Abstract
Objectives. The aetiology and outcome of constrictive pericarditis vary between geographic regions and has changed over time. We describe the diagnostic work-up and outcome in a contemporary cohort of Danish patients with constrictive pericarditis. Design. Hospital databases were searched for patients undergoing cardiac catheterisation for suspected constrictive pericarditis or for patients undergoing pericardiectomy or discharged with the diagnosis of constrictive pericarditis. Results. We identified 57 patients with constrictive pericarditis diagnosed from 1998 to 2012. Previous surgery and inflammatory disease were the most prevalent underlying conditions. Diagnosis was made primarily by echocardiography and right- and left-sided cardiac catheterisation. Echocardiography was particularly notable for dilated inferior caval vein, increased E/A ratio, and high septal tissue velocity in addition to the presence of septal bounce. Pericardiectomy was performed in 47 patients with a 30-day mortality of 8.5%. Clinical improvement was noted in 69% of cases. Several echocardiographic parameters normalised with time, including markers of diastolic function. Conclusions. Long-term outcome after pericardiectomy is acceptable with clinical improvement and partial resolution of the echocardiographic parameters in the majority of patients.
Introduction
Constrictive pericarditis is the presence of a fibrous or calcific pericardium limiting myocardial expansion, adversely influencing cardiac function. Diagnosis is based upon clinical features of cardiac failure with supportive echocardiographic and haemodynamic findings of a restrictive filling pattern and increased and equalised diastolic pressures. Computerised tomography or magnetic resonance imaging can be used diagnostically by visualising the thickened pericardium (Citation1).
Several causes of constrictive pericarditis have been described, including inflammation or infection, previous irradiation of the thoracic region, or previous cardiac surgery, but many cases are idiopathic (Citation2,Citation3). The aetiologies of constrictive pericarditis have changed with time, and a recent study suggests that the frequency of post-radiation constrictive pericarditis has declined, whereas constrictive pericarditis secondary to cardiac surgery has increased (Citation2). Tuberculosis is still the major single cause of constrictive pericarditis in Asia (Citation4–7), but the incidence of tuberculosis and tuberculous pericarditis has declined to low levels in the Western World (Citation8,Citation9).
Provided that the outcome of constrictive pericarditis is related to aetiology, it must be assumed that the survival of patients with constrictive pericarditis has changed during later years and differs throughout the World. There is, however, conflicting evidence as to whether the outcome of constrictive pericarditis is related to the aetiology (Citation2,Citation9,Citation10), and only one recent report of constrictive pericarditis in Scandinavia (Citation9).
The preferred treatment of constrictive pericarditis is surgical pericardiectomy. With few exceptions (Citation11), conservative management with diuretics and drainage of effusions (pleural, pericardial, and ascites) is considered mere palliation (Citation6). Outcome in conservatively managed patients is not known as most studies consider only patients undergoing pericardiectomy (Citation2,Citation4–7). In this study, we aim to describe the aetiologies of constrictive pericarditis, the haemodynamic status, and outcomes of surgical as well as conservative treatment in a well-characterised population of Danish patients.
Materials and methods
Patients
The study was conducted as a retrospective analysis of patients referred to the Department of Cardiology, Rigshospitalet, Copenhagen for evaluation of constrictive pericarditis. Patients were identified by searching the hospital databases for
| – | Patients evaluated with right-sided cardiac catheterisation, with a pre- or postevaluation suspicion or diagnosis of constrictive pericarditis | ||||
| – | Patients discharged from hospital with a diagnosis of constrictive pericarditis | ||||
| – | Patients undergoing pericardiectomy | ||||
Patients’ hospital files were reviewed for medical history concerning predisposing factors for constrictive pericarditis (previous cardiac surgery, malignancy, chemotherapy, irradiation of thoracic region, and rheumatologic/systems disease). Symptoms and clinical status at presentation were recorded, as well as coexisting ischaemic heart disease, heart failure, renal failure, diabetes, and chronic obstructive pulmonary disease.
