Abstract
Background. We sought to investigate left ventricular (LV) function and mechanics assessed by three-dimensional echocardiography (3DE) and speckle tracking in patients with subclinical hyperthyroidism (SCH). Methods. We included 35 untreated women with SCH and 35 healthy control women matched by age. All participants underwent laboratory analyses which included thyroid hormone levels, and complete 2DE and 3DE examination. Results. 2DE LV longitudinal and circumferential strain was significantly decreased in the SCH subjects. 2DE LV systolic and early diastolic strain rates in longitudinal and circumferential directions were reduced, whereas late diastolic strain rates were increased in SCH individuals. 3DE LV end-diastolic volume and cardiac output were significantly elevated in the SCH patients. 3DE LV deformation in all three directions, as well as 3DE area strain, were significantly lower in the SCH group. Serum thyroid-stimulating hormone (TSH) and free thyroxine (FT4) levels correlated with 2DE LV mass index, 2DE longitudinal strain, and 3DE LV area strain in the whole study population. Conclusion. LV deformation evaluated by 3DE and speckle tracking imaging are significantly impaired in SCH subjects. TSH and FT4 levels correlate with 2DE and 3DE LV structure and mechanics.
Introduction
Subclinical hyperthyroidism (SCH) is characterized by low or undetectable serum thyroid-stimulating hormone (TSH) levels, with normal ranges of free thyroxine (T4) and total or free triiodothyronine (T3) levels (Citation1,Citation2). However, the cutoff values for thyroid hormone levels have not been established yet. The prevalence of SCH in the USA is estimated at less than 0.7% when SCH is defined with TSH level less than 0.1 mIU/l, and 3.2% when TSH cutoff is 0.4 mIU (l,3).
The effect of SCH on cardiovascular morbidity and mortality has not been clarified because results on this topic are still conflicting. On one side, authors have shown that SCH is associated with increased all-cause and cardiovascular morbidity and mortality (Citation4). On the other side, investigators demonstrated that SCH was associated with atrial fibrillation development, but not with other cardiovascular diseases or mortality (Citation5).
The remodeling of the left ventricle in SCH has not been investigated enough; results are inconsistent and authors mainly included patients with exogenous or long-lasting SCH and used traditional two-dimensional echocardiographic (2DE) parameters (Citation6–10). Only recently the same group of authors reported the results of left ventricular (LV) remodeling obtained by 2DE strain analysis in subjects with exogenous SCH due to differentiated thyroid carcinoma and long-term TSH-suppressive levothyroxine substitution therapy (Citation11,Citation12).
We hypothesized that SCH could significantly impact LV mechanical function, thus the aim of the present study was to investigate LV function and deformation by comprehensive two- and three-dimensional echocardiography and speckle tracking analysis in individuals with endogenous SCH.
Methodology
Our study enrolled 41 female subjects with untreated subclinical hyperthyroidism and 35 healthy female controls of similar age. The SCH and control subjects were included between January 17, 2011 and January 29, 2014. The inclusion criteria were the decreased serum TSH level (< 0.4 mIU/ml) with normal levels of T4 and free thyroxine (FT4) in at least two separate measurements taken 3–6 months apart. However, 2 subjects refused to perform further echocardiographic investigation, whereas 4 persons were excluded due to concomitant disease (arterial hypertension, diabetes mellitus, and mitral stenosis). Thus, investigation included 35 SCH participants. The control group included individuals who were referred to our outpatient clinic due to innocent murmur, palpitations, or within annual screening examination. All subjects from the control group were included simultaneously with the SCH subjects. Exclusion criteria were cardiovascular disease (arterial hypertension, myocardial infarction, atrial fibrillation, heart failure, congenital heart disease, and valvular disease), obesity (body mass index [BMI] ≥ 30 kg/m2), asthma, chronic obstructive lung disease, neoplastic disease, cirrhosis of the liver, kidney failure, sleeping disorders, type 2 diabetes mellitus, and treatment with L-thyroxine due to hypothyroidism.
