Abstract
Objective. The clinical data considering the bone marrow mononuclear cell (BMMNC) therapy in treatment for acute myocardial infarction (AMI) are controversial and the mechanisms remain unknown. Our objective was to study the cardiac function and changes in cytokine levels after administration of BMMNC in experimental AMI model. Design. Unlabeled or Super-Paramagnetic-Iron-Oxide-labeled BMMNCs or saline was injected into myocardium of 31 pigs after circumflex artery occlusion. Ejection fraction (EF) was measured preoperatively, postoperatively and at 21 days by echocardiography. Cardiac MRI was performed postoperatively and after 21 days in 7 BMMNC animals. Serum cytokine levels were measured at baseline, 24 h and 21 days. Cellular homing was evaluated comparing MRI and histology. Results. From baseline to 21 days EF decreased less in BMMNC group (EF mean control -19 SD 12 vs. BMMNC -4 SD 15 percentage points p = 0.02). Cytokine concentrations showed high variability between the animals. MRI correlated with histology in cell detection and revealed BMMNCs in the infarction area. By MRI, EF improved 11 percentage points. The improvement in EF was associated with the number of transplanted BMMNCs detected in the myocardium. Conclusion. BMMNC injection after AMI improved cardiac function. Quantity of transplanted BMMNCs correlated with the improvement in cardiac function after AMI.
| Abbreviations | ||
| AMI | = | Acute Myocardial Infarction |
| BMMNC | = | Bone Marrow Mononuclear Cell |
| CX | = | Circumflex Coronary Artery |
| DE | = | Delayed Enhancement |
| EF | = | Ejection Fraction |
| FGF | = | Fibroblast Growth Factor |
| FOV | = | Field of View |
| FS | = | Fractional Shortening |
| G-CSF | = | Granulocyte Colony Stimulating Factor |
| Gm-CSF | = | Granulocyte-macrophage Colony Stimulating Factor |
| IC | = | Intracoronary |
| IFN-γ | = | Interferon-γ |
| IL | = | Interleukin |
| IM | = | Intramuscular |
| MRI | = | Magnetic Resonance Imaging |
| PDGF | = | Platelet-Derived Growth Factor |
| SPIO | = | Super Paramagnetic Iron Oxide |
| TE | = | Echo Time |
| TNFα | = | Tumor Necrosis Factor-α |
| TR | = | Repetition Time |
| VEGF | = | Vascular Endothelial Growth Factor |
Introduction
Acute myocardial infarction (AMI) evokes myocardial apoptosis and triggers necrosis leading to contractile dysfunction and a decrease in ejection fraction (EF) (Citation1–3). Subsequently, inflammation, degradation of the extracellular matrix, and formation of granulation tissue and a scar are regulated by the cytokines (Citation2,Citation3). Cell therapy represents a novel approach to treat this process of AMI. According to both clinical and pre-clinical studies, stem cell therapy either by application of bone marrow mononuclear cells (BMMNCs) or in vitro expanded mesenchymal stem cells (MSCs) can contribute to the cardiac function and attenuate the detrimental postinfarction remodeling process mediated by cytokines and trophic factors (Citation4,Citation5).
Although the results from stem cell therapy in AMI appear potential, there is an extensive variation in the extent of recovery (Citation6,Citation7). It is known that several factors, such as timing of therapy, time points of follow-up, and finally the number and quality of transplanted cells, all have significant effects on the data. These differences in studies can make it difficult to estimate the final therapeutic outcome end especially to compare different studies. After AMI, cytokines regulate the survival of cardiomyocytes and the extent of the inflammatory cell response (Citation2). Over the long term, multiple inflammatory mechanisms and cytokines mediate simultaneously the myocardial remodeling process and wound healing (Citation3). Optimization of transplantation methods are required to direct stem cells to the infarction area for improved recovery. Our previous study pointed to the superiority of intramuscular (IM) transplantation method in stem cell directing compared with the intracoronary (IC) injections (Citation8). The number of administered BMMNCs was seven-fold higher using an IM transplantation method than could be achieved with IC. In addition, the cell type may influence recovery, and pre-treatment of stem cells has been suggested to increase their therapeutic ability (Citation9). However, in clinical trials where the same cell type (bone-marrow-derived cells) and transplantation method (IC) were used, there was yet a large variation encountered in the extent of recovery. The difference in EF improvement between the study groups and the benefit of BMMNC administration varied in two separate trials from 1.1% to 12% (Citation10,Citation11). Given that the optimal transplantation method or cell type alone does not provide the optimal recovery, we hypothesize that accurate delivery of the cells, that is, cell targeting has to be performed to ensure optimal recovery.
The aim of this study was to further characterize the effect of cell targeting and measure the EF recovery between the controls and BMMNC-treated pigs after AMI and study the variation in cytokine levels during the recovery process. For cell detection, the transplanted BMMNCs were labeled with Super Paramagnetic Iron Oxides (SPIO) and visualized using MRI and histopathology after transplantation to myocardium. Previous studies suggest that SPIO label does not alter the function of the stem cells significantly, and BMMNC or MSC transplantation increases the heart function after AMI (Citation12,Citation13). In this study, the actual cell number in myocardium associates with the improvement in EF. As far as we are aware, this is the first study to describe association between the recovery in cardiac function and transplanted cells detected in myocardium, highlighting the importance of cell targeting and homing in therapy.