The use of diagnostic modalities (echocardiography, cardiac catheterisation, computed tomography, and magnetic resonance imaging) was recorded. Echocardiography and cardiac catheterisation examinations were reviewed as described below.
Echocardiography
All echocardiography examinations were reviewed. In most cases (80%) the examinations were saved in original data in Philips Xcelera (Xcelera software, Philips Medical Systems) and the examinations were re-read by one person (NI) blinded to the time of the examination in relation to diagnosis or surgery (if performed). In the remaining cases the examinations were too old to be stored as loops in the echocardiographic database and the original written report was used to extract the data available.
In general, three echocardiography examinations were sought for each patient: one at the time of cardiac catheterisation (‘Diagnostic echocardiography’), one early after pericardiectomy during admittance (‘Early post-operative echocardiography’), and one late after pericardiectomy, years later (‘Late post-operative echocardiography’).
Echocardiographic variables were collected as follows: Diameter of inferior caval vein (IVC), retrograde liver flow, right atrium size, left atrium size, right ventricle diameter, left ventricular end-diastolic diameter, the presence of septal bounce, mitral flow profile, early (E) and late (A) diastolic flow, mitral valve deceleration time, mitral inflow respiratory changes, tissue Doppler velocity of septal and lateral annulus s’ and e’. Additionally, respiratory changes in transmitral flow (more than 25% considered abnormal) and presence of abnormal pericardium (defined as thickened or echogenic) was noted. Valvular disease was quantified using the European Society of Cardiology guidelines (Citation12).
Cardiac catheterisation
All cardiac catheterisation reports were reviewed. Wherever possible, original examination reports were reviewed. Parameters noted were as follows: Cardiac output, cardiac index, pulmonary artery systolic and diastolic pressures, mean right atrial pressure, right and left ventricular systolic and diastolic pressures, pulmonary capillary wedge pressure, and pulmonary vascular resistance. The presence of dip-plateau was noted.
Intervention
The operative procedure was performed through, most often, a median sternotomy or via a left anterolateral approach; the latter was used when exploring the posterior aspects of the pericardium. Cardiopulmonary bypass was necessary in seven patients in case of haemodynamic instability during dissection of the heart or when other cardiac procedures were performed. The pericardium was resected down to approximately 1 cm from the phrenic nerve on both sides and where possible the posterior part of the pericardium was removed too. The procedure was subtotal in 26 cases.
Outcome
Only patients who were diagnosed with constrictive pericarditis were considered in the further analyses.
Survival was calculated as the time from diagnosis (defined as the date of right-sided cardiac catheterisation or echocardiography if the former had not been performed) to death or until December 31st 2012. Date of death was found in the cardiac catheterisation database. Cause of death was sought from hospital records.
Patients’ most recent echocardiography record was reviewed for reversal or persistence of features associated with constrictive pericarditis as mentioned above. Clinical status was sought from hospital records.
Statistics
Continuous data are presented as mean (+/− SD) or proportions. Patients with constrictive pericarditis treated with pericardiectomy or managed conservatively were compared using Student's t-test for continuous variables, and in the case of categorical data, Fisher's exact test or Chi-square test was used.
Survival curves were generated according to the Kaplan–Meier method and the two patient groups were compared with log-rank statistics. Prognostic effects of age and previous sternotomy were evaluated using Cox regression analyses. All analyses were two-tailed with a significance level of P < 0.05.
Analyses were performed using SPSS 19.0.
Ethics
The study was entirely registry-based without direct participation of patients and informed consent from the patients was not required.
Results
Patients
Between January 1st 1998 and December 14th 2012, 86 patients were identified. Information regarding two patients was so scarce, that the patients were excluded from the study. Of the remaining patients, 57 patients were diagnosed with constrictive pericarditis, including one patient with an organised pericardial haematoma causing a constrictive physiology. Another 27 patients were evaluated with right-sided cardiac catheterisation due to a suspicion of constrictive pericarditis, but the diagnosis rejected. Patients in whom the evaluation led to a rejection of the clinical suspicion of constrictive pericarditis were most often diagnosed with ischaemic heart disease and/or heart failure from other causes. Only one patient was identified with restrictive cardiomyopathy. Only patients diagnosed with constrictive pericarditis were considered for further study. The incidence rate of constrictive pericarditis in Eastern Denmark is estimated at 0.202 per 100,000 person years. Demographics are given in .
