Abstract
Objectives. The objective was to investigate the potential protective effects of two conditioning methods, on myocardial ischemic and reperfusion injury in relation to cardiac surgery. Design. Totally 68 patients were randomly assigned to either a control group (n = 23), a remote ischemic preconditioning (RIPC) group (n = 23) or a glucagon-like peptide-1 (GLP-1) analogue group (n = 22). The RIPC protocol consisted of three cycles of upper limb ischemia. The GLP-1 analogue protocol consisted of intravenous infusion with exenatide. The primary endpoint was postoperative cardiac enzyme release. The other secondary endpoints were metabolic parameters related to myocardial ischemia, measured using microdialysis technique, as well as other operative- and postoperative data. Results. Postoperative cardiac enzyme release indicated a possible beneficial effect of the interventions, but the difference did not reach statistical significance. RIPC showed a trend toward lower levels (p = 0.07). We managed to establish a functional myocardial microdialysis model, but we were unable to demonstrate clear protective effects. Conclusions. We were in this prospective randomized proof-of-concept trial, unable to show distinct protective effects of the studied conditioning methods. However, this trial can hopefully contribute to generate a productive discussion concerning limitations and future use of cardiac conditioning as well as microdialysis technique.
Introduction
When performing cardiac surgery using cardiopulmonary bypass (CPB) technique, the process of reperfusion at the end of the surgical procedure can paradoxically damage the coronary vascular endothelium and subcellular structures (Citation1,Citation2). This process is known as myocardial ischemic reperfusion injury.
The purpose of this trial was to investigate two conditioning methods and potential protective effects against myocardial ischemic and reperfusion injury during on-pump cardiac surgery. The trial focused on remote ischemic preconditioning (RIPC) and pharmacological conditioning using a glucagon-like peptide-1(GLP-1) analogue. The effect of GLP-1 analogue infusion has never previously been investigated in relation to cardiac surgery.
Cardiac enzyme release as well as microdialysis technique were used to determine the effects of the interventions. Microdialysis technique has to our knowledge never before been used to monitor the potential effects of RIPC and GLP-1 analogue infusion on myocardial reperfusion injury.
RIPC
RIPC was first described by Przyklenk et al. in dogs (Citation3). Several studies have subsequently shown that brief cycles of ischemia and reperfusion outside the heart, such as in the upper limb, prior to a cardiac ischemic episode, can protect the heart against subsequent ischemia and reperfusion injury (Citation4–6). The underlying mechanisms behind this phenomenon are still unknown. However, both humoral and neural pathways have been suggested (Citation7,Citation8).
Pharmacological conditioning
GLP-1 is an intestinal incretin hormone and its receptors are located in human pancreas, lung, kidney, brain, and heart (Citation9–11).The role of the cardiac receptor has been investigated in both animal and human studies. A human study proved that a 72-h GLP-1 analogue infusion after PCI, in patients with myocardial infarction, reduced the size of the infarction by 30%, assessed using echocardiography (Citation12). Furthermore, infusion of the GLP-1 analogue exenatide (Byetta®, Eli Lilly, USA), during primary PCI in STEMI-patients, reduced the following reperfusion injury (Citation13). This effect has been called pharmacological conditioning. It is believed that the cardio-protective effect is mediated through cAMP-induced PKA activation (Citation14), but potentially also through GLP-1 receptor-independent effects (Citation9).
Materials and methods
Design
This trial was designed as a prospective randomized proof-of-concept trial. All patients received written and oral information, and gave their written consent before inclusion. The patients were included in a consecutive order over a period of 6 months and were randomly allocated to the different interventional groups using sealed envelopes which were opened after informed consent was obtained. The surgical staff was not blinded during the trial. The postoperative staffs at the intensive care unit and at the ward, as well as the patients themselves were however blinded throughout the entire trial.
The trial was approved by The Danish National Committee of Biomedical Research Ethics., journal no. H-4-2010-115.
Trial population
Adult patients undergoing first time elective on-pump cardiac surgery, with an intended aortic clamp time of at least 60 min, were eligible for inclusion. The following exclusion criteria were used: diabetic ketoacidosis, hypoglycemia, pancreatitis, peripheral vascular disease involving the right upper limb, harvesting of right radial artery during the surgical procedure, immune defects, and previous treatment with a GLP-1 analogue.
