Abstract
Objectives: Stromal cell-derived factor 1a (SDF-1α), is a chemokine and is able to home hematopoietic progenitor cells to injured areas of heart tissue for structural repair. Previous studies have found increased levels of SDF-1α in several cardiac diseases, but only few studies have investigated SDF-1α in patients with atrial fibrillation (AF). We aimed to test SDF-1α in a large cohort of patients with AF and its role as a prognostic marker. Design: Between January 1st 2008 to December 1st 2012, 290 patients with ECG documented AF were enrolled from the in- and outpatient clinics at the Department of Cardiology, Hvidovre Hospital, University of Copenhagen, Hvidovre, Denmark. Plasma levels of SDF-1α were measured using ELISA technique. Clinical data were registered and patient follow-up was conducted. Results: Patients with permanent AF had significantly higher SDF-1α levels (2199.5 pg/ml) than the patients with paroxysmal AF (1982.0 pg/ml) and persistent AF (1906.0 pg/ml), p < 0.0005. Higher SDF-1α level was associated with longer time spent in the hospital per readmission, p < 0.05. Conclusion: In AF patients, a higher SDF-1α level was found in patients with a more progressive state of arrhythmia and was associated with longer hospitalizations. These findings suggest that SDF-1α could prove valuable in risk stratification and evaluating the disease burden in AF patients.
Introduction
Stromal cell-derived factor-1α (SDF-1α) is a chemokine with many vital functions, such as stem cell survival, proliferation [Citation1] and homing of hematopoietic progenitor cells to injured areas of heart tissue for structural repair. [Citation2] As reviewed, [Citation3] several studies have linked tissue damage in cardiovascular diseases, such as myocardial infarction, to an increase in SDF-1α secretion, and suggested that SDF-1α has a pivotal role in mobilization of stem cells that are associated with improved left ventricular function after an ischemic event. [Citation4] But only few studies have examined the role of SDF-1α in atrial fibrillation (AF). [Citation5,Citation6] Atrial fibrillation (AF) is the most common arrhythmia in clinical practice often occurring in the presence of other cardiovascular diseases and often involves structural changes in atrial tissue. [Citation7] Recent studies found association between levels of SDF-1α and the type of AF disease: increased plasma SDF-1α level was seen in patients with permanent (PermAF) or persistent AF (PeAF) compared with paroxysmal AF (PAF) [Citation5] and in patients with PeAF compared with PAF and controls with sinus rhythm (SR). [Citation6] Former studies have also linked the recurrence of AF after cardioversion (CV) with low baseline SDF-1α level and high age. [Citation8] However, the prognostic role of SDF-1α have, to the best of our knowledge, only been studied in one large cohort study, [Citation9] demonstrating a correlation between SDF-1α and heart failure (CHF) and all-cause mortality. Previous studies also suggest that illness severity is associated with the duration of hospital admissions. [Citation10] As hospitalization due to AF or complications thereof could reflect the severity of the disease, it is likely that the level of SDF-1α could also be associated with the duration of hospitalization.
We aim to (i) investigate the SDF-1α level in relation to the degree of arrhythmia (PAF, PeAF and PermAF) while adjusting for relevant covariates, (ii) explore the prognostic power of SDF-1α in relation to risk of cardiovascular readmissions, (iii) investigate the level of SDF-1α compared to duration of hospitalizations during follow-up and (iv) explore the relationship between mortality and SDF-1α.