Materials and methods
The animals were maintained and the procedures were performed in accordance with the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes (ETS 123, Council of Europe 2006) and the European Union recommendation on guidelines for the accommodation and care of animals used for experimental and other scientific purposes (2007/526/EC). The study was approved by the Finnish Animal Ethics Committee. Authorships comply with the Vancouver guidelines and each author has a significant role in substantial contribution, article drafting and final approval.
Experimental setting
A total of 24 landrace pigs (8–10 weeks of age) (control n = 9; BMMNC n = 15) and 7 randomly selected animals (control n = 4; BMMNC n = 3) from previously reported data were used according to power estimation (Power EF baseline vs. 21 days 71%) (Citation14). BMMNC group was divided to two subgroups based on the random selection: BMMNC group and BMMNC + MRI (n = 7). In a later subgroup detailed cell detection was performed using MRI and histology. Before the study, the animals were made adapted to conditions in the laboratory animal center for one week.
Anesthesia
The animals were sedated by IM injection of ketamine hydrochloride (350 mg) and midazolam (45 mg). A peripheral catheter was inserted into an ear vein for administration of drugs and to maintain the fluid balance with Ringer acetate solution. After induction with fentanyl (25 μg/kg) and pancuronium (0.2 mg/kg) intravenously (IV) (Hameln Pharmaceuticals, Gloucester UK), the pigs were intubated, and the balanced anesthesia was maintained by a continuous infusion of midazolam (0.25 mg/kg/h IV) (Pfizer Inc), fentanyl (25 μg/kg/h IV), pancuronium (0.2 mg/kg/h IV), and inhaled isoflurane (0.5%) in both study groups throughout the experiment. The animals were maintained on positive pressure ventilation with 50% oxygen throughout the experiment. Antibiotic prophylaxis with 1.5 g of cefuroxime was administered IV at anesthesia induction and during extubation.
Cardiac function monitoring
As a way of monitoring the function of the heart, transthoracic echocardiography was performed blinded in all experimental animals by a cardiologist. Echocardiography was performed preoperatively and 30 min after instituting reflow with the Acuson Seqouia 512 echocardiography system with a 3.5-MHz transducer (Siemens AG, Munich, Germany). M-mode view was used to measure the thicknesses of the ventricular walls: interventricular septum, left ventricular posterior wall, left ventricular end-systolic, and left ventricular end-diastolic diameters. The left ventricular mass was calculated by using the method described by Reichek and Devereux and the EF was calculated by using the Teichholz formula, which use the end-diastolic and end-systolic diameters on the left ventricle. Transmitral flow peaks E and A and their ratio were measured from the 4-chamber view with the pulsed-wave Doppler sample volume between mitral leaflet tips, and the isovolumetric relaxation time was calculated from the aortic valve with continuous-wave Doppler scanning. By using the velocity-time integral of the pulsed-wave Doppler sample from the left outflow tract, the diameter of the left outflow tract, the heart rate, and the cardiac output could be measured. Electrocardiographic monitoring was performed throughout the experiment to monitor the ischemic alterations.
Isolation of mononuclear BMMNCs
While in the supine position, 2 × 10 ml of pig bone marrow was aspirated beneath the tibial tuberosity into a syringe containing 5000IU of heparin to harvest mononuclear cells from the bone marrow. The bone marrow aspirate was diluted 1:6 in phosphate-buffered saline (Sigma-Aldrich, St.Luis, USA) and subjected to a density-gradient centrifugation (Ficoll-Paque Plus, Amersham Biosciences, Inc.) to exclude granulocytes and erythrocytes. After collecting mononuclear cells from the interphase, they were washed three times with phosphate-buffered saline.
Labeling of mononuclear cells
SPIO labeling (39 mg/ml ferucarbotran corresponding to 36 mM of iron) was done by incubating the BMMNCs in Resovist (Bayer Healthcare, Berlin, Germany) at 37°C for 3 h. After labeling, the BMMNCs were washed 3–4 times with PBS. The overall cell amount was counted and suspended in a saline solution at a density of 6.2–14.3 × 107cells/2 ml.
Hemodynamic monitoring
Blood sampling and arterial pressure monitoring were conducted via an arterial cannula positioned into the left femoral artery. A thermodilution catheter (CritiCath, 7F; Ohmeda GmbH &Co, Erlangen, Germany) was placed through the right femoral vein to allow monitoring of central venous pressure, pulmonary artery pressure, pulmonary capillary wedge pressure, and blood sampling as well as for recording the cardiac output and blood temperature. Hemodynamic parameters were measured at the baseline, every 15 min during the occlusion of the circumflex artery, and after 90 min of reflow. A temperature probe was placed in the rectum and a 10 Fr catheter was placed in the urinary bladder to monitor urinary output.
Biochemical analysis
Systemic arterial and venous blood samples were taken and hemoglobin, hematocrit, blood gases, electrolytes, pH, glucose, and serum ionized calcium levels (i-STAT analyzer, Abbot, Inc.) were measured at the baseline, 90 min after the infarction, and 30 min after initiation of reperfusion. Basic blood count, white blood cell differential count, and troponin-I sampling were performed at the same time points and also at the 21 st postoperative day. The cytokine levels were measured from all study animals in the baseline, from 14 animals (controls n = 6, BMMNC n = 8) 24 h postoperatively, and from 11 animals (control n = 7, BMMNC n = 4) 21 days after AMI. Cases were excluded analysis by analysis in statistical calculations.