Anthropometric measures (height and weight) and laboratory analyses (the level of thyroid hormones, total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein cholesterol, and triglycerides) were taken from all the subjects included in the study. Fasting venous blood samples were drawn between eight and nine o'clock in the morning. None of the participants used any medications before inclusion in the study. Normal ranges for T3, T4, FT4, and TSH were 1.3–2.6 nmol/L, 58–161 nmol/L, 11.5–22.7 pmol/l, and 0.4–4 mIU/l, respectively. T3, T4, and FT4 levels were assessed by IMMULITE 2000 enzyme-labeled chemiluminescent competitive immunoassay; TSH level was determined using IMMULITE 2000, third-generation TSH, two-site chemiluminescent immunometric assay.
BMI and body surface area (BSA) were calculated for each patient. The study was approved by the Local Ethics Committee, and informed consent was obtained from all the participants.
Echocardiography
Echocardiographic examination was performed using a 2.5-MHz transducer and 3D data set acquisition of the left ventricle obtained using the 3 V matrix probe and a Vivid 7 ultrasound machine (GE Healthcare, Horten, Norway).
Standard two-dimensional echocardiographic examination
Values of all 2DE parameters were obtained as the average value of three consecutive cardiac cycles. The LV end-systolic and end-diastolic diameters (LVEDD), LV posterior wall thickness (PWT), and interventricular septum thickness were determined according to the current recommendations (Citation13). Relative wall thickness was calculated as (2xPWT)/LVEDD. LV ejection fraction was estimated by the biplane method. LV mass was calculated using the Devereux formula (Citation14), and indexed for BSA. Left atrial volume was measured just before the mitral valve opening, according to the biplane method in four- and two-chamber views, and all the values were indexed for BSA (Citation13).
Transmitral Doppler inflow and tissue pulsed Doppler were obtained in the apical four-chamber view. Pulsed Doppler measurements included the transmitral early diastolic peak flow velocity (E), late diastolic flow velocity (A), and their ratio (E/A)(Citation15). Tissue Doppler imaging was used to obtain LV myocardial velocities in the apical four-chamber view, with a sample volume placed at the septal and lateral segment of the mitral annulus during systole, and early and late diastole (s, a´, and e´). The average of the peak early diastolic relaxation velocity (e´) of the septal and lateral mitral annulus obtained by the tissue Doppler was calculated, and the E/e´ ratio was computed.
Two-dimensional left ventricular strain and strain rate
2DE strain analysis was performed using three consecutive cardiac cycles of 2DE LV images in apical (long-axis four-chamber and two-chamber view) and parasternal short-axis views (just below the mitral level) (Citation16). The frame rate ranged between 50 and 70 Hz. A commercially available software 2DE Auto LVQ (EchoPAC 112, GE-Healthcare, Horten, Norway) was used for 2DE strain analysis.
The 2DE longitudinal strain and strain rate were calculated by averaging all the values of the regional peak longitudinal strain and strain rate obtained in two-chamber and four-chamber apical views. 2DE circumferential strain and strain rate, as well as 2DE radial strain and strain rate, were assessed as the average of the LV six regional values measured in the parasternal short-axis view, just below the mitral valve. LV twist values, which represent the difference between average peak degree of rotation at six basal segments and four apical segments, as well as untwisting rate, were provided by the software (Citation16).
Three-dimensional echocardiographic examination
A full-volume acquisition of the LV required for further analyses was obtained by harmonic imaging from an apical approach. Six electrocardiogram-gated consecutive beats were acquired during end-expiratory breath hold to generate full volume. The frame rate was higher than 30 frames/s.
