- Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2005;2:536–43.
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6.
- Miyata K, Shimokawa H, Kandabashi T, Higo T, Morishige K, Eto Y, et al. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol. 2000;20:2351–8.
- Prati F, Arbustini E, Labellarte A, Sommariva L, Pawlowski T, Manzoli A, et al. Eccentric atherosclerotic plaques with positive remodeling have a pericardial distribution: a permissive role of epicardial fat? Eur Heart J. 2003;24:291–3.
- Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kälsch H, Seibel RM, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010; 211:195–9.
- Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H460–5.
- Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–7.
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–70.
- Aydin H, Toprak A, Deyneli O, Yazici D, Tarçin O, Sancak S, et al. Epicardial fat tissue thickness correlates with endothelial dysfunction and other cardiovascular risk factors in patients with metabolic syndrome. Metab Syndr Relat Disord. 2010;8:229–34.
- Sade LE, Eroglu S, Bozbaş H, Ozbiçer S, Hayran M, Haberal A, Müderrisoğlu H. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009;204:580–5.
- Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–8.
- Willens HJ, Gómez-Marín O, Chirinos JA, Goldberg R, Lowery MH, Iacobellis G. Comparison of epicardial and pericardial fat thickness assessed by echocardiography in African American and non-Hispanic White men: a pilot study. Ethn Dis. 2008;18:311–6.