The cytokine concentrations were determined with Bio-Plex Pro Human Cytokine Assay (Bio-Rad Laboratories Inc.) according to manufacturer's instructions. Briefly, the sample was centrifuged (13,300 g, 10 min), diluted 1:2, and analyzed by Bio-Plex 200 instrument based on Luminex xMAP Technology (Bio-Rad Laboratories). The results were automatically calculated with Bio-Plex Manager Software version 6.0 with five-parameter logistic equations. The concentrations under the threshold of the standard line were included in the data as half of the standard threshold value. Due to large number of samples, two Cytokine Assay kits were used. Intra-assay variations (variation among the replicates within the assays) were < 15% and inter-assay variations, that is, variability across three independents assays were < 11% according to manufacturer`s announcement.
Myocardial infarction and BMMNC transplantation
The left anterolateral thoracotomy was done in the fourth intercostal space and the heart was exposed after opening the pericardium. A removable silicone vascular loop (Surg-I-Loop, Scanlan Inc.) was coiled around the circumflex coronary artery (CX) just proximal to the left anterior descending coronary artery for 90 min to bring occlusion and myocardial infarction. Infarction was ascertained visually by monitoring the CX and typical electrocardiographic changes. After removal of the silicone loop, 40 mg of papaverine (Parenta Pharmaceuticals Inc) was administered into the vessel to avoid spasm. Reperfusion was confirmed visually and BMMNCs were transplanted into 5 different locations in and around the infarcted area with a needle (26-gauge) attached to a syringe. For draining, a single pleural tube was used and the thoracic cavity was closed. Transthoracic echocardiography was performed 30 min after instituting reflow.
MR imaging in vivo
MR imaging was performed (n = 7) at 2 h and after 21 days of BMMNC transplantation. T1 CINE MR, perfusion, and delayed enhancement (DE) sequences were used. A 1.5 T scanner (Signa Twinspeed, GE Healthcare, Milwaukee, USA) with a 4-channel cardiac coil was used. The animals were under general anesthesia and ECG and respiratory gated. T1 CINE MR images were obtained in three orientations (vertical long axis, horizontal long axis, and short axis) with the following parameters: pulse repetition time (TR) 4.2 ms, echo time (TE) 1.8 ms, matrix size 256 × 256 mm, view size 805 × 340 mm2, slice thickness 8–11 mm, and field of view (FOV) 340 mm2 × 340 mm2. A gradient-echo planar inversion–recovery, multishot, T1-weighted images were obtained using a perfusion sequence with the following parameters: TR 8 ms, TE 1.2 ms, matrix size 256 × 256 mm, FOV 340 mm2 × 340 mm2, and 3–5 slices with slice thickness 11 mm. Segmented inversion recovery fast gradient echo images were obtained using a DE sequence with the following parameters: TR 6.8 ms, TE 3.2 ms, matrix size 256 × 256 mm, FOV 340 mm2 × 340 mm2, thickness 10 mm, and inversion time 200–150 ms. For each sequence, twenty cardiac phases were reconstructed per slice location. An entire left ventricle was covered with the slice locations. A dose of 0.2 mmol/kg of gadopentetate dimeglumine (Magnevist;Schering AG, Berlin, Germany) was used as the contrast agent, and IV injected just prior to perfusion imaging. DE imaging was conducted 10–15 min after the contrast administration.
MR image analyses
All radiological measurements were evaluated independently by a radiologist (R.B.S) and a trained researcher (R.M.K) blinded to both histological and clinical data. A cardiac segmentation scheme based on the coronary vascular territories () was used for visual assessment of the extent of infarcted area and detection the BMMNC label at in-vivo MRI. An extent of infarcted area was evaluated from the DE images. Stem cell label visualization was evaluated from the T1-weighted short axis images. The SPIO label causes hypointense regions in the MRI images ( and ). A qualitative analysis of the extent of intensity change was evaluated and marked with a three step scaling system as follows: 1, slight; 2, moderate; and 3, strong. Later, the first two scales were combined in the assessment to assist in the correlation analyses with the histological quantitative analyses. Contractility was analyzed using the T1-weighted short axis images and was subdivided as follows: 0, normal; 1, hypokinesia; 2, akinesia; and 3, dyskinesia. The change in perfusion was visually observed and rated as either normal or abnormal perfusion. In the evaluation of the EF, the GE Healthcare advantage workstation AW4.2 and analysis software (MASS v.5.2, Medis Inc.) were used and data were presented as mean values from two independent readings.
Histopathologic examination
All surviving animals were followed up for 21 days. After blood sampling, echocardiography, and heparin administration (500IU/kg), the animals were sacrificed with an IV injection of pentobarbital (60 mg/kg) and the hearts were removed and fixed in 10% formaldehyde. The detailed histopathologic examination was performed to seven BMMNC + MRI subgroup animals that were initially randomly selected. The heart was divided according to the specific segmentation scheme based on the coronary vascular territories, similarly to the MRI evaluation (). The left ventricle was divided into 16 segments that were embedded in paraffin, sectioned (5 μm) and deparaffinized for stainings.