All data sets were stored digitally and analyzed by a commercially available software 4D Auto LVQ software (EchoPAC 112, GE-Healthcare, Horten, Norway), which was used for the off-line analysis. 3DE-derived LV mass was also indexed for BSA. The software automatically identified the LV cavity endocardial border in 3DE and provided the LV volumes, cardiac output, stroke volume, ejection fraction, and LV sphericity index (Citation17). After that, an automatic trace of the epicardial border was displayed to detect the region of interest required for LV mass and 3DE myocardial deformation parameters (speckle tracking). The 3DE deformation parameters, including global longitudinal strain, global circumferential strain, global radial strain, and global area strain, were calculated as weighted averages of the regional values from the 17 myocardial segments at end-systole (Citation17). If ≥ 3 segments were rejected and global strain values were not calculated, that patient was excluded.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) and were compared using the two-tailed Student's t-test in case of normal distribution of data or by Mann–Whitney U test in case where the data were not distributed normally. Comparisons between the controls and the patients were performed by an independent-samples t-test. The differences in proportions were compared using the χ² test. The correlations were determined by the Pearson's correlation test, after TSH values were transformed by natural logarithm logarithmic in order to obtain normal distribution. Inter- and intraobserver variability was examined using Pearson's bivariate two-tailed correlations for randomly selected 10 SCH subjects and 10 controls. The p value < 0.05 was considered statistically significant.
Results
We did not find any difference in age, BSA, BMI, blood pressure, heart rate, and lipid levels, except for HDL, between the controls and the SCH group (). HDL level was higher among the SCH subjects. Regarding the thyroid hormone level, T3 level was similar between the observed groups, but T4 and FT4 levels were higher in the SCH group, although in normal ranges (). TSH was significantly lower among the SCH individuals, by definition ().
Table I. Demographic and clinical parameters of study population.
Conventional two-dimensional echocardiographic parameters
The observed groups did not differ in diameters of aorta, left atrium, and left ventricle. However, the thicknesses of interventricular septum and posterior wall of the LV, as well as LV mass index, were increased in the SCH group (). Left atrial volume and volume index were also increased in the SCH patients ().
Table II. Echocardiographic parameters of LV structure and function in study population.
LV systolic function, assessed by 2DE ejection fraction, was normal in both groups, without any difference (). On the other hand, tissue Doppler assessment indicated reduced LV systolic function in the SCH participants. Parameters of LV diastolic function (E/A and E/e’) were worse in the SCH subjects comparing with healthy controls, but still in the normal ranges (). There was no difference in pulsed Doppler-derived transmitral velocities during early and late diastole (E and A) between the observed groups, whereas tissue Doppler-derived e’ was significantly lower in the SCH subjects ().
Two-dimensional strain analysis
2DE LV mechanics was decreased in longitudinal and circumferential direction in the subjects with SCH, whereas there was no difference in radial strain and strain rates between the two observed groups (). Systolic deformation of the LV, evaluated by systolic strain rates, was decreased in the SCH comparing with the healthy individuals. Additionally, LV diastolic mechanical function, estimated by early and late diastolic strain rate, was impaired in patients with SCH (). LV twist and untwisting rate were increased in the SCH subjects in comparison with the healthy controls ().
Table III. Two- and three-dimensional speckle tracking assessment of the LV function in the study population.
Three-dimensional echocardiography
End-diastolic LV volume obtained by 3DE was higher in the SCH than in the controls (). On the other hand, there was no statistically significant difference in LV end-systolic and stroke volumes, although both were higher among the SCH subjects (). This resulted in significantly higher cardiac output in the SCH group. There was no difference in 3DE LV ejection fraction or sphericity index between the groups, suggesting preserved LV systolic function ().
3DE LV strains in all three directions, as well as area strain that represents a combination of longitudinal and circumferential strain, were significantly decreased in the SCH subjects, indicating the impairment of LV deformation in this group ().