Experimental treatment protocol
Group 1 (n = 23): This group served as a control group and patients received the department's standard treatment protocol.
Group 2 (n = 23): After induction of anesthesia, RIPC protocol was commenced, consisting of three cycles, each consisting of 5 min of right upper limb ischemia, followed by 5 min of reperfusion. Ischemia was induced by inflating a standard blood pressure cuff to 200 mmHg. Corresponding algorithm has previously been shown to limit reperfusion injury in relation to coronary artery bypass graft (CABG) procedures (Citation4,Citation5).
Group 3 (n = 22): Thirty minutes before the aortic cross clamp was placed, intravenous infusion of the GLP-1 analogue exenatide (Byetta®, Eli Lilly, USA) was commenced. The GLP-1 analogue and the algorithm used were the same as previously found cardio-protective in a setting of global ischemia and reperfusion (Citation15). Exenatide was diluted in isotonic saline (50 μg exenatide in 500 ml isotonic saline). Human serum albumin was added to the solution in order to prevent the active substance from binding to the infusion materials. The first 15 min, an infusion rate of 72 ml/h was used to obtain an exenatide plasma concentration between 0.03 and 0.30 nmol/L (Citation13,Citation15). Afterwards the infusion rate was reduced to 26 ml/h for the remaining duration of the infusion, to keep the exenatide concentration within the before mentioned interval. The exenatide dose and infusion rate was calculated based on exenatide's distribution volume of 64 ml/kg body weight and a plasma half time of 26 min (Citation16). The infusion was continued for 6 h after aortic clamp removal and initiation of cardiac reperfusion.
Plasma amylase concentration was determined before and after the exenatide infusion, in order to monitor potential pancreatic effects. Blood glucose was continuously monitored in order to avoid severe hypoglycemic episodes. If blood glucose levels bordered to hypoglycemic levels, arbitrarily defined as 4 mM, the exenatide infusion was either stopped or an isotonic glucose infusion was put up in order to keep the blood glucose at a suitable level.
Anesthesia
The anesthetic procedure was the same in all three patient groups, according to the department's standard protocol for open heart surgery. Patients were given midazolam 1 hour before surgery. The anesthesia was induced with midazolam 1–5 mg and fentanyl 0.5–1 mg intravenously. It was thereafter maintained with sevoflurane 0.5–1.5% and remifentanil.
CPB
The CPB procedure was the same in all three interventional groups and according to the department's standard procedure. CPB was performed in normothermia.
In order to induce cardioplegic arrest, 1000 ml oxygenated cold blood cardioplegic solution (4:1 ratio) was infused in to the myocardial circulation. In order to maintain myocardial protection as well as cardioplegic arrest, 300 ml were infused every 20 min throughout the surgical procedure.
Data collection
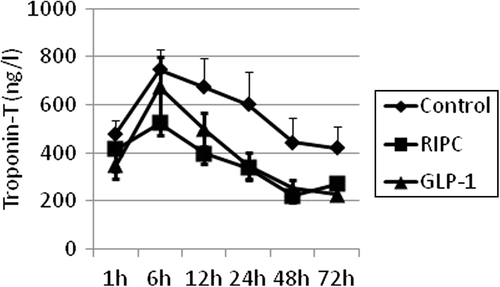
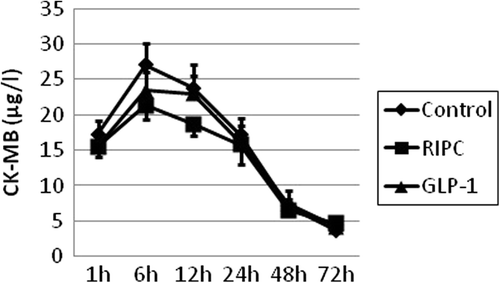
The primary endpoint was postoperative cardiac enzyme release. Plasma concentrations of troponin-T and CK-MB were measured 1, 6, 12, 24, 48, and 72 h after initiation of reperfusion.