Histochemistry
Histologic staining with hematoxylin–eosin (H&E) and Preussian blue was done using a standard protocol. In brief, the Preussian blue staining sections were incubated for 30 min in 2% potassium ferrocyanide to label intracellular iron of the SPIO-labeled BMMNCs in blue.
Analysis of the histochemistry
Histological analyses were conducted by two trained researchers (K.A., P.L.), and verified by a consulting pathologist (E.L-B). H&E staining was used to assess the injury on each section as follows: 0 = normal tissue, 1 = granulation tissue, 2 = necrotic areas, and 3 = fibrotic areas.
Prussian blue was used to detect the SPIO-labeled BMMNCs and to evaluate their number in the cardiac tissue. The evaluation was performed according to their color intensity, shape, and size. The number of the BMMNCs on each Prussian-blue-stained section was semiquantitatively evaluated with the following scoring: 0 = no BMMNCs; 1 = small amount of BMMNCs; and 2 = large amount of BMMNCs.
Histology was compared with the MRI data obtained 2 h after transplantation in order to determine the homing of BMMNCs to the infarction area. For each set of cardiac segment MR images, there were three stained sections available for histological analysis. Hence, when comparing segmental signal loss of MRI and sectional staining in histology, the highest measured value from the sections on both MRI and histology was noted.
Statistical analysis
Statistical analysis was performed using statistical program (SPSS SmartViewer version 18.0, SPSS, Inc.) and SAS (version 9.2, SAS Institute Inc.). To test normality, Shapiro–Wilk test was used. Student`s t-test or Mann–Whitney U test was used to assess the distribution of variables between the study groups. The SAS procedure “Mixed” was used for repeated measurements. Cohen`s kappa coefficient was used to measure the agreement between two raters and a value of 0.6 was considered as a significant finding. All results are presented as mean and standard deviation (SD) unless otherwise stated. Reported p values are as follows: p difference between the groups, p(t*g) indicates group–time interaction. A p-value less than 0.05 was considered statistically significant.
Results
Characterization of the study groups
The median weight of the pigs was 26.1 kg (± 1.9 kg) in the BMMNC group and 27.5 kg (± 1.2 kg) in the control group. Three pigs (control n = 1 BMMNC n = 2) died during CX occlusion or soon after and they were excluded from further analysis. At the baseline the mean diastolic pulmonary artery pressure and diastolic artery pressure differed significantly between the study groups (, diastolic pulmonary artery pressure p = 0.026; diastolic artery pressure p = .04). There were no statistically significant differences in the cytokine levels between the study groups at the baseline.
Table I. Weight and hemodynamic data.
Hemodynamic and metabolic data
During the experiment, hemodynamic and metabolic variables displayed no significant differences between the study groups over time. During 90 min of CX occlusion, pulmonary artery pressures moderately increased and arterial pressures decreased in both groups (). After occlusion, cardiac output decreased in BMMNC and control groups. The infarction caused eminent release of troponin in both groups indicating a critical-sized heart injury (). The concentration of heart enzyme was similar in both study groups pre- and postoperatively.
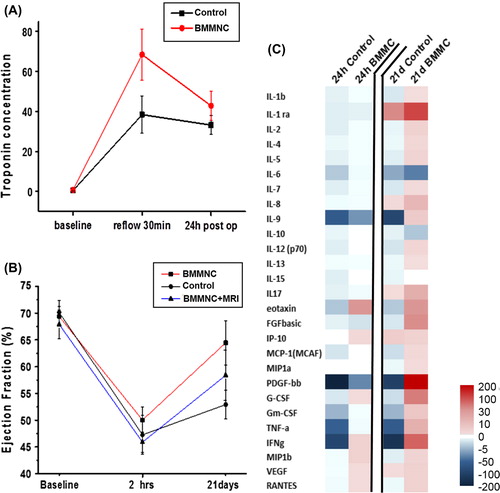
Figure 1. Effects of AMI and BMMNC transplantation. Increase in troponin concentration (A) and decrease in EF (B) reveals myocardial infarction in both groups 2 h after CX occlusion. After 21 days, the EF improvement was better in BMMNC group and BMMNC + MRI subgroup (control 5.6 percentage points vs. BMMNC 14.4). (C) The cytokine levels changed in different ways between the time points baseline vs. 24 h or 21 days in the two study groups.
Cardiac function
Transthoracic echocardiography was used to evaluate the change in cardiac function during the experiment. At the baseline, the cardiac function was similar between the study groups and there was no statistically significant difference in EF (p = 0.8, mean control group 70%, SD 8 vs. BMMNC 70%, SD 7) or in fractional shortening (FS) (mean control group 41% SD 7 vs. BBMNC 39% SD 6 p = 0.28. Two hours after CX occlusion, EF decreased similarly in all study groups due to infarction: 23 percentage points in the control group and 18 percentage points in the BMMNC group (mean control group EF = 47%, SD 13 vs. BMMNC group EF = 52%, SD 10). There was no statistically significant difference between the study groups in EF at that time point (p = 0.30). Mean FS in control group was 24% SD 8 and in BMMNC group 26% SD 7 (p = 0.35). After 21 days, significantly improved EF recovery was observed in BMMNC group, whereas only a moderate recovery appeared in controls (mean control group EF = 53%, SD 9 vs. BMMNC EF = 65%, SD 14 p = 0.02). In the control group, EF increased only 7 percentage points (SD 17) compared with 14 percentage points (SD 17) in BMMNC group (p = 0.30 ). At that time point mean FS differed between the study groups: control group 27% SD 6 and BMMNC 40% SD 14 (p = 0.018). Difference in FS between the groups at time points baseline vs. 21 days was statistically significant (mean control -14 SD 9.9 vs. BMMNC 1 SD 14 p = 0.011). No statistically significant differences existed in the measurements of the left ventricular mass, cardiac output, or thicknesses of the ventricular walls.