Correlation and regression analyses
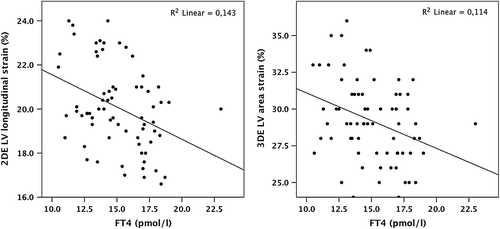
We found a correlation between the TSH level and 2DE LV mass index (r = − 0.276, p = 0.046), 3DE LV mass index (r = − 0.3, p = 0.032), transmitral E/A ratio (r = 0.38, p < 0.01), 2DE longitudinal strain (r = 0.43, p < 0.01), 2DE circumferential strain (r = 0.35, p = 0.017), LV twist (r = − 0.32, p = 0.024), 3DE LV longitudinal (r = 0.41, p < 0.01), and 3DE LV area strain (r = 0.4, p < 0.01) in the whole study population.
The correlation was also revealed between FT4 level and 2DE LV mass index (r = 0.29, p = 0.045), 3DE LV mass index (r = 0.33, p = 0.01), 2DE LV longitudinal strain (r = − 0.38, p < 0.01), and 3DE LV area strain (r = − 0.34, p < 0.01) ().
Interobserver variability
Pearson's correlations—3DE LV longitudinal strain: r = 0.9, p < 0.001; 3DE LV circumferential strain: 0.83, p < 0.001; 3DE LV radial strain: r = 0.57, p < 0.001; 3DE LV area strain: r = 0.8, p < 0.001.
Intraobserver variability
Pearson's correlations—3DE LV longitudinal strain: r = 0.93, p < 0.001; 3DE LV circumferential strain: 0.88, p < 0.001; 3DE LV radial strain: r = 0.64, p < 0.001; 3DE LV area strain: r = 0.86, p < 0.001.
Discussion
Our investigation revealed several new findings: (i) LV cardiac deformation in all directions, assessed by 2DE and 3DE strain, was impaired in the SCH subjects; (ii) systolic and diastolic mechanical LV functions were affected by SCH; (iii) LV twist and untwisting rate were elevated in the SCH patients; (iv) LV volumes and cardiac output, evaluated by 3DE, were increased in the SCH individuals; (v) TSH and FT4 levels correlated with 2DE and 3DE structural and mechanical parameters in the whole study population.
To our knowledge, there are only two studies by the same group of authors that used 2DE strain (Citation11,Citation12). They included 25 patients with a history of differentiated thyroid carcinoma on long-term TSH-suppressive levothyroxine substitution and found that SCH affected LV circumferential and longitudinal myocardial function assessed by strain and strain rate (Citation11). Additionally, they observed a U-shaped relationship between a range of thyroid hormone levels (from hyper- to hypothyroid concentrations) and myocardial longitudinal and circumferential strain (Citation12). Our findings in the present study and the previous study about subclinical hypothyroidism (Citation20) are completely in line with these results. However, we went a step further and determined LV twist, untwisting rate, and 3DE LV deformation. Considering the fact that LV twist mostly represents a marker of LV systolic function (Citation18), while LV untwisting rate is commonly used as a parameter of LV diastolic dysfunction (Citation19), we could conclude that both systolic and diastolic LV mechanical functions are influenced by SCH. Namely, our findings show that LV systolic function, estimated by 2DE and 3DE LV ejection fraction, in SCH is normal and does not differ from the healthy controls; on the other hand, LV systolic mechanics, estimated by 2DE and 3DE strain, is impaired in the SCH subjects. The reason for preserved LV systolic function, despite decreased LV systolic deformation, could actually lie in the paradoxically elevated LV twist that we detected in the SCH patients. LV twist represents a complex movement that involves circumferential motion of the apex with respect to the base of the heart and enables the most efficient contraction of the LV myocardial fibers; it could be increased in overload condition (Citation18). Our results indicate that 3DE LV volumes and cardiac output are increased in the SCH individuals, which suggests hemodynamic overload condition and possibly explains elevated LV twist in this group. Considering our findings that 2DE and 3DE LV ejection fractions were similar between the observed groups, it seems that this conventional echocardiographic parameter is not quite suitable for the estimation of LV systolic function in the SCH subjects.