Materials and methods
Patients
Patients with ECG documented AF were consecutively enrolled in the Atrial Fibrillation Survey – Copenhagen (ATLAS-CPH) from January 1st 2008 to December 1st 2012. In- and outpatients were enrolled from the Department of Cardiology at Hvidovre Hospital, University of Copenhagen, Hvidovre, Denmark. Inclusion criteria were: >18 years old; ECG-documented AF or a Holter recording of at least 30 s of AF and ability to give oral and written consents. PAF was defined as at least one recorded AF with spontaneous conversion to SR and excluding other forms of AF in same patient. PeAF was characterized as recorded episodes of AF where either medical or electrical cardioversion (CV) was needed to restore SR. PermAF was defined as AF where medical or electrical CV had been unsuccessful in restoring SR or, in some cases, without attempting CV if a cardiologist accepted the arrhythmia as permanent at the time of the diagnosis. Patients were excluded if estimated survival was less than one year from inclusion date. Included patients were asked to fill out self-administered questionnaires about their health. Questions included whether or not they had: hypertension, diabetes, stroke or transitory cerebral ischemia, chronic obstructive pulmonary disease, ischemic heart disease (IHD), CHF, smoking and alcohol consumption. Gender and age was also registered in the database. Peripheral venous blood samples were taken in stable condition at discharge or within 1 week after an outpatient clinical control.
Measurement of plasma levels of SDF-1α
Peripheral venous blood was sampled and anticoagulated with heparin. Samples were spun for 15 min at 1000 g within 30 min of collection. Plasma was stored at −80 °C until analysis.
Levels of SDF-1α were determined using a commercially available ELISA kit (Quantikine®, Human CXCL12/SDF-1α Immunoassay, RnD, Minneapolis, MN; #DSA00) according to manufacturer’s instructions.
Follow-up
Since 2010 every medical journal notation and information from patient administrative systems has been registered in the Danish Electronic Medical Journal. Information from paper-based medical records and administrative systems from before 2010 have been scanned and uploaded to the electronic medical journal. Between March and April 2014, senior medical students registered every hospital readmission after inclusion date and duration of each of the readmissions if the discharge diagnosis was: AF, unstable angina, stroke or transitory cerebral ischemia, myocardial infarction, CHF, CV, radiofrequency catheter ablation, pacemaker implantation, coronary catheterization, percutaneous coronary intervention, coronary artery bypass graft, bleeding/unusual INR levels, observation for anti-arrhythmic drug therapy or anti-arrhythmic drug side effects through the electronic medical journal. The senior medical students assigned a discharge diagnosis based on the journal notations from each hospital readmission of every patient. Definitions for the discharge diagnoses were: AF: patients with ECG-documented AF attacks or Holter monitoring for min; 30 seconds, hypertension: a systolic pressure of 140 mmHg or greater and a diastolic pressure of 90 mmHg or greater; unstable angina: Troponin T-negative (TnT-negative) patients with ECG-recorded ischemia; stroke or transitory cerebral ischemia: patients admitted with CT documented stroke or suspected transitory cerebral ischemia excluding other differential diagnoses; myocardial infarction: TnT-positive with ECG-recorded ischemia; CHF: patients with left ventricular ejection fraction < 35% or patients treated with diuretics due to edema or dyspnea at rest; CV: patients hospitalized due to elective cardioversion or patients hospitalized due to AF and then treated with CV; radiofrequency catheter ablation: admitted and performed radiofrequency catheter ablation; pacemaker implantation: hospitalization due to implantation of or because of pacemaker/implantable cardioverter defibrillator, coronary catheterization; percutaneous coronary intervention or coronary artery bypass graft: admission due to one of these procedures; bleeding/unusual INR levels: INR level > 3; observation for anti-arrhythmic drug therapy or anti-arrhythmic drug side effects: hospitalized for either start of new anti-arrhythmic drug or side effect of an anti-arrhythmic drug. New comorbidities that occurred after inclusion date were registered if these were registered in the electronic medical journal. Comorbidities included: hypertension, IHD, CHF, chronic obstructive pulmonary disease or diabetes. Vital status was recorded as alive or dead and was investigated for each patient through the Central Patient Registry.
Ethics
The study complies with the Declaration of Helsinki. Establishment of the database and bio bank was approved by the Danish Data Protection Agency (J. No. 2007-41-1199) and the study was approved by the Danish Standards Ethical Committees (J. No. H-C-2009-014). Written informed consents were obtained from all participants.