Inflammation
There were no significant differences in group–time interaction of white blood cell counts: lymphocytes p(t*g) = 0.798, monocytes p(t*g) = 0.229, and neutrophils p(t*g) = 0.346.
The cytokine concentrations at the baseline are shown in and the change in the concentrations are shown in and . Although no statistically significant differences appeared, tendency in the absolute cytokine levels differed between the study groups. At 24 h, mean change in interferon-γ (IFN-γ) was as follows: controls -260 SD 576 vs. BMMNC + 5 SD 38 (p = 0.66). Mean change in platelet-derived growth factor (PDGF) level was -554 SD 908 in the control group and -36 SD 108 in the BMMNC group (p = 0.08). Increase in several cytokine levels appeared in BMMNC group at 21 days: PDGF (control -315 SD 513 vs. BMMNC + 363 SD504 p = 0.08), IFN-γ (control -411 SD 1001 vs. BMMNC + 165 SD 242 p = 0.20), and IL-1ra (control + 59 SD 161 vs. BMMNC 211 SD 263 p = 0.52). In addition, the level of granulocyte colony stimulating factor (G-CSF) increased in BMMNC group but declined in controls (control -10 SD 24 vs. BMMNC 92 SD 183 p = 0.39). It is notable that cytokines showed large variation between animals and therefore the changes are not statistically significant.
Figure 2. The change in the cytokine concentrations at 24 h and 21 days. Significant decrease in IL-9, PDGF, and IFN-γ levels in the control group at 24 h suggest that BMMNC administration may have an impact on the inflammation reaction after AMI. At 21 days, the significant changes in the cytokine concentrations appeared in the BMMNC group.
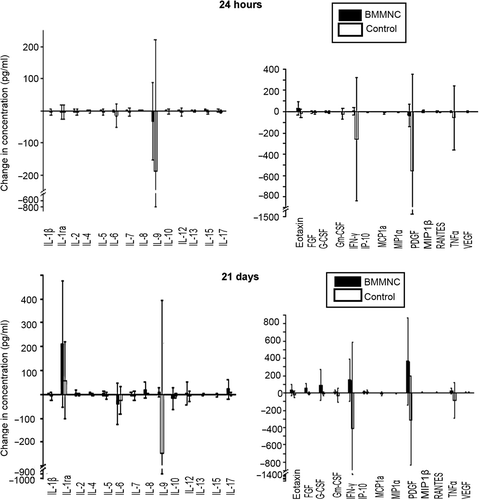
Table II. The mean cytokine concentrations at the baseline.
Cell detection based on histochemistry and MRI
In order to study in detail the role of cell homing, the left ventricle was divided into 16 segments, 5 of them being supplied by the circumflex artery (segments 5, 6, 11, 12 and 16)(). In the histological examination, either small or large amounts of SPIO-labeled BMMNCs were detected in at least two segments in each BMMNC group animal (, , ). Histological analysis revealed that the transplanted BMMNCs were situated in the infarction areas (segments 5, 6, 11, 12, and 16) ().
Figure 3. Cell detection using histology and MRI. (A) The left ventricle was divided into 16 segments based on the vascular territories in order to detect the injected BMMNC. The infarction area is designated by arrows. (B) BMMNCs were detected from the same segment area using MRI or histology (C–D) Hypointense area in MRI is interpreted as BMMNCs (arrows). The left ventricle has become thinner after 21 days due to AMI.
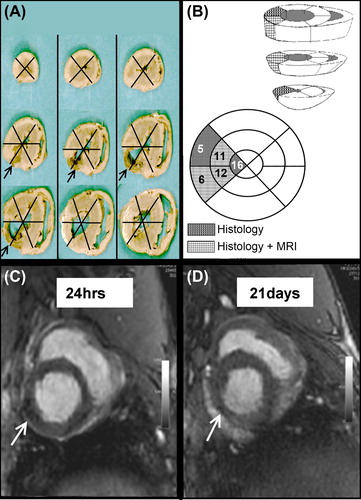
Table III. BMMCs detected using histology or MRI.
Visualized by MRI 2 h after CX occlusion, BMMNCs were detected in the hearts of 4 pigs out of 7 at the segments 6, 11, and 12 ( and , ). Cohen`s kappa coefficient was used to indicate the correspondence between histology and MRI for detection of BMMNCs. In segments 6 and 12, Cohen`s kappa coefficient was 0.571 when visualized by MRI 2 h after BMMNC transplantation. All segments could not be assessed due to statistical reasons. Combining three or four adjacent segments (7 combinations), kappa coefficient was 0.667 when using a histologically large amount of BMMNCs and MRI 2 h postoperatively.