Namely, untwisting is the recoil of the systolic torsional deformation; it happens mostly during isovolumic relaxation and contributes to early diastolic filling (Citation19). The evaluation of untwisting may provide an accurate evaluation of LV relaxation. In the present study, untwisting rate is significantly increased in the SCH subjects, which indicates the prolongation of the isovolumic relaxation time and deterioration of LV diastolic function. This is in line with our results regarding LV early and late diastolic strain rates that also show the impairment of LV diastolic mechanical function in the SCH patients.
The assessment of LV myocardial deformation by 3DE has not been done so far. Our findings showed that 3DE LV strain has been impaired in all directions (longitudinal, circumferential, and radial), along with 3DE area strain, in the SCH subjects comparing with the controls. Using this sophisticated method we showed that LV radial strain is also affected by SCH, which was not shown by 2DE strain analysis in our investigation or previously by Abdulrahman et al. (Citation11,Citation12). The fact that 3DE area strain, the parameter of both longitudinal and circumferential function, and accurate index for quantitative assessment of global and regional LV function (Citation20), is reduced among the SCH patients in the present study is more significant.
Our results also indicate the correlation between the TSH and FT4 levels and LV structure, function, and mechanics, assessed by 2DE and 3DE. The progressive reduction of TSH has been associated with increase of LV mass and LV twist, and with decrease of mitral E/A ratio, and LV longitudinal, circumferential, and area strain. Abdulrahman et al. showed a borderline association of 2DE longitudinal and circumferential strain with the TSH levels (Citation11). FT4 inversely correlates with 2DE mass index and longitudinal, as well as 3DE area, strain.
Although the mechanism of LV remodeling in SCH is unclear, there are several possibilities that could explain cardiovascular changes: (i) reduced peripheral resistance and peripheral vasodilatation; (ii) increased activity of renin–angiotensin–aldosterone system; (iii) decreased renal perfusion; and (iv) increased LV volumes and cardiac output. All these changes could induce impairment of LV systolic and diastolic mechanical function, as well as LV hypertrophy (Citation21).
There are several important clinical implications of our study. First, our findings about decreased LV strain could potentially explain cardiovascular morbidity and mortality in SCH patients, especially in long-lasting SCH, because it has been already shown that LV deformation is a good predictor of the outcome (Citation22). Second, LV strain analysis could help us to determine the necessity of therapy introduction. Previous investigations indicate that LV remodeling is reversible (Citation7,Citation8); however, strain analysis could demonstrate improvement much faster and therapy could be stopped earlier. Third, evaluation of LV deformation, due to high sensitivity, may facilitate the follow-up of these patients.
Limitations
The present investigation has several limitations: (i) 3DE estimation of LV structure and function could be significantly influenced by the quality of ultrasound images, especially during the full-volume acquisition; (ii) a small number of patients; (iii) our investigation included only women, which restricts our results to this population; and (iv) coronary artery disease (CAD) was not excluded by coronary angiography, but we included young females without cardiovascular risk factors and free of any symptom of CAD, thus it is unlikely that CAD could explain the LV mechanical myocardial changes that we found.
Conclusion
LV myocardial deformation, evaluated by 2DE and 3DE strain, is impaired in the SCH subjects. There is no difference in LV systolic function, assessed by 2DE and 3DE ejection fraction, between the SCH and the controls. However, other parameters of LV systolic function obtained by tissue Doppler and strain analysis indicate impaired LV systolic function in the SCH subjects, which refers to the inefficacy of ejection fraction in the estimation of LV systolic function in the SCH subjects. It has been shown that the TSH and FT4 levels correlated with LV mass index and LV deformation. Future longitudinal studies with a larger number of patients are necessary to confirm the unfavorable influence of endogenous SCH on LV mechanics and investigate the influence of these changes on long-term outcome. Further research is essential for the assessment of time when therapy should be initiated in the SCH subjects, as well as the possible effect of therapy on LV deformation improvement.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bahn Chair RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593–646.
- Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–38.
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002; 87:489–99.
- Selmer C, Olesen JB, Hansen ML, von Kappelgaard LM, Madsen JC, Hansen PR, et al. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab. 2014;99:2372–82.
- Singh S, Duggal J, Molnar J, Maldonado F, Barsano CP, Arora R. Impact of subclinical thyroid disorders on coronary heart disease, cardiovascular and all-cause mortality: a meta-analysis. Int J Cardiol. 2008;125:41–8.
- Donatelli M, Assennato P, Abbadi V, Bucalo ML, Compagno V, Lo Vecchio S, et al. Cardiac changes in subclinical and overt hyperthyroid women: retrospective study. Int J Cardiol. 2003;90:159–64.
- Kaminski G, Michalkiewicz D, Makowski K, Podgajny Z, Szalus N, Ruchala M, et al. Prospective echocardiographic evaluation of patients with endogenous subclinical hyperthyroidism and after restoring euthyroidism. Clin Endocrinol (Oxf). 2011;74:501–7.
- Smit JW, Eustatia-Rutten CF, Corssmit EP, Pereira AM, Frölich M, Bleeker GB, et al. Reversible diastolic dysfunction after long-term exogenous subclinical hyperthyroidism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2005;90:6041–7.
- Dörr M, Ittermann T, Aumann N, Obst A, Reffelmann T, Nauck M, et al. Subclinical hyperthyroidism is not associated with progression of cardiac mass and development of left ventricular hypertrophy in middle-aged and older subjects: results from a 5-year follow-up. Clin Endocrinol (Oxf). 2010;73:821–6.
- Rodondi N, Bauer DC, Cappola AR, Cornuz J, Robbins J, Fried LP, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol. 2008;52:1152–9.
- Abdulrahman RM, Delgado V, Ng AC, Ewe SH, Bertini M, Holman ER, et al. Abnormal cardiac contractility in long-term exogenous subclinical hyperthyroid patients as demonstrated by two-dimensional echocardiography speckle tracking imaging. Eur J Endocrinol. 2010;163:435–41.
- Abdulrahman RM, Delgado V, Hoftijzer HC, Ng AC, Ewe SH, Marsan NA, et al. Both exogenous subclinical hyperthyroidism and short-term overt hypothyroidism affect myocardial strain in patients with differentiated thyroid carcinoma. Thyroid. 2011;21:471–6.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA. American Society of Echocardiography's Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108.
- de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60.
- Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: A report from the Doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84.
- Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography—from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20:234–43.
- Biswas M, Sudhakar S, Nanda NC, Buckberg G, Pradhan M, Roomi AU, et al. Two- and three-dimensional speckle tracking echocardiography: clinical applications and future directions. Echocardiography. 2013;30:88–105.
- Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008;1:366–76.
- Burns AT, La Gerche A, Prior DL, Macisaac AI. Left ventricular untwisting is an important determinant of early diastolic function. JACC Cardiovasc Imaging. 2009;2:709–16.
- Kleijn SA, Aly MF, Terwee CB, van Rossum AC, Kamp O. Three-dimensional speckle tracking echocardiography for automatic assessment of global and regional left ventricular function based on area strain. J Am Soc Echocardiogr. 2011;24:314–21.
- Biondi B, Palmieri EA, Klain M, Schlumberger M, Filetti S, Lombardi G. Subclinical hyperthyroidism: clinical features and treatment options. Eur J Endocrinol. 2005;152:1–9.
- Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–64.