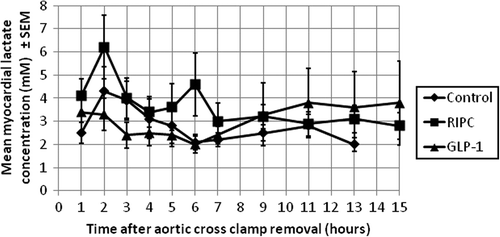
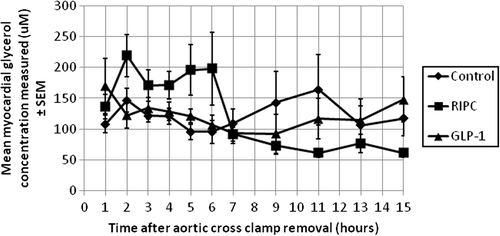
The secondary endpoints included local left ventricular myocardial concentrations of glycerol, glucose, lactate, and pyruvate assessed using myocardial microdialysis technique. Custom-made microdialysis probes were used (CMA Microdialysis AB, Sweden). The probes had the following specifications; membrane length 10 mm, cut-off 100 kDalton, inlet 600 mm, outlet 220 mm, and shaft length 30 mm.
Before the aortic cross clamp was removed, a microdialysis probe was introduced into the left ventricular myocardium using a cannula, as described by Mantovani et al. (Citation17). The tip of the probe was inserted into the distal end of the cannula and by retracting the cannula, the probe was implanted into the myocardium. The probe was then fixed using a 5-0 prolene suture, and connected to CMA107 pump (CMA Microdialysis AB, Sweden). The pump perfused the microdialysis system with perfusion fluid T1(CMA Microdialysis AB, Sweden) at a flow rate of 0.3 μl/min. Samples were collected in microvials through the outlet. The vials were changed every hour for 7 h after initiation of reperfusion and thereafter every second hour for another 8 h. The vials were analyzed using automated CMA600 Microdialysis Analyzer (CMA Microdialysis AB, Sweden).
Other secondary endpoints were perioperative bleeding, administered blood products, DC-conversion, blood glucose levels as well as postoperative need of inotropic agents after 12 and 24 h, reoperation, temporary pacemaker duration, atrial fibrillation, duration of respirator treatment, time spent in intensive care, ischemic complications, and length of hospital stay.
Furthermore total CPB duration, end CPB–fluid balance, aortic cross-clamp duration, amount of cardioplegia. and reperfusion time were registered.
Data analysis
The sample size analysis indicated that 17 experimental subjects and 17 control subjects were required in order to be able to reject the null hypothesis and show a reduction in cardiac enzyme release of 0.40, with a standard deviation of 0.40 and a power of 0.80. This was based on data from similar studies previously conducted (Citation4,Citation5). In total, 68 patients were included in this trial to make up for potential patient exclusion.
Six patients were excluded due to complications which impeded the use of the acquired data in the data analysis (two patients from the control group, three patients from the RIPC group, and one patient from the GLP-1 group). The complications were the use of cardiac massage and/or peri- or postoperative infarctions defined as occurrence of plasma CKMB greater than 80 μg/l or troponin-T greater than 3.0 μg/l, within the first 48 h after surgery (Citation18).
The statistical analysis was performed using IBM-SPSS 20 software. Comparisons between groups were performed using one-way ANOVA test for continuous variables and chi-squared test or fisher's exact test for categorical variables. To compare continuous variables over time, area under the curve (AUC) was calculated. It was calculated using the trapezoidal rule. Results were considered statistically significant at a P value of less than 0.05.
Results
Demographic data
Baseline characteristics are shown in . Patients did not differ with respects to their demographics.
Table I. Demographic data of the trial population.
Operative data
The different types of procedures were uniformly distributed between three groups ().
Table II. Procedure types.
Technical operative data are shown in . No statistical significant differences were seen in these parameters.
Table III. Technical operative data.
Hypoglycemia was observed in eight patients (35%) during the exenatide infusion. In two cases the infusion was stopped to avoid severe hypoglycemic episodes. In the remaining cases, normal glucose levels were easily maintained using isotonic glucose infusion without any complications. No pancreatic adverse effects were observed.
In two cases where reoperations were necessary due to bleeding, the insertion points of the microdialysis probes were contributing causes. Both reoperations progressed without any complications or sequelae.
Postoperative data
Postoperative care and technical data are shown in . No significant differences were detected.
Table IV. Postoperative care- and technical data.
Postoperative mean concentrations of troponin-T () indicated a beneficial effect of both the interventions. AUC values for troponin-T were consequently 25–30% lower in the interventional groups than those in the control group, but failed to reach statistical significance. RIPC: 21059 ± 10168 h × ng/L (p = 0.07), GLP-1: 23483 ± 14488 h × ng/L (p = 0.21) and Control: 30803 ± 18708 h × ng/L.
No statistically significant differences were observed in the mean total AUC values for CK-MB () or in the postoperative microdialysis metabolites ( and ).
Discussion
We managed to establish a functional myocardial microdialysis model in a clinical setting consisting of different types of surgical procedures. Microdialysis technique has to our knowledge never before been used to monitor the effects of RIPC on the myocardial metabolic changes related to ischemia.
We were in this trial unable to show clear protective effect, when applying RIPC to a more general surgical population. However, there was a trend toward lower levels of postoperative cardiac enzyme release. It is hard to determine the specific reason for these results. A potential lack of, or insufficient effect of the intervention itself cannot be dismissed, but methodological reasons must also be considered. Previous studies have shown a large variation of findings for RIPC. Several studies have demonstrated beneficial effects of RIPC in patients undergoing CABG. However, there are also studies, which have failed to demonstrate any reduction in enzyme release following RIPC (Citation19,Citation20). This indicates that both correct intervention and patient cohort are necessary to accomplish cardio-protection.
After our trial was conducted, a meta-analysis on the subject was published showing reduced levels of troponin in patients undergoing CABG (Citation21).
The effect of GLP-1 analogue infusion has never before been investigated in relation to cardiac surgery. We were in our trial unable to show a clear cardio-protective effect of exenatide infusion. The ischemic injury is significantly smaller during elective surgical procedures than during reperfusion after myocardial infarction. Thus, less ischemia most likely results in less reperfusion injury, which in turn makes the effect of any cardio-protective strategy more difficult to show. During cardiac surgery, the heart is cooled using cold blood cardioplegia, in order to reduce the ischemic effects. This can potentially reduce the effects of the GLP-1 analogue, either by inhibiting the GLP-1 effect directly or by offering protection per se that blunts any effect of exenatide. The myocardium was conditioned with the GLP-1 analogue before the placement of the aortic cross clamp, as well as after the removal of it. Potentially, adding the GLP-1 analogue to the cardioplegic solution, in order to accomplish perioperative conditioning of the myocardium, could potentially result in a different effect. However, according to a previous trial using exenatide as a protectant, the time window around reperfusion is critical and thus the compound has to be in circulation at the time of reperfusion (Citation13). There is also a possibility that another GLP-1 target concentration should be used in a surgical setting or perhaps even another GLP-1 analogue (e.g., lixenatide, liraglutide, and curaglutide).
The previous studies with GLP-1 analogues have not reported any cases of hypoglycemia. In our trial, we did however observe eight cases (35%). Even though easily manageable, the risk of hypoglycemic episodes adds another aspect to the usefulness of GLP-1 analogues in an elective surgical setting, when patients fast preoperative.
Research on possible pharmacological conditioning effects of anesthetic agents such as sevoflurane and remifentanil indicates that they potentially could have a cardio-protective effect (Citation22–24). However, such effects would have been present in all three interventional groups in this trial and would therefore not have affected the results.
The microdialysis data did not show any clear beneficial effects of the studied interventions. In this trial, the probes were placed in the same location every time. By doing so, that location acted as surrogate marker, representing the entire myocardium. This was done under the assumption that ischemic and reperfusion injury affects the entire myocardium evenly. Although this assumption is not entirely accurate, we decided on this design in order to investigate the general effects of ischemic and reperfusion injury in a standardized manner. We focused on parameters directly related to myocardial cell membrane damage and metabolic changes.
With further development of microdialysis technique for cardiac use, real-time microdialysis could constitute a new way to measure heart function postoperatively and it could potentially complement ECG, echocardiography, cardiac enzymes etc. In the excluded cases where peri- or postoperative infarctions where detected, corresponding ischemic changes were clearly seen in the microdialysis data, with sharp increase in lactate and glycerol levels. This indicates that the microdialysis technique definitely can be used to detect ischemic changes in the myocardium, and add early indication for angiographic reinvestigation of the cause. However, when inserting the probe, there is a small implantation trauma as well as a somewhat increased risk of bleeding. There is no doubt that the surgical technique regarding insertion and fixing of the microdialysis probes can and should be improved, in order to decrease the risk of such complications. Due to the risk of complications related to the microdialysis catheter, it has been concluded, in the light of the negative findings, that a large-scale trial using the current microdialysis technique cannot be justified at this point.
Trial limitations
The inclusion criteria used and the size of the trial resulted in a heterogeneous population when it comes to surgical procedures. This in turn resulted in a large spread of the data. It is evident that, although based on previous studies, our sample size calculations were too optimistic.
Microdialysis probes specifically designed for myocardial use does not exist and the current probes are very fragile. The contractions of the myocardium can sometimes damage the probe membrane, resulting in inaccurate measurements or failure of the probe itself.
Since the probe gets removed percutaneously, it must not be too well fixed. Therefore, there is a risk that the myocardial contractions drive the probe out of its desired location, which can result in inaccurate measurements.
Conclusion
We were unable to clearly demonstrate significant protective effects of the suggested cardio-protective interventions. However, the almost 30% reduction in troponin release following RIPC and a borderline significant p value indicates that a larger trial may reach statistical significance and that RIPC may have a place as a method of cardio-protection.
It will in the future be crucial to determine if the potential reduction in enzyme release, translates into a real a clinical benefit.
The risk of hypoglycemic episodes when using GLP-1 analogues needs to be taken into account in a surgical setting.
Microdialysis is a promising but not yet fully reliable method to assess myocardial markers in a general surgical population. This trial can hopefully contribute to generate a productive discussion concerning limitations and future use of cardiac conditioning as well as microdialysis technique.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work was funded by the Department of Cardiovascular & Thoracic Surgery and the Department of Cardiology and the Department of Cardiothoracic Anesthesiology, Copenhagen University Hospital, Rigshospitalet, Denmark.
References
- Vinten-Johansen J, Zhao ZQ, Jiang R, Zatta AJ, Dobson GP. Preconditioning and postconditioning: innate cardioprotection from ischemia-reperfusion injury. J Appl Physiol. 2007;103:1441–8.
- Verma S, Fedak PW, Weisel RD, Butany J, Rao V, Maitland A, et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105:2332–6.
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–9.
- Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–64.
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–9.
- Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–34.
- Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–86.
- Tapuria N, Kumar Y, Habib MM, Abu AM, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury—a review. J Surg Res. 2008;150:304–30.
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–50.
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
- Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–24.
- Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5.
- Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2011;33:1491–9
- Birnbaum Y, Ye Y, Bajaj M. Myocardial protection against ischemia-reperfusion injury by GLP-1: molecular mechanisms. Metab Syndr Relat Disord. 2012;10:387–90.
- Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9–36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–9.
- Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab. 2001;281:E155–E161.
- Mantovani V, Kennergren C, Goiny M, Ungerstedt U, Lonnroth P, Sala A, et al. Microdialysis for myocardial metabolic surveillance: developing a clinical technique. Clin Physiol Funct Imaging. 2006;26:224–31.
- Moller CH, Perko MJ, Lund JT, Andersen LW, Kelbaek H, Madsen JK, et al. Three-year follow-up in a subset of high-risk patients randomly assigned to off-pump versus on-pump coronary artery bypass surgery: the Best Bypass Surgery trial. Heart. 2011;97:907–13.
- Lomivorotov VV, Shmyrev VA, Nepomnyaschih VA, Ponomarev DN, Knyazkova LG, Lomivorotov VN, et al. Remote ischaemic preconditioning does not protect the heart in patients undergoing coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. 2012;15:18–22.
- Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, et al. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment?Circulation. 2010;122:S53–S59.
- D’Ascenzo F, Cavallero E, Moretti C, Omede P, Sciuto F, Rahman IA, et al. Remote ischaemic preconditioning in coronary artery bypass surgery: a meta-analysis. Heart. 2012;98:1267–71.
- Zaugg M. Is protection by inhalation agents volatile?Controversies in cardioprotection. Br J Anaesth. 2007;99: 603–6.
- Frassdorf J, Borowski A, Ebel D, Feindt P, Hermes M, Meemann T, et al. Impact of preconditioning protocol on anesthetic-induced cardioprotection in patients having coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2009;137:1436–42.
- Lemoine S, Zhu L, Massetti M, Gerard JL, Hanouz JL. Continuous administration of remifentanil and sufentanil induces cardioprotection in human myocardium, in vitro. Acta Anaesthesiol Scand. 2011;55:758–64.