We were not able to reliably detect the injected BMMNCs in MRI at the 21 st postoperative day due to infraction-related changes in the tissue, such as fibrosis ().
EF and BMMNC visualization
At 2 h mean EF was 63% SD 9 and at 21 days 75% SD 7 according to MRI. The absolute difference in EF between the time points was 18% and 11 percentage points that was kept as cutoff point to measure recovery in . shows the proportional area of BMMNC on each segments. The median value of the measured segments is higher in animals with the EF increase greater than 11,5 units. The BMMNCs were also located in a wider area both in the histological analysis (≥ 3 segments), and in the MRI taken 2 h after CX occlusion and BMMNC transplantation (≥ 2 segments).
Contractility of the heart
Abnormal kinetics were found in the segments supplied by the circumflex artery in every animal in the BMMNC group in MRI at both 2 h and after 21 days of infarction. Myocardial, hypokinesia, or akinesia was detected in four to five segments (total of 5) after 2 h of infarction, and in 0 to 5 segments (total of 5) at the 21st postoperative day. The abnormal kinetics (hypokinesia, akinesia, dyskinesia) was compared with myocardial injury (granulation tissue, necrosis, and fibrosis) evaluated histologically segment by segment. The abnormal kinetics did not correspond to the histological changes (Cohen`s kappa < 0.5).
Table IV. The proportional (%) area of BMMCs on each segment.
Histology: Morphological changes in the myocardium
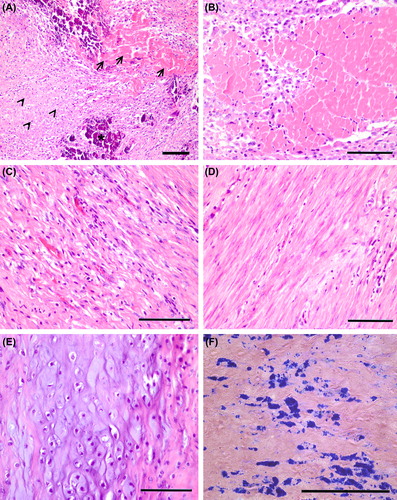
There was mainly scar-like fibrosis but also granulation tissue and necrosis in the 5 segments supplied by arteria circumflex in the BMMNC group and control group animals corresponding to the age of the infarcts (). Calcification was observed in both 50% of controls and 50% BMMNC-treated pigs. The presence of cartilage was observed in one BMMNC group animal and one control group animal in one segment ().
Figure 4. Histological changes in myocardium after 3 weeks of AMI. (A) Mainly fibrosis (arrow heads) but also necrosis (arrows) and calcification (asterix) were observed in an injured area of both BMMNC group and control group animals. (B) Necrosis was detected in a few segments, but mainly there was granulation tissue with newly formed fibrosis (C) or fibrotic scar (D, E) Cartilage was also found in the myocardium of both controls and BMMNC group animals. (F) Prussian blue staining detected the SPIO-labeled BMMNCs. (A–E) H&E staining, scale bars 100 μm.
Discussion
In this paper we demonstrate the beneficial therapeutic effect of direct myocardial BMMNC administration after AMI and characterize the cytokine profile after BMMNC therapy. Our primary echocardiography results showed better improvement in EF after AMI in the BMMNC-treated pigs. This result is in line with reports from other studies showing improved cardiac function after stem cell transplantation (Citation5,Citation6).
In our study, the change in the cytokine levels differed between the controls and BMMNC but the differences were not statistically significant due to large variation. Especially, the level of PDGF, G-CSF, IL-1Ra, and IFNγ increased in the BMMNC group at 21 days. Indeed, the recent research has highlighted the involvement of the inflammatory cytokines in recovery of AMI (Citation15,Citation16). However, the role of individual cytokines in remodeling after AMI requires further studies. The PDGF and G-CSF levels increased in the BMMNC group and declined in controls at 21 days. MSCs have been reported to secrete both PDGF and G-CSF that have an effect on neovascularization and may promote cardiac repair through reduced apoptosis and inflammation () (Citation17,Citation18). In clinical studies, G-CSF has shown to increase the functional potential of stem cells and attenuate ventricular remodeling (Citation19,Citation20). The angiogenesis in granulation tissue observed in this study is in line with the previous reports of PDGF- and G-CSF-induced neovascularization. Consequently, BMMNC transplantation and the observed elevations in the PDGF and G-CSF levels support beneficial effect on cardiac recovery.
Figure 5. The major changes in the cytokine levels at 24 h and 21 days after AMI and the possible effects. The IL-9 and PDGF levels declined in both groups at 24 h. At 21 days, a more complex cytokine network was apparent in the BMMNC group. IL-1ra, PDGF, INF-γ, and G-CSF levels were elevated. Dotted line indicates the possible effect of the cytokine.
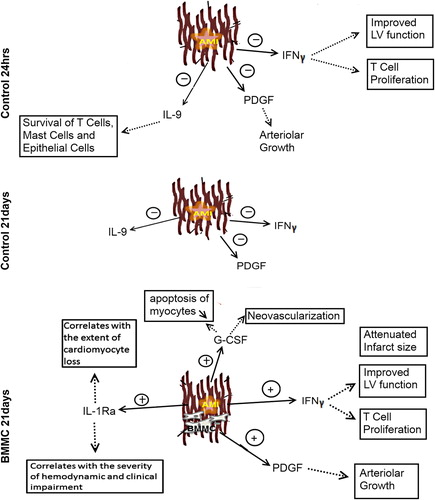
Not only were there differences in EF and in the cytokine levels between the study groups but also large, unexplained differences detected between the individual cases of the BMMNC group. We hypothesized that the variation in the cell distribution could explain at least in part the variations observed in the different cytokines, MRI, and histology. The preliminary comparative analysis of MRI and histology which was possible to perform using the current limited samples, suggested that the number and distribution of the injected BMMNCs in myocardium would correlate with the EF improvement. Between the individual porcines, the variable amount of cells detected after stem cell injection may be due to stem cell leakage during delivery or rapid elimination due to entry to circulation via heart arterioles or lymphatic vessels. This may consequently lead to diminished number of the transplanted stem cells in myocardium. Previous study suggests that the true stem cell number delivered into the myocardium is markedly smaller than the planned dose, especially if the procedure is performed with the beating heart and using multiple injections (Citation21). Hence, in order to interpret the relation of functional and biochemical variables, evaluation of stem cell homing using MRI or histology is advisable. In later phase the transplanted cells can be rejected actively by immunological activation. Concerning the high variation in the initial cytokine levels, this might also reflect different individual stress responses of animals that ultimately may have an impact on the final outcome and infarction severity, an issue that warrants further study with larger sample size.
MRI data collected either 2 h after CX occlusion or after 21 days showed abnormal kinetics in segments supplied by the circumflex artery in every experimental animal analyzed. Histopathological analysis revealed that the abnormal kinetics observed in MRI localized not only to the area of the ischemic injury but also to segments with apparent normal histology. It is known, that focal AMI has remote effect in the heart and also other organs (Citation22). Consequently, the area of abnormal kinetics is larger than the true ischemic injury with its self-evident morphological changes. In our view this reflects the well-known phenomena of stunned or hibernating myocardium (Citation23). Reflecting our results of inflammatory responses, it is possible that the inflammatory status of the myocardium has a role in restoring ventricular contractile force of stunned myocardium and recovery in general.
In this study, cartilage and calcification were observed in the myocardium of both control and BMMNC-treated animals (). Calcification has also been observed in our previous studies in which BMMNC transplantation has been used after AMI (Citation24). There was a clear tendency toward less calcification in BMMNC-treated animals suggesting that BMMNC administration does not promote the incidence of calcification. Consequently the appearance of both cartilage and calcification was interpreted as an unspecific finding in the myocardium reflecting probably apposition of inadequate tissue repair (Citation25). Similar phenomenon also occurs in human induced membranes, another example of reparative granulomatous tissue (Citation26).
One limitation of this study is that the presence of iron-containing BMMNCs could not be definitely evaluated with MRI at 21 days due to multiple, mainly infarction-related tissue issues. Here, the infarction-related trans-mural necrosis and consequent progression of scarring caused marked reduction of ventricular thickness and water content leading to decreased signal making detection of label impossible. However, in histology at 21 days, the morphology of BMMNCs was typical for SPIO-labeled cells. Hardly any signs of BMMNC disintegration appeared in the histological analysis suggesting that there had been minimal leakage of iron from the labeled BMMNCs. Another obvious limitation in this study is the cytokine multiplex–assay, which is optimized for human use and does not necessarily translate to cross species investigation, especially when low values are obtained () (Citation27).
Due to the anatomical reasons, especially after the infarction and thoracotomy, the visibility was not optimal to use standard apical 2-chamber and 4-chamber views to estimate the functional recovery by echocardiography. Instead it was estimated by using the EF obtained from the measurements of FS, that is, the movements of the lateral and the septal myocardium. This was considered to be accurate enough as the infarction was caused by the ligation of left circumflex artery, which causes the infarction in the lateral wall.
Conclusions
We conclude that the number and distribution of the injected BMMNCs in myocardium could associate with the EF improvement after AMI. Consequently detection of cell homing is essential in the assessment of the intramyocardial stem cell injection and can be performed using MRI. The present study also shows that the BMMNC injection seems to modulate the cytokine profiles in AMI and may contribute to the recovery. The study was designed and powered to detect effects of stem cell therapy on EF after CX-occlusion. Power is inadequate to show change in cytokine values. Further research is required to unravel the complexities of the cytokine network and quantification of cellular detection in myocardial tissue.
Acknowledgments
The study was supported by Finnish Foundation for Cardiovascular Research and the state subsidy to the University Hospital of Oulu. The skillful assistance of laboratory technicians Riitta Vuento and Minna Savilampi, biostatistician Pasi Ohtonen, and RN Seija Seljänperä is gratefully acknowledged.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Research project was sponsored by Finnish Foundation for Cardiovascular Research and the state subsidy for the University Hospital of Oulu. No competing financial interests exist.
References
- Krijnen PA, Nijmeijer R, Meijer CJ, Visser CA, Hack CE, Niessen HW. Apoptosis in myocardial ischaemia and infarction. J Clin Pathol. 2002;55:801–11.
- Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–53.
- Srinivas G, Anversa P, Frishman WH. Cytokines and myocardial regeneration: a novel treatment option for acute myocardial infarction. Cardiol Rev. 2009;17:1–9.
- Huikuri HV, Kervinen K, Niemela M, Ylitalo K, Saily M, Koistinen P, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29:2723–32.
- Herreros J, Prosper F, Perez A, Gavira JJ, Garcia-Velloso MJ, Barba J, et al. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur Heart J. 2003;24:2012–20.
- Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Clarke MJ, et al. Long-term effects of autologous bone marrow stem cell treatment in acute myocardial infarction: factors that may influence outcomes. PLoS One. 2012; 7:e37373.
- Mathiasen AB, Jorgensen E, Qayyum AA, Haack-Sorensen M, Ekblond A, Kastrup J. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous bone-marrow derived Mesenchymal Stromal Cells in chronic ischemic Heart Failure (MSC-HF Trial). Am Heart J. 2012;164:285–91.
- Makela J, Anttila V, Ylitalo K, Takalo R, Lehtonen S, Makikallio T, et al. Acute homing of bone marrow-derived mononuclear cells in intramyocardial vs. intracoronary transplantation. Scand Cardiovasc J. 2009;43:366–73.
- Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–43.
- Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–5.
- Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21.
- Balakumaran A, Pawelczyk E, Ren J, Sworder B, Chaudhry A, Sabatino M, et al. Superparamagnetic iron oxide nanoparticles labeling of bone marrow stromal (mesenchymal) cells does not affect their “stemness”. PLoS ONE. 2010;5:e11462.
- Peng C, Yang K, Xiang P, Zhang C, Zou L, Wu X, et al. Effect of transplantation with autologous bone marrow stem cells on acute myocardial infarction. Int J Cardiol. 2013;162:158–65.
- Makela J, Ylitalo K, Lehtonen S, Dahlbacka S, Niemela E, Kiviluoma K, et al. Bone marrow-derived mononuclear cell transplantation improves myocardial recovery by enhancing cellular recruitment and differentiation at the infarction site. J Thorac Cardiovasc Surg. 2007;134:565–73.
- Patti G, Mega S, Pasceri V, Nusca A, Giorgi G, Zardi EM, et al. Interleukin-1 receptor antagonist levels correlate with extent of myocardial loss in patients with acute myocardial infarction. Clin Cardiol. 2005;28:193–96.
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–98.
- Takano H, Ohtsuka M, Akazawa H, Toko H, Harada M, Hasegawa H, et al. Pleiotropic effects of cytokines on acute myocardial infarction: G-CSF as a novel therapy for acute myocardial infarction. Curr Pharm Des. 2003;9:1121–7.
- Hao X, Mansson-Broberg A, Blomberg P, Dellgren G, Siddiqui AJ, Grinnemo KH, et al. Angiogenic and cardiac functional effects of dual gene transfer of VEGF-A165 and PDGF-BB after myocardial infarction. Biochem Biophys Res Commun. 2004;322:292–6.
- Achilli F, Malafronte C, Maggiolini S, Lenatti L, Squadroni L, Gibelli G, et al. G-CSF treatment for STEMI: final 3-year follow-up of the randomised placebo-controlled STEM-AMI trial. Heart. 2014;100:574–81.
- Mozid AM, Jones D, Arnous S, Saunders N, Wragg A, Martin J, et al. The effects of age, disease state, and granulocyte colony-stimulating factor on progenitor cell count and function in patients undergoing cell therapy for cardiac disease. Stem Cells Dev. 2013;22:216–23.
- Teng CJ, Luo J, Chiu RC, Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132:628–32.
- Ruparelia N, Digby JE, Jefferson A, Medway DJ, Neubauer S, Lygate CA, et al. Myocardial infarction causes inflammation and leukocyte recruitment at remote sites in the myocardium and in the renal glomerulus. Inflamm Res. 2013;62: 515–25.
- Kloner RA, Allen J, Cox TA, Zheng Y, Ruiz CE. Stunned left ventricular myocardium after exercise treadmill testing in coronary artery disease. Am J Cardiol. 1991;68:329–34.
- Makela J, Yannopoulos F, Ylitalo K, Makikallio T, Lehtonen S, Lappi-Blanco E, et al. Granulation tissue is altered after intramyocardial and intracoronary bone marrow-derived cell transfer for experimental acute myocardial infarction. Cardiovasc Pathol. 2012;21:132–42.
- Ribeiro KC, Mattos EC, Werneck-de-castro JP, Ribeiro VP, Costa-e-Sousa RH, Miranda A, et al. Ectopic ossification in the scar tissue of rats with myocardial infarction. Cell Transplant. 2006;15:389–97.
- Aho OM, Lehenkari P, Ristiniemi J, Lehtonen S, Risteli J, Leskela HV. The mechanism of action of induced membranes in bone repair. J Bone Joint Surg Am. 2013;95:597–604.
- Merilainen S, Makela J, Jensen HA, Dahlbacka S, Lehtonen S, Karhu T, et al. Portal vein cytokines in the early phase of acute experimental oedematous and necrotizing porcine pancreatitis. Scand J Gastroenterol. 2012;47:1375–85.