Table I. Demographics and symptoms at diagnosis. Information was not available for all patients.
Of the 57 patients at some point diagnosed with constrictive pericarditis, ten were re-evaluated due to repeated clinical suspicion of constrictive pericarditis. One patient was not diagnosed until second evaluation. Six of the re-evaluations concerned suspected recurrence after pericardiectomy and two of these patients were diagnosed with recurrence and underwent repeated pericardiectomy.
Symptoms at presentation were those of heart failure with pleural effusions, ascites, and considerable dyspnoea with most patients exhibiting symptoms consistent with New York Heart Association (NYHA) functional class II-III ().
In 36 cases, it was possible to identify one or more probable causes of constrictive pericarditis. Rheumatologic disease (such as rheumatoid arthritis) and previous cardiac/thoracic surgery were most frequent. A significant proportion of cases were idiopathic and in some cases, information was insufficient as to decide a cause ().
Diagnostic work-up
Patients were evaluated with right- and left sided cardiac catheterisation, and results were available for 47 of the patients diagnosed with constrictive pericarditis. Haemodynamic data are given in . Haemodynamic findings consistent with constrictive pericarditis were pressure curves with a ‘dip-plateau’ configuration, increased and equalised diastolic pressures, and decreased cardiac output/cardiac index (). When only a qualitative report was available, these typically noted increased and equalised diastolic pressures.
Table II. Diagnostics and outcome.
Computed tomography was performed in 61% of cases and magnetic resonance imaging in 17% of cases (). The former was useful in demonstrating effusions, pericardial calcifications, and malignancy (or to rule out malignancy) whereas magnetic resonance imaging aided the diagnosis through demonstration of cardiac anatomy and physiology consistent with constrictive pericarditis.
Key echocardiographic parameters are shown in . Echocardiography was available in 44 patients at baseline (‘diagnostic echocardiography’), in 22 patients at ‘early post-operative echocardiography’ (mean of 8.8 ± 8.0 days) after surgery and in 22 patients at the ‘late post-operative echocardiography’ (mean of 32 ± 27 months after surgery). There was no follow-up echocardiography in the medically treated group. One patient had severe mitral regurgitation and underwent mitral valve repair during the pericardiectomy. The patient was excluded from the analysis before and after surgery. The remaining patients were all without significant valvular disease.
Table III. Echocardiography parameters at the time of cardiac catheterisation (‘Diagnostic’) and early (days) after surgery (‘Early post-op’) and late (months) after surgery (‘Late post-op’).
A number of echocardiographic abnormalities were present at time of cardiac catheterisation (Citation13–15): dilated inferior caval vein in 100% of patients, diastolic retrograde flow in liver veins in 90% of patients, left atrial dilatation in 78% (mean left atrial volume/BSA of 48.7 ± 24.5 ml), right atrial dilatation in 64% of patients, E/A ratio of more than 1 in 96% of patients, short mitral deceleration time (135 ± 31 ms) as compared with normal, high septal tissue velocity (13.2 ± 3.9 cm/sec) as compared with normal, and a high ratio of early diastolic septal versus lateral tissue velocity at the mitral annulus (usual below 1). The pericardium was noted abnormal (thickened or echogenic) in 98% of patients and the characteristic septal ‘bounce’ was present in 98% of patients. Less prevalent was abnormal respiratory mitral flow variation (more than 25%) which was only present in 53% of patients.
Choice of treatment
Of the patients diagnosed with constrictive pericarditis, ten were managed conservatively, whereas 46 were treated with pericardiectomy and one patient with pericardiectomy and mitral valve repair. One of the patients had pericardiectomy after a failed course of immunosuppression. The decision not to perform pericardiectomy was based on anticipated prohibitive surgical risk (five patients) or limited symptoms, allowing for conservative management with symptomatic treatment and control (four patients). One patient was successfully treated for an underlying inflammatory condition. Finally, one patient declined surgery.