Data presentation and statistical analysis
Continuous variables were tested for normal distribution with the Shapiro-Wilk test. Data were normalized using logarithmic transformation if strongly positively skewed and squared transformation if moderately positively skewed. Baseline tests for parametric data included Student’s t-test and ANOVA followed by a Tukey post-hoc analysis. These were used to assess differences between two or three groups, respectively. For non-parametric data, Mann-Whitney U tests were used to assess differences between two groups. Two-way ANOVA was used to assess any interaction between two independent variables on the dependent variable. Individual correlations were assessed with Pearson’s correlation coefficient test for parametric data and Spearman’s Rank Order test for non-parametric data. Multiple regression analysis was applied to identify any independent explanatory variables that could predict the levels of SDF-1α. A multivariate survival analysis was conducted with the use of a Cox proportional-hazard model for the time between inclusion date and first date of readmission after inclusion date. If patients did not experience any readmission event, the end date was set to be 1 April 2014, where the follow-up information was gathered. Logistic regression where death was set as outcome was also performed. All tests were two-tailed and statistical significance was considered for p < 0.05 if nothing else is stated. All statistical analyses were performed using SPSS version 21 (Armonk, NY).
Results
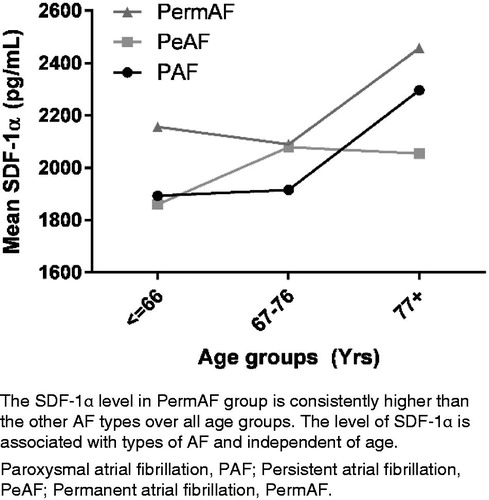
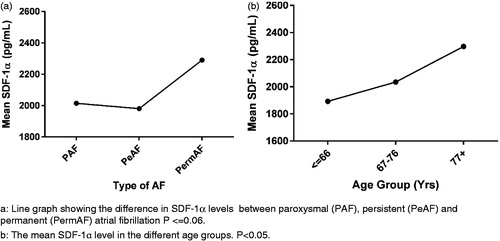
Between 1 January 2008 and 1 December 2012, 310 patients with AF were included in the study. Follow-up was accomplished in 310 patients and measurement of SDF-1α was completed in 290 patients. Further analysis was done only on patients with measureable SDF-1α. Of these, 74 patients were diagnosed with PAF, 128 were diagnosed with PeAF, and 68 with PermAF. We were not able to obtain information regarding AF type for the remaining 20 patients. Median SDF-1α levels in PAF, PeAF and PermAF was 1982.0 pg/ml [1176.0 to 3450.0 pg/ml], 1906.0 pg/ml [1137.0 to 3370.0 pg/ml] and 2199.5 pg/ml [1212 to 4163 pg/ml], respectively. The age ranged from 24 to 94 years. For further analyses, patients were age-grouped in even tertiles. Conventional cardiovascular risk factors and levels of SDF-1α at the baseline examination are presented in . Mean follow-up was 4.12 years [range 2.4 to 5.2 years]. SDF-1α showed a positively skewed distribution and was normalized by logarithmic transformation (Shapiro–Wilk’s test, p > 0.05). Data are given in exact SDF-1α values but statistical calculations were performed with the transformed values.
Table I. Patients baseline characteristics (N=290).
SDF-1α in patients with different types of AF
The level of SDF-1α was significantly different between patients with different types of AF (p < 0.0005) (). The SDF-1α levels in were a back-calculation from the logarithmic transformed values. The Tukey post-hoc analysis () showed that the difference was statistically significant (p < 0.001) between PAF and PermAF and between PeAF and PermAF but no other group differences were statistically significant. There was no statistically significant interaction between AF type and age group on the SDF-1α levels, p = 0.113 ().
Table II. One-way ANOVA of the difference in SDF-1α between patients with different types of AF.
Table III. Tukey post-hoc test of One-way ANOVA for AF types and age groups.
Determinants of plasma SDF-1α level
The multiple regression was performed to predict SDF-1α from conventional risk factors for heart disease, diabetes, alcohol, tobacco, age, gender, type of AF, IHD, CHF and hypertension. In the multiple regression analysis only age, alcohol, hypertension, and the presence of CHF significantly contributed to the SDF-1α level (p < 0.005). One-way ANOVA showed that the levels of SDF-1α increased with increasing age group: 1892.7 ± 430.8 pg/ml in patients below 66 years, 2035.1 ± 380.9 pg/ml in the age group 67-76 years and 2297.8 ± 551.3 pg/ml in patients aged 77 years and older (p < 0.001) (). Patients with CHF or IHD had significantly higher mean SDF-1α levels (2437.9 and 2147.2 pg/ml, respectively) compared to patients without CHF or IHD (2017.4 and 2053.6 pg/ml, respectively).
Readmissions
During the follow-up period, 162 (mean SDF-1α =2089.3 pg/ml) patients had one or more readmissions due to cardiovascular disease, whereas 125 (mean SDF-1α =2040.1 pg/ml) patients had no readmissions. No information was available regarding hospital admissions for the last three patients. Variables for inclusion in the multivariate Cox regression analysis were age, sex, hypertension, IHD, CHF, diabetes and SDF-1α. Only age (HR = 0.986, 95% CI 0.973 to 0.999, p = 0.039) and IHD (HR = 1.718, 95% CI 1.128 to 2.617, p = 0.012) was significantly associated with increased risk of readmission. SDF-1α was not an individual significant factor for the prediction of number of readmissions in patients with AF. Patients with PeAF were more often hospitalized during follow-up compared with patients with PermAF and PAF (2.7, 1.6 and 1.7 readmissions, respectively; one-way ANOVA, p < 0.05). No other differences between AF types were statistically significant.
SDF-1α and duration of hospital admission
There was a positive correlation between an increased level of SDF-1α and the time spent in the hospital per readmission [rs (288) = 0.125, p < 0.05, Spearman’s Rank Order test]. Using Mann-Whitney U-test, it was found that average duration of readmissions were different for patients with and without IHD (5 and 1.7 days, respectively; p = 0.004). Similarly, patients with CHF had longer duration of hospital admissions compared with patients without CHF (6.2 and 1.7 days, respectively, p = 0.011).
Mortality
During follow-up, 41 of 290 patients died. The SDF-1α level was higher in patients who died (2266 ± 461.5 pg/ml) compared with those who survived (2035.5 ± 479 pg/ml) (p = 0.002). Logistic regression demonstrated that age and CHF significantly predicted death during follow-up (p < 0.0005). Gender, IHD, diabetes, stroke and SDF-1α levels did not influence survival. The model explained 20.2% of the variance (Nagelkerke R2).
Discussion
The major findings of this study are: (i) patients with PermAF have higher levels of SDF-1α compared with PAF and PeAF, (ii) patients with PeAF have more readmissions compared to patients with other types of AF and IHD significantly contributes to higher readmission rate, (iii) a positive correlation between higher SDF-1α levels and longer duration of hospital stay and (iv) higher SDF-1α level in patients who died during follow-up compared to patients who survived follow-up time.
Our finding of increased SDF-1α levels with more progressive stages of AF is in accordance with previous studies. [Citation5,Citation6] It has been widely accepted that the incidence of AF increases with age and that with age, AF is more likely to become permanent. [Citation11] Previous studies adjusting for age [Citation5] or using age-matched controls [Citation6] suggest that the increase in SDF-1α levels with more permanent types of AF is independent of age. Our results support that the SDF-1α is related to the arrhythmia itself. [Citation1] Our patients were screened for major illnesses prior to inclusion in the study. Baseline values of the different patient groups suggest that there is no major difference in severe comorbidities among the different groups of AF types.
The finding, that patients with PeAF had more readmissions compared with patients with PAF and PermAF, is in accordance with the findings of Qvist et al. [Citation12] This demonstrates that patients with PeAF tended to be hospitalized more often for either medical or electrical cardioversion, whereas patients with PermAF were admitted less often, but due to more severe complications, such as ischemic stroke or CHF. Kim et al. [Citation8] suggested that a low baseline level of SDF-1α was an independent risk factor for AF recurrence after CV in patients with PeAF and in this particular group, a low level of SDF-1α might predict hospitalization.
To our knowledge, this is the first study evaluating the relationship between the level of SDF-1α and the average length of hospital stay per hospitalization, demonstrating a correlation between SDF-1α levels and average length of hospital stay per hospitalization. The presence of CHF and/or IHD also contributed to the average length of hospital stay per hospitalization, in accordance with previous studies. [Citation13] The results agrees with former studies suggesting that increased SDF-1α is correlated to progressive CHF [Citation9,Citation14] and that ischemia mobilizes progenitor cells to injured areas of heart tissue through SDF-1α signaling. [Citation15]
SDF-1α was not an independent factor in the logistic regression but it was still statistically significantly different in patients who died during follow-up compared to patients who survived. Previous studies regarding cancer have suggested that high SDF-1α levels are an independent factor for poor survival [Citation16] possibly because SDF-1α facilitates neovascularization in neoplasm. [Citation17] As mentioned before, SDF-1α is known to be elevated after ischemia, and injection of SDF-1α into ischemic myocardium in mice has been demonstrated to reduce infarct size and improve cardiac function, possibly due to the ability of SDF-1α to promote angiogenesis. [Citation18] The relation of SDF-1α and survival of patients with cardiovascular disease has, to the best of our knowledge, only been studied once: demonstrating an association between SDF-1α and all-cause mortality in a population free of cardiovascular disease at baseline. [Citation9] Our findings of an association between SDF-1α and increased mortality, IHD and CHF are in accordance with this. Altogether, previous research and our findings suggest that SDF-1α is increased in response to cardiac disease and that, since hypoxia stimulates SDF-1α secretion in several circumstances, cardiac ischemia may contribute to the increase in SDF-1α levels. Although SDF-1α confers protection against ischemia, [Citation1] it may also indicate ongoing cardiac injury and thus may be a marker of disease burden and possibly indicate the risk of progression of disease and death.
The strengths of this study include a large cohort of AF patients and extensive, systematical gathering of follow-up data. Possible limitations could be a selected patient group due to the inclusion criteria of an expected survival of more than one year, thus they may not represent the general population of AF patients. We did not select patients based on specific use of medication and this may influence data on readmission. As we did not include non-cardiac discharge diagnoses in the follow-up, we could neither evaluate the possible influence of non-cardiac conditions on outcome nor can we exclude the possibility that non-cardiac disease may influence the SDF-1α levels.
Conclusion
Patients with permanent atrial fibrillation had significantly increased SDF-1α levels compared with patients with paroxysmal and persistent atrial fibrillation, independent of age. Moreover, an increased SDF-1α level related to longer hospital stay per hospitalization and a higher mortality risk. SDF-1α could prove valuable in risk stratification and evaluation of the disease burden in patients with atrial fibrillation, but further studies are needed to field-test SDF-1α in relation to disease burden in a clinical setting.
| Abbreviations | ||
| SDF-1α | = | stromal cell-derived factor-1α |
| AF | = | atrial fibrillation |
| PAF | = | paroxysmal atrial fibrillation |
| PeAF | = | persistent atrial fibrillation |
| PermAF | