Abstract
Background. As patients with severe aortic valve stenosis (AS) develop symptoms their survival decreases rapidly, if treated conservatively. Transcatheter aortic valve implantation (TAVI) has been introduced as a less invasive treatment alternative, especially in inoperable patients, who often have severe comorbidities, including chronic obstructive pulmonary disease (COPD). Materials and methods. Since the beginning of our TAVI program in March 2008, data on all 131 TAVI patients were prospectively and consecutively collected in this registry with complete follow-up. COPD was present in 37 patients. By January 2012 survival data were collected from the Danish Civil Registration System. Median follow-up duration was 559 days. Results. Overall survival and survival from cardiac death was equivalent in both patients with and without COPD (p = 0.98 and p = 0.26) in the follow-up period. Further, patients with COPD had higher New York Heart Association (NYHA) class prior to intervention compared with those without (3.1 ± 0.5 vs. 2.9 ± 0.5, p = 0.02). In multivariate regression analysis COPD was associated with 30-day postoperative NYHA class (0.43; 95% confidence interval (CI): 0.10–0.75; p = 0.01), but not to NYHA class improvement from pre- to postintervention (0.25; 95% CI: − 0.12 to −0.63; p = 0.18). Conclusions. In patients with symptomatic severe AS treated with TAVI, the presence of COPD neither affects overall survival nor survival from cardiac death. Patients with COPD had, however, both higher pre- and postoperative NYHA class compared with patients without COPD, but NYHA class improvement from pre- to postintervention was equivalent in both groups.
Introduction
As patients with severe aortic valve stenosis (AS) develop symptoms their survival decreases rapidly, if treated conservatively (Citation1,Citation2). Surgical aortic valve replacement (sAVR) is the conventional treatment that improves both survival and morbidity in these patients (Citation3). Recently, transcatheter aortic valve implantation (TAVI) has been introduced as a less invasive treatment alternative, especially in inoperable patients (Citation4–8), who often have severe comorbidities, including chronic obstructive pulmonary disease (COPD) (Citation9). Further, short-term follow-up data in high-risk operative patients have shown that TAVI is a good treatment alternative; however, long-term data are still not available (Citation2,Citation6–8,Citation10).
The aim of this study was twofold. First, to evaluate if TAVI can improve New York Heart Association (NYHA) class in patients with symptomatic severe AS and a history of COPD. Second, to evaluate survival after TAVI in these patients with symptomatic severe AS.
Materials and methods
Patients referred for TAVI between March 2008 and December 2011 at our center were prospectively and consecutively enrolled in a single-center registry. Patients were considered eligible for TAVI in the presence of symptomatic severe AS (aortic valve area (AVA): < 1.0 cm2) and high surgical risk, as judged by consensus between senior cardiologists, cardiac surgeons, and anesthesiologists based on a composite evaluation of EuroScore and non-scored data, for example, frailty, lung capacity, and liver function. In addition, high-risk patients were further subdivided into potential operable, but TAVI deemed the better option, and inoperable patients using the aforementioned variables.
If the patient was selected for TAVI at the above-mentioned team conference, a senior cardiologist evaluated the NYHA class prior to the TAVI procedure and inserted the NYHA class in the registry. Further, all patients underwent a lung function test prior to the TAVI procedure.
At enrollment each patient was evaluated for COPD. Patients with COPD were diagnosed by either their general physician or pulmonologist prior to referral for cardiac evaluation using the standard criteria for COPD in Denmark (chronic, slowly progressive disorder characterized by airflow obstruction; forced expiratory volume (FEV1): < 80% of predicted and FEV1/forced vital capacity ratio: < 0.70) and further at referral, each patient diagnosed with COPD should also have had a long-term use of bronchodilators or steroids using the EuroScore definition of COPD (Citation11). Two of the patients with COPD were on long-term oxygen therapy (continuous home oxygen therapy 24 h a day).
Transcatheter aortic valve implantation
All TAVIs were performed using standard techniques. Two commercially available valves were used: The balloon-expandable Edwards SAPIEN (Edwards Lifesciences) (n = 23) or the self-expandable CoreValve (Medtronic) (n = 108). At our center the transfemoral approach was the first method of choice (n = 123). In some cases (n = 8) this was not possible due to tortuosity, calcification, and/or atheroma of the aortoiliofemoral tree as demonstrated by angiography and/or computed tomography. In these cases (n = 8), a left subclavian approach was used. General anesthesia was used in the beginning of the registrations (n = 62); however, this strategy was changed to local anesthesia without any change in complications (data not shown). Postprocedure, each patient was observed in the intensive care unit (ICU). The first 20 patients were observed in the ICU for 12 h, the next 22 patients for 6 h, and since then for 4 h. After the ICU observation, patients were transferred from the ICU to the department of cardiology for further 4 days—especially for the detection of arrhythmia. None of the patients needed postprocedural intubation; however, two patients were treated with non-invasive ventilation, and one of them had COPD.
Echocardiography
All echocardiograms were performed on a GE Vivid 7 Ultrasound Machine (GE Medical System, Horten, Norway) and Philips iE33 (KoninKlijke Philips Electronics, Eindhoven, Netherlands), digitally stored and later analyzed blinded for all clinical and survival data.
AVA was estimated with quantitative Doppler using the continuity equation. The diameter of the left ventricular (LV) outflow tract was measured 5 mm below the annulus from a zoomed image of the LV outflow tract obtained in the parasternal long axis view. Peak flow velocity across the valve was determined in the apical window or the echocardiographic window where the highest peak velocity could be obtained by placing the continuous-wave Doppler cursor as parallel as possible with the flow across the valve. Peak transvalvular gradient was estimated using the modified Bernoulli equation (Citation12). Finally, the peak systolic flow velocity in the outflow tract was estimated with pulsed-wave Doppler. LV ejection fraction (LVEF) was estimated using Simpson's biplane method (Citation13).
LV mass was estimated according to the joint recommendations of the American (ASE) and European (EAE) associations of echocardiography (Citation13). LV wall thickness and dimensions were estimated from 2D images obtained in the parasternal long axis view according to guidelines. Left atrial volume index (LAVi) was measured in LV end-systole (maximum LA size). The volume was measured using the biplane area–length method and corrected for body surface area (Citation14).
Clinical follow-up
Short-term outcome was registered 30 days postoperatively at a clinical visit performed by a senior cardiologist. At this visit NYHA class was evaluated and an echocardiogram was performed as described above. Further, long-term outcome on survival was collected from the Danish Civil Registration System and from discharge notes available in the Danish Admission Registry in January 2012. In case of ambiguous information, local hospitals were contacted and detailed patient charts were reviewed.
Statistical methods
Continuous variables are presented as mean and standard deviation, and categorical variables as numbers and percentages. We used Student's t-test to test for differences between independent continuous variables and Fischer's exact test for differences between categorical variables. We analyzed the association between each outcome variable (postoperative NYHA class and NYHA class improvement from pre- to postintervention), and the predefined clinical parameters using linear regression analysis. Due to significant colinearity of FEV1 and postoperative NYHA class, this parameter was excluded in the multivariable analysis. Results are reported as regression coefficient (β) with 95% confidence intervals (CIs) and corresponding p values.
Mortality and event rates were calculated using the product limit method and was plotted according to the Kaplan–Meier method, and death rates were compared using the log-rank test. Further, estimation of risk was performed using Cox proportional hazard models. The assumptions (proportional hazard assumption, linearity of continuous variables, and lack of interaction) were tested and found to be valid. A p value < 0.05 was considered significant using STATA/SE 12.0.
Results
TAVI was performed in 131 patients with symptomatic severe AS, in whom 56 were categorized as high-risk operable and 75 were categorized as inoperable. Combined symptomatic severe AS and COPD was present in 37 patients. provides baseline clinical data.
Table I. Patient characteristics.
For all patients, COPD status at enrollment, NYHA class pre- and postinterventional lung function test results were available.
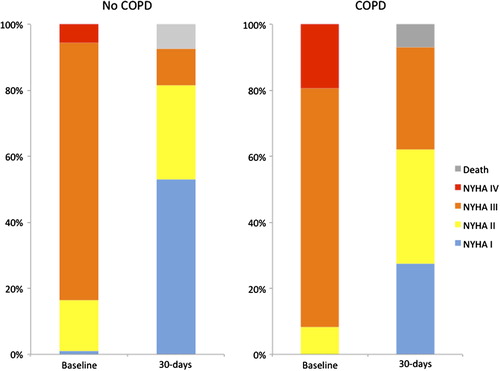
As patients with symptomatic severe AS and COPD (n = 37) were compared with patients without COPD (n = 94), patients with COPD were younger (79.6 ± 6.0 vs. 82.0 ± 4.2 years, p = 0.01); had lower FEV1 (1.1 ± 0.5 vs. 1.8 ± 0.7l, p < 0.001), lower FEV1% (56 ± 17 vs. 78 ± 22%, p < 0.001), and higher preoperative NYHA class (3.1 ± 0.5 vs. 2.9 ± 0.5, p = 0.02); and were less often categorized as operable (n = 9 (24%) vs. 47 (50%), p = 0.007).
Echocardiography data are shown in . Patients with COPD had smaller aortic valve area (0.65 ± 0.13 vs. 0.73 ± 0.24 cm2, p = 0.04) and higher LVEF (55 ± 15 vs. 53 ± 12%, p = 0.03).
Table II. Echocardiographic data.
30-day postoperative outcome
Six patients died during the first 30 days after TAVI with no difference between patients with or without COPD (n = 1 (3%) vs. n = 5 (5%), p = 0.59) (). No difference was observed between patients with or without COPD with respect to postoperative stroke (n = 4 (11%) vs. n = 7 (7%), p = 0.53) or number of reoperations (n = 0 (0%) vs. 2(2%), p = 0.37).
Table III. Postoperative data.
Although implanted valve sizes were smaller in patients with COPD (26 ± 2 vs. 27 ± 2, p = 0.013), hemodynamic flow characteristics were similar in both groups with respect to peak gradients (15.4 ± 6.8 vs. 15.3 ± 6.0 mmHg, p = 0.94) and effective orifice areas (1.7 ± 0.4 vs. 1.7 ± 0.3 cm2, p = 0.57). Further, postoperative LVEFs were similar (54 ± 11 vs. 52 ± 13%, p = 0.47). Finally, postoperative NYHA class was higher among patient with COPD versus those without COPD (2.0 ± 0.8 vs. 1.5 ± 0.7, p = 0.004).
In a univariable regression analysis, preoperative NYHA class, COPD, FEV1, and EuroScore were predictors for postoperative NYHA class (). In a multivariable regression analysis, excluding FEV1 (due to significant colinearity), COPD was the only predictor of postoperative NYHA class ().
Table IV. Regression analysis including factors associated with postoperative NYHA class.
Furthermore, in a univariable regression analysis preoperative NYHA class was the only predictor for NYHA class improvement from pre- to postintervention ().
Table V. Regression analysis including factors associated with NYHA class improvement.
Improvement in NYHA class after TAVI was demonstrated in both patients with and without COPD (), although patients with COPD had higher NYHA class prior to intervention (3.1 ± 0.5 vs. 2.9 ± 0.5, p = 0.02). Finally, COPD was associated with postoperative NYHA class (); however, COPD was not associated with NYHA class improvement from pre- to postintervention (), demonstrating that patients with COPD improved equivalently in NYHA class compared with patients without COPD.
Survival data
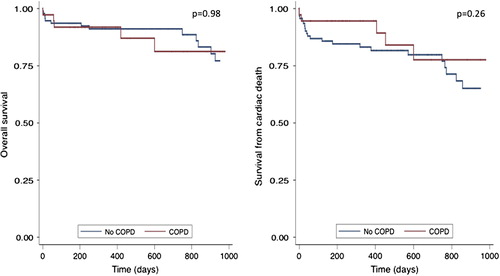
Median follow-up duration was 559 (249–951) days. Survival status was available for all patients. Overall, there were 19 deaths, 14 (15%) in patients without COPD and 5 (14%) in patients with COPD (p = 0.84).
The Kaplan–Meier plot for overall survival and survival from cardiac death in the follow-up period demonstrated no difference in patients with COPD versus those without COPD (p = 0.98 and p = 0.26, respectively) (). Finally, patients with COPD were younger than those without COPD (79.6 ± 6.0 vs. 82.0 ± 4.2 years, p = 0.01); however, age did not significantly affect the overall survival when these two groups were compared using a multivariable Cox regression analysis (COPD: hazard ratio—0.80 (0.28–2.33), p = 0.69 and age: hazard ratio—0.92 (0.84–1.01), p = 0.09).
Discussion
In patients with COPD and severe AS it may be difficult to distinguish the precise contribution of each pathology, when progressive dyspnea appears (Citation6). In the present prospective consecutive registry study of 131 patients with symptomatic severe AS treated with TAVI, medium-term follow-up demonstrated equivalent overall survival and survival from cardiac death in patients with or without COPD (). Further, patients with COPD had both higher pre- and postoperative NYHA class compared with patients without COPD, respectively, but NYHA class improvement from pre- to postintervention was equivalent in both groups.
The PARTNER trial demonstrated that TAVI was a non-inferior option to sAVR in high-risk patients with symptomatic severe AS, with a 30-day mortality of 3.7% and a 1-year mortality of 24.2% (Citation15). Further, the FRANCE2 trial showed similar 30-day and 1-year mortalities (9.7% and 24.0%, respectively) in inoperable patients with severe AS undergoing TAVI (Citation4). Our single-center patients had similar 30-day mortality (4.6%), and lower 1-year mortality rate (8.4%) than in the above-mentioned multicenter studies (Citation4,Citation15). This difference may reflect the fact that patients undergoing TAVI in our registry were younger and had lower EuroScore. The prevalence of COPD was, however, similar to the ones reported in the PARTNER and FRANCE2 trials (Citation4,Citation15).
It has previously been shown that patients with symptomatic severe AS could be subdivided according to comorbidity. High-risk patients were characterized as having advanced age and COPD, and renal failure—or both (Citation9). Grossi et al., further, demonstrated COPD as being a long-term prognostic predictor of worse outcome in patients undergoing sAVR (Citation16), which the PARTNER and FRANCE2 trials also reported at 1-year follow-up for patients undergoing TAVI (Citation17,Citation18). Although the PARTNER trial found that patients with COPD undergoing TAVI had worse outcome compared with those without COPD, COPD patients who had TAVI performed had better survival compared with those receiving standard therapy (Citation18). Finally, these studies with symptomatic severe AS patients treated with TAVI demonstrated that COPD was an independent predictor of 1-year survival postimplant in their multivariate regression analysis (Citation6,Citation7). Both the PARTNER (high-risk patients) and the FRANCE2 (inoperable patients) trials evaluated patients with severe symptomatic AS undergoing TAVI and demonstrated that NYHA class improved within 30 days postoperatively (Citation4,Citation15). The authors, however, were not able to demonstrate any further functional class improvement beyond 30 days (Citation4,Citation15). Mok et al. reported improvement in both NYHA class and 6-minute walk test 6 months after TAVI in patients with and without COPD (Citation19). We demonstrate a similar postoperative NYHA class improvement in our population, and further we also demonstrated that NYHA class improvement from pre- to postintervention was independent of COPD ( and ) (). These findings demonstrate that symptom improvement after TAVI is already observed within 30 days postoperatively and also occurs in patients with COPD, which further favors TAVI as a fast symptom relief strategy, especially in patients with high preoperative NYHA class including patients with COPD. NYHA class improvement from pre- to postintervention in our registry was similar among operable and inoperable patients (1.3 ± 0.8 vs. 1.3 ± 0.9, p = 0.88), suggesting that TAVI, after careful individual patient evaluation, should be considered even in this very-high-risk group.
Study limitations
Although data were prospectively and consecutively collected, no prespecified case report form was designated for the purpose of this study. Cases were, however, presented in a similar presentation format in a team conference (attended by senior cardiologists, cardiac surgeons, and anesthesiologists) called twice weekly, partially compensating for this limitation. The sample size was small with relatively few events, which make our models unstable with a potential risk of overfitting the models. The present study should be considered hypothesis generating, and clearly larger studies are warranted.
Conclusions
In this single-center prospective consecutive registry, patients with symptomatic severe AS and COPD undergoing TAVI demonstrated no difference in overall survival and survival from cardiac death when compared with patients without COPD during the follow-up period. Further, patients with COPD undergoing TAVI had both higher pre- and postintervention NYHA class, but NYHA class improvement from pre- to postintervention was similar in patients with and without COPD. Therefore, we propose that TAVI should be considered even in patients with both symptomatic severe AS and COPD.
Acknowledgements
Thanks to the laboratory nurses and secretaries from the Department of Cardiology, Odense University Hospital. The study has not been funded.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ross J Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–7.
- Wenaweser P, Pilgrim T, Kadner A, Huber C, Stortecky S, Buellesfeld L, et al. Clinical outcomes of patients with severe aortic stenosis at increased surgical risk according to treatment modality. J Am Coll Cardiol. 2011;58:2151–62.
- Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451–96.
- Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–15.
- Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–704.
- Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–8.
- Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–90.
- Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308.
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43.
- Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–95.
- Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–22; discussion 22–3.
- Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
- Moller JE, Pellikka PA, Hillis GS, Oh JK. Prognostic importance of diastolic function and filling pressure in patients with acute myocardial infarction. Circulation. 2006;114:438–44.
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98.
- Grossi EA, Schwartz CF, Yu PJ, Jorde UP, Crooke GA, Grau JB, et al. High-risk aortic valve replacement: are the outcomes as bad as predicted? Ann Thorac Surg. 2008; 85:102–6.
- Chopard R, Meneveau N, Chocron S, Gilard M, Laskar M, Eltchaninoff H, et al. Impact of chronic obstructive pulmonary disease on Valve Academic Research Consortium- defined outcomes after transcatheter aortic valve implantation (from the FRANCE 2 Registry). Am J Cardiol. 2014; 113:1543–9.
- Dvir D, Waksman R, Barbash IM, Kodali SK, Svensson LG, Tuzcu EM, et al. Outcomes of patients with chronic lung disease and severe aortic stenosis treated with transcatheter versus surgical aortic valve replacement or standard therapy: insights from the PARTNER trial (placement of AoRTic TraNscathetER Valve). J Am Coll Cardiol. 2014;63:269–79.
- Mok M, Nombela-Franco L, Dumont E, Urena M, DeLarochelliere R, Doyle D, et al. Chronic obstructive pulmonary disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes, prognostic markers, and functional status changes. JACC Cardiovasc Interv. 2013;6:1072–84.