Patients managed conservatively were comparable with those undergoing pericardiectomy as regards age and haemodynamic status, except for higher right atrial pressures in the patients undergoing surgery (P = 0.0007) ( and ).
Outcome
The patients who underwent pericardiectomy were followed for median 3.5 years (range 9 days–14.2 years) after diagnosis. During this time 11 (23.4%) patients died with median time from diagnosis to death of 190 days (range 9 days–9.4 years) (). Four deaths (8.5%) occurred within 30 days after operation; these deaths were due to circulatory or respiratory failure. It was seldom possible to ascertain the cause of death in the cases of later death because the deaths mainly occurred outside our institution. Survival did not differ between patients undergoing total versus subtotal pericardiectomy (data not shown).
Figure 1. Survival curves representing survival in patients managed with pericardiectomy or conservatively (a) overall survival (a) and influence of previous sternotomy on survival after pericardiectomy (b).
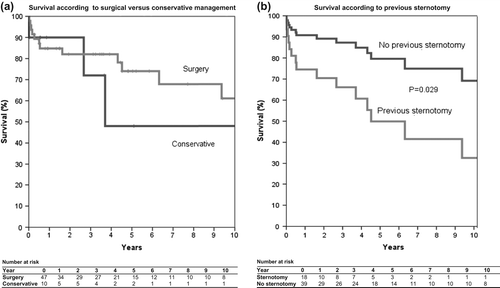
Regarding the patients who were managed conservatively, 4 (40%) died during a median follow- up time of 1.8 years (range 2 days–11.3 years) (). Two patients died from progressive heart failure, one from circulatory collapse secondary to cardiac invasion of lymphoma. The cause of death was unknown for the last case.
Mortality was not significantly different between the patients undergoing pericardiectomy and those managed conservatively. On the other hand, mortality was higher for patients who had undergone previous cardiac/thoracic surgery (Hazard Ratio 0.33, 95% CI 0.12–0.89, P = 0.029) (), indicating that the aetiology of constrictive pericarditis may influence outcome. This difference in survival was found not to be caused by differences in age between the two groups. Regarding the patients, for whom data were available after pericardiectomy, 22 (69%) had clinical improvement ranging from less dyspnoea to complete remission.
Two patients had repeat pericardiectomy performed. Both were alive at the time of follow-up (after 1 month and 1.7 years, respectively).
Post-operative echocardiography
Early after surgery few echocardiographic variables changed: tricuspid annular plane systolic excursion was reduced by a mean of 3 ± 4 mm. Of the diastolic parameters, only mitral deceleration time (increase of 22.1 ± 30.7 ms) and septal early diastolic tissue velocity (decrease of 3.9 ± 2.8 cm/sec) changed. All other parameters were unchanged relative to the diagnostic echocardiography ().
At late follow-up a number of parameters normalised, but of note septal bounce was still present in 70% of patients and the pericardium (or pericardial space) remained visually abnormal on 2-D echocardiography in almost all patients (95%). The IVC diameter decreased but remained dilated in 82% of patients. Both left and right ventricle increased in size and so did left atrium. The diastolic measures all changed towards normal including E/A ratio, septal early diastolic tissue velocity, ratio of septal versus lateral early diastolic tissue velocity, and mitral flow deceleration. Abnormal respiratory mitral variation was not present in any patient late after surgery ().
Discussion
In summary, we characterise a contemporary population of patients with constrictive pericarditis. Our main findings are that pericardiectomy in a selected group of patients is associated with good clinical outcome and that certain echocardiographic signs appear to have a high specificity for constrictive pericarditis but that the time course of improvement of the variables following surgery differs.
The diagnostic work-up for patients suspected of suffering from constrictive pericarditis consisted mainly of cardiac catheterisation and echocardiography. Magnetic resonance imaging, when employed, contributed with diagnostic information and might be considered in difficult cases (Citation16).
