Abstract
Introduction. Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with a variety of clinical features. Cardiac involvement is present in more than half of the patients with SLE. Fragmentation of QRS (fQRS) is presumed marker of cardiovascular risk and has not been previously evaluated in SLE. Methods. A total of 56 women previously diagnosed with SLE were recruited. In addition, a control group consisting of 51 healthy people was formed. QRS complexes were also evaluated in terms of fragmentations. All patients with SLE and control subjects underwent transthoracic echocardiographic examination. Erythrocyte sedimentation rate and C-reactive protein levels were also obtained. Results. Frequency of fQRS was higher in patients with SLE (41% vs. 21%, p = 0.03). Left ventricular posterior wall thickness and mass index were higher in the patients with SLE. CRP levels and age were significantly higher, and disease duration was significantly longer in the fQRS(+) group (p = 0.02, 0.01, and 0.006, respectively). Conclusion. A careful cardiovascular evaluation and follow-up is essential to continuously improve survival in SLE. For this purpose, fQRS may be used for the early detection in patients with SLE.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease of unknown etiology that predominantly affects women of childbearing age (Citation1,Citation2). The normal immune responses are redirected against healthy organs and tissues. The dysregulated immune system produces antibodies that attack the skin, joints, kidneys, heart, and brain (Citation3). The most common cause of death in SLE patients is cardiovascular disease (Citation4). Arrhythmias and conduction disturbances are frequently seen. Fibrosis plays a major role in cardiac arrhythmogenicity and reduces cardiac function (Citation5). Multiple small areas of fibrosis can affect ventricular repolarization and electrocardiographic abnormalities may be the first sign of a cardiac problem. Late potentials related to these histopathological changes may increase the risk of malignant ventricular arrhythmias (Citation6). Developing cardiac fibrosis in patients with SLE may lead to increased left ventricular mass and hypertrophy (Citation7).
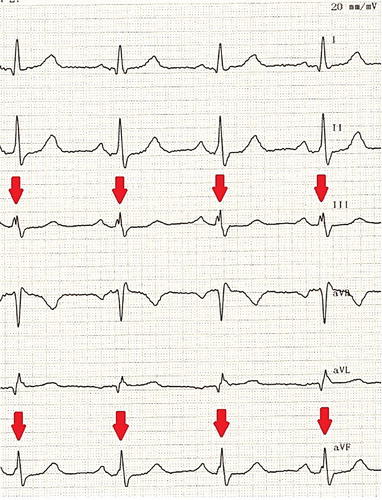
Fragmented QRS (fQRS) is bridging of an additional R’ wave with an R wave or upward or downward part of the S wave, or more than 1 R’ wave on two consecutive electrocardiogram (ECG) leads (Citation8). The fQRS complex is related to myocardial fibrosis and is associated with sudden cardiac death and predicts mortality (Citation9–11). The presence of a fQRS complex is easily evaluated using electrocardiograpy. In various rheumatic diseases, including rheumatoid arthritis, anklylosing spondylitis, and Behçet's disease the prevalence of fQRS appears to be higher than in controls (Citation12–14). fQRS might play a role as a screening and prognostic tool in the patients with SLE as well, and since this has not yet been reported, the present study examined the fQRS waves on ECGs of SLE patients.
Patients and methods
A total of 56 (56 females, mean age 35.9 ± 10.6) recruited, who were followed up at Selcuk University, diagnosed with SLE according to the 1982 revised criteria of the American College of Rheumatology for classification of SLE. A healty control group consisting of 51 age-matched and demographic feature-matched healty women were formed. The demographic and clinical features of the patients with SLE and the control group are given in . SLE patients were divided into two subgroups according to the presence or absence of fQRS.
Table I. Clinical characteristics of the study population.
Patients with ischemic heart disease, moderate or severe valve disease, cardiovascular medication, diabetes mellitus, malignancy, renal failure, hepatic failure, congestive heart failure, atrial fibrillation, history of arrhythmia, bundle branch block, incomplete right bundle branch block (QRS < 120 ms and RSR’ pattern on V1-V2), and other ECG abnormalities or abnormal serum electrolytes were excluded from the study.
The disease duration was estimated by considering onset as the day of the initial examination, on which the patient fulfilled all the American Rheumatism Association criteria. This study complies with the Helsinki declaration of 1975, as revised in 2000, and was approved by the Ethics Committee and the institutional review board of Selcuk University Medical School.
Electrocardiographic assessment
All standard 12-lead ECGs were obtained simultaneously using a recorder, which was set at a 50-mm/s paper speed and 20-mm/mV standardization. All ECGs were analyzed by two independent experienced cardiologists blinded both to the name and group of patients. All patients were sinus rhythm. QRS duration was measured from the onset to the end of the QRS complex. fQRS was defined as the presence of an additional R wave or R or S wave bridging, or the presence of fragmentation (more than 1 R’) on two consecutive leads that corresponded to the major coronary artery regions in a normal QRS intervals ().
Echocardiographic assessment
All patients and control subjects underwent transthoracic echocardiographic examination with Vivid E9 system using a 1,5-4.6 MHz probe (GE-Vingmed Ultrasound AS, Horten, Norway). Left ventricular (LV) dimensions and wall thickness were obtained from the parasternal long axis view with the M-mode cursor positioned just below the mitral leaflet tips, perpendicular to the long axis of the LV. LV ejection fraction was measured in accordance with Simpson's method. Left ventricular mass (LVM) was measured using the formula defined by Devereux. LVM index was calculated LVM divided by the body surface area. All Standard conventional echocardiographic assessments were performed according to published criteria of the American Society of Echocardiography.
Statistical analysis
SPSS 18.0 for Windows was used for statistical analyses (SPSS, Chicago, Illinois, USA). Quantitative data were expressed as mean ± SD. Categorical data were presented as frequencies and percentages. Categorical variables were compared using the X² test. A P value less than 0.05 was considered statistically significant.
Results
There was no significant difference in clinical and demographic data in SLE and control group. Systolic and diastolic blood pressure were higher in patients with SLE, but statistically insignificant. However, CRP and ESR levels in the patients with SLE were significantly higher compared with those of the control group (5.2 ± 3.7 vs. 3.3 ± 0.3 mg/L, p = 0.001; 22.3 ± 16.8 vs. 9.2 ± 5.7 mm/h, p < 0.001 respectively; ).
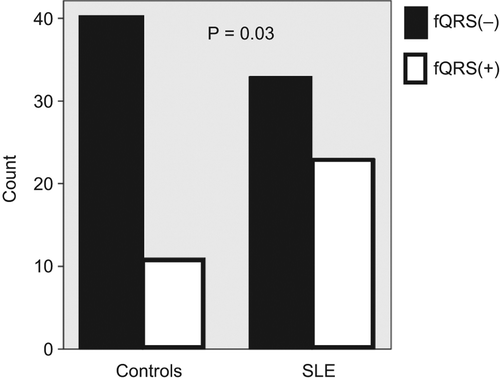
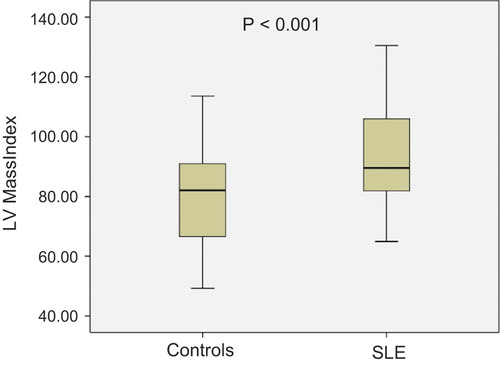
The prevalence of fQRS was significantly higher in patients with SLE (41% vs 21 p = 0.03) but QRS durations were similar in both groups (, ). There was no difference regarding LV diameters and ejection fractions. However, interventricular septum, posterior wall thickness, and LVMI were higher in the patients with SLE than in the control group (all p < 0.001; , ).
Table II. Comparison of the electrocardiographic and echocardiographic parameters.
SLE patients were divided into two subgroups according to the presence or absence of fQRS. The clinical and demographic characteristics features for the fQRS (+) and fQRS (−) patients are presented in . The differences in clinical characteristics, anti-SLE medication, electrocardiographic, and echocardiographic findings between the fQRS (+) and fQRS (-) subgroups of SLE patients were not statistically significant (p > 0.05). However, CRP levels and age were significantly higher, and disease duration was significantly longer in the fQRS (+) group (p = 0.02, 0.01, and 0.006, respectively). Echocardiographic parameters for fragmented and non-fragmented QRS are presented in . LVMI was higher in the fQRS (+) group than that in the fQRS (−) group, but the difference was not statistically significant.
Table III. Clinical and demographic features for fragmented and non-fragmented QRS.
Table IV. Echocardiographic paremeters for fragmented and non-fragmented QRS.
Discussion
To the best of our knowledge, this study is the first report on a higher prevalence of fQRS in patients with SLE compared to a control group.
The contemporary definition of fQRS is additional spikes within the QRS complex (Citation15). The specific cause of fractionations is incompletely understood, but frequently seen on routine surface ECG both with narrow and wide QRS complexes, and more often in paced rhythm, bundle branch block, or ventricular premature beats (Citation16). fQRS can be caused by zigzag conduction around a scarred myocardium, resulting in multiple spikes within the QRS complex (Citation8,Citation17). These fragmentations on surface ECG were found to be associated with increased adverse cardiac events in previous studies (Citation8,Citation10). Pathophysiologically, fQRS is generally believed to derive from regional myocardial fibrosis/scar and ischemia, which cause non-homogeneous myocardial electrical activation (Citation18). In patients with ischemic or non-ischemic LV dysfunction, fQRS has been shown to be related to myocardial fibrosis (Citation19). Cetin et al. observed an association between fQRS and CRP levels in patients with stable angina pectoris and suggested that fQRS was independently related to systemic inflammation (Citation20). In our study CRP levels were higher in patients with SLE having fQRS.
Clinical cardiac manifestations occur in less than 10% of SLE patients, and myocardial involvement can be seen in SLE patients even in their absence (Citation21). Previous studies with Tc-99 m sestamibi myocardial perfusion single-photon emission computed tomography showed a high incidence of myocardial perfusion abnormalities in asymptomatic lupus patients without clinical signs of cardiac involvement (Citation16). The possible use of fQRS as a screening tool in a systemic disease with cardiac involvement has not yet been investigated.
We found increased left ventricular wall thickness and LVMI in SLE group compared to controls confirming a recent study (Citation7). Pieretti et al. found that SLE was associated with could predict increased LVM in the absence of valvular and clinical coronary artery disease (Citation22) but, the clinical use of LVM measurements has not been firmly established in patients with cardiovascular disease (Citation23). There was, however, no significant relationship between echocardiographic abnormalities and the presence of fQRS.
In our study, a greater prevalence of fQRS in patients with SLE may be the result from myocardial fibrosis and increased LVM. In the present study, we found that duration of disease was higher in the fQRS (+) group. Moreover, duration of disease was a predictor of the presence of fQRS on ECGs. This finding might be indicate that long-standing chronic inflammation in SLE causes fQRS on ECGs, possibly signifying myocardial fibrosis.
Study limitations
The relatively low number of subjects in our study population is a limitation. The absence of qualitative measures of myocardial fibrosis, such as cardiac magnetic resonance imaging, is another one limitation.
Conclusion
In conclusion, clinical studies are required for confirmation of this relationship and assessment of the prognostic value of fQRS in SLE.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Foster MH. T cells and B cells in lupus nephritis. Semin Nephrol. 2007;27:47–58.
- Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308–18.
- Askanase A, Shum K, Mitnick H. Systemic lupus eythematosus: an overview. Soc Work Health Care. 2012;51: 576–86.
- Knight JS, Kaplan MJ. Cardiovascular disease in lupus: insights and updates. Curr Opin Rheumatol. 2013;25: 597–605.
- Teixeira RA, Borba EF, Bonfa E, Martinelli FM. Arrhythmias in systemic lupus erythematosus. Rev Bras Reumatol. 2010;50:81–9.
- Lazzerini PE, Acampa M, Guideri F, Capecchi PL, Campanella V, Morozzi G, et al. Prolongation of the corrected QT interval in adult patients with anti-Ro/SSA-positive connective tissue diseases. Arthritis Rheum. 2004;50:1248–52.
- Shang Q, Yip GW, Tam LS, Zhang Q, Sanderson JE, Lam YY, et al. SLICC/ACR damage index independently associated with left ventricular diastolic dysfunction in patients with systemic lupus erythematosus. Lupus. 2012;21:1057–62.
- Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–501.
- Das MK, Michael MA, Suradi H, Peng J, Sinha A, Shen C, et al. Usefulness of fragmented QRS on a 12-lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol. 2009;104:1631–7.
- Korhonen P, Husa T, Konttila T, Tierala I, Makijarvi M, Vaananen H, et al. Fragmented QRS in prediction of cardiac deaths and heart failure hospitalizations after myocardial infarction. Ann Noninvasive Electrocardiol. 2010;15:130–7.
- Das MK, Saha C, El Masry H, Peng J, Dandamudi G, Mahenthiran J, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–92.
- Kadi H, Inanır A, Habiboğlu A, Ceyhan K, Koc F, Celik A, et al. Frequency of fragmented QRS on ECG is increased in patients with rheumatoid arthritis without cardiovascular disease: a pilot study. Mod Rheumatol. 2012;22:238–42.
- Inanir A, Ceyhan K, Okan S, Kadi H. Frequency of fragmented QRS in ankylosing spondylitis: a prospective controlled study. Z Rheumatol. 2013;72:468–73.
- Sayin MR, Akpınar I, Gursoy YC, Kiran S, Gudul NE, Karabag T, et al. Assessment of QRS duration and presence of fragmented QRS in patients with Behçet's disease. Coron Artery Dis. 2013;24:398–403.
- Das MK, El Masry H. Fragmented QRS and other depolarization abnormalities as a predictor of mortality and sudden cardiac death. Curr Opin Cardiol. 2010;25:59–64.
- Das MK, Suradi H, Maskoun W, Michael MA, Shen C, Peng J, et al. Fragmented wide QRS on a 12-lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008;1:258–68.
- Morita H, Kusano KF, Miura D, Nagase S, Nakamura K, Morita ST, et al. Fragmented QRS as amarker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–704.
- Basaran Y, Tigen K, Karaahmet T, Isiklar I, Cevik C, Gurel E, et al. Fragmented QRS complexes are associated with cardiac fibrosis and significant intraventricular systolic dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Echocardiography. 2011;28:62–8.
- Calore C, Cacciavillani L, Boffa GM, Silva C, Tiso E, Marra MP, et al. Contrast-enhanced cardiovascular magnetic resonance in primary and ischemic dilated cardiomyopathy. J Cardiovasc Med. 2007;8:821–9.
- Cetin M, Kocaman SA, Canga A, Durakoglugil ME, Erdogan T, Satiroglu O, et al. The independent relationship between systemic inflammation and fragmented QRS complexes in patients with stable angina pectoris. Kardiol Pol. 2012;70:668–75.
- Kojuri J, Nazarinia MA, Gahahartas M, Mahmoody Y, Rezaian GR, Liaghat L. QT dispersion in patients with systemic lupus erythematosus: the impact of disease activity. BMC Cardiovasc Disord. 2012;12:11.
- Pieretti J, Roman MJ, Devereux RB, Lockshin MD, Crow MK, Paget SA, et al. Systemic lupus erythematosus predicts increased left ventricular mass. Circulation. 2007;116:419–26.
- Armstrong AC, Gidding S, Gjesdal O, Wu C, Bluemke DA, Lima JA. LV Mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC: Cardiovascular Imaging. 2012;5:837–48.