Using echocardiography routinely in the diagnosis and follow-up of patients, we have identified echocardiographic markers that are almost always present at diagnosis, like dilated IVC, E/A ratio of more than 1, septal tissue velocity that are equal or higher than the lateral and septal bounce. Less prevalent is abnormal transmitral respiratory changes. These observations are in accordance with recent findings (Citation17). Further we identify which variables are subject to early post-operative changes like increase in mitral deceleration time and reduction in septal tissue velocity and which parameters are persistent at late follow-up like septal bounce and increased size of the left atrium. While the reason for the persistent septal bounce remains obscure, the persistent increase in left atrial size might reflect residual diastolic dysfunction and in some cases relief of atrial constriction allowing for further dilatation of the chamber after surgery. Such postoperative echocardiographic changes have been described only a few times, but are comparable with our findings (Citation18).
Recurrence does occur after pericardiectomy and is obviously related to performing subtotal rather than complete pericardiectomy (Citation19). Recurrence rate obtained by us was 4% which is comparable with previous reports (Citation19–21). Echocardiography may be of less value for diagnosing recurrence of constrictive pericarditis as the echocardiographic signs of constrictive physiology to some extent – as discussed above – remain after pericardiectomy. Clearly, cardiac catheterisation is mandatory in such cases.
The causes of constrictive pericarditis in our series correspond well with those reported by others (Citation2,Citation5,Citation7,Citation9, Citation10,Citation11,Citation22), except for a higher prevalence of malignancy and cardiac or thoracic surgery and a lower prevalence of tuberculous constrictive pericarditis (1.8%) reflecting the low incidence of tuberculosis in Denmark [incidence 6.9 per 100,000 in 2012; (Citation23)]. Of note, the single patient identified with tuberculous constrictive pericarditis originated from Greenland where the prevalence of tuberculosis remains high.
Pericardiectomy was found to be a safe procedure in selected patients, that is symptomatic patients who are considered fit for surgery. Early and late mortality was comparable with most other reports (Citation2,Citation9,Citation10,Citation19,Citation22,Citation24). Mortality did not differ between the surgically and conservatively treated patients. This probably reflects that the group of conservatively managed patients consisted equally of patients with few symptoms and patients with anticipated prohibitive surgical risk. Furthermore the low number of patients in this group calls for caution when drawing conclusions about their outcome and the lack of difference in outcome between conservatively and surgically managed patients may simply reflect a type II error. Larger studies are required to determine if a subgroup of patients with constrictive pericarditis and moderate symptoms may safely be treated conservatively.
Limitations
The current study was based on retrospective analysis of hospital files which explains the less-than-complete diagnostic information regarding haemodynamics and comorbidities as well as the aetiology of constrictive pericarditis – especially the possibility of discerning between idiopathic constrictive pericarditis and lack of information. It is probable that some patients with constrictive pericarditis were not identified, especially those who did not have cardiac catheterisation performed (as most patients were identified from the cardiac catheterisation database), but we probably reliably identified all patients undergoing pericardiectomy within the study period.
Conclusion
In a contemporary Danish cohort of patients with constrictive pericarditis the most common cause was underlying chronic inflammatory disease, whereas tuberculosis was rare. Most reliable echocardiographic signs were dilated inferior caval vein, increase E/A ration, high septal tissue velocity, and the presence of septal bounce. Outcome after pericardiectomy was satisfactory, and survival appeared to be influenced by previous sternotomy.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Khandaker MH, Espinosa RE, Nishimura RA, Sinak LJ, Hayes SN, Melduni RM, et al. Pericardial disease: diagnosis and management. Mayo Clin Proc. 2010;85:572–93.
- George TJ, Arnaoutakis GJ, Beaty CA, Kilic A, Baumgartner WA, Conte JV. Contemporary etiologies, risk factors, and outcomes after pericardiectomy. Ann Thorac Surg. 2012; 94:445–51.
- Oreto L, Mayer A, Todaro MC, Mori N, Kress DC, Kleinman LH, et al. Contemporary clinical spectrum of constrictive pericarditis: a 10-year experience. Int J Cardiol. 2013;163:339–41.
- Yetkin U, Kestelli M, Yilik L, Ergunes K, Kanlioglu N, Emrecan B, et al. Recent surgical experience in chronic constrictive pericarditis. Tex Heart Inst J. 2003;30:27–30.
- Ghavidel AA, Gholampour M, Kyavar M, Mirmesdagh Y, Tabatabaie MB. Constrictive pericarditis treated by surgery. Tex Heart Inst J. 2012;39:199–205.
- Lin Y, Zhou M, Xiao J, Wang B, Wang Z. Treating constrictive pericarditis in a chinese single-center study: a five-year experience. Ann Thorac Surg. 2012;94:1235–40.
- Kang SH, Song JM, Kim M, Choo SJ, Chung CH, Kang DH, et al. Prognostic predictors in pericardiectomy for chronic constrictive pericarditis. J Thorac Cardiovasc Surg. 2014;147:598–605.
- Winqvist N, Björk J, Miörner H, Björkman P. Long-term course of Mycobacterium tuberculosis infection in Swedish birth cohorts during the twentieth century. Int J Tuberc Lung Dis. 2011;15:736–40.
- Kytö V, Sipiläauml; J, Rautava P. Chronic constrictive pericarditis in general adult population. Int J Cardiol. 2014;176:1158–60.
- Bertog SC, Thambidorai SK, Parakh K, Schoenhagen P, Ozduran V, Houghtaling PL, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445–52.
- Ariyoshi T, Hashizume K, Taniguchi S, Miura T, Tanigawa K, Matsukuma S, et al. Surgical experience with chronic constrictive pericarditis. Gen Thorac Cardiovasc Surg. 2012;60:796–802.
- Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (2012 version). Eur Heart J. 2012;33:2451–96.
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009b;22:107–33.
- Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Normative reference values for the tissue Doppler imaging parameters of left ventricular function: a population-based study. Eur J Echocardiogr. 2010;11:51–6.
- Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G, et al. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging. 2014;15:680–90.
- Zwas DR, Gotsman I, Admon D, Keren A. Advances in the differentiation of constrictive pericarditis and restrictive cardiomyopathy. Herz. 2012;37:664–73.
- Welch TD, Ling LH, Espinosa RE, Anavekar NS, Wiste HJ, Lahr BD, et al. Echocardiographic diagnosis of constrictive pericarditis. Mayo Clinic criteria. Circ Cardiovasc Imaging. 2014;7:526–34.
- Kusunose K, Dahiya A, Popović ZB, Motoki H, Alraies MC, Zurick AO, et al. Biventricular mechanics in constrictive pericarditis comparison with restrictive cardiomyopathy and impact of pericardiectomy. Circ Cardiovasc Imaging. 2013; 6:399–406.
- Chowdhury UK, Subramaniam GK, Kumar AS, Airan B, Singh R, Talwar S, et al. Pericardiectomy for constrictive pericarditis: a clinical, echocardiographic, and hemodynamic evaluation of two surgical techniques. Ann Thorac Surg. 2006;81:522–9.
- Elmistekawy EM, Veinot JP, Dennie CJ, Rubens FD. Recurrent cardiac constriction after complete pericardiectomy. Heart Lung Circ. 2011;20:766–8.
- Cho YH, Schaff HV, Dearani JA, Daly RC, Park SJ, Li Z, et al. Completion pericardiectomy for recurrent constrictive pericarditis: importance of timing of recurrence on late clinical outcome of operation. Ann Thorac Surg. 2012;93: 1236–40.
- Ling LH, Oh JK, Schaff HV, Danielson GK, Mahoney DW, Seward JB, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380–6.
- SSI: Tuberkulose 2012. EPI-NYT 2014–4. Danish
- Gopaldas RR, Dao TK, Caron NR, Markley JG. Predictors of in-hospital complications after pericardiectomy: a nationwide outcomes study. J Thorac Cardiovasc Surg. 2013;145: 1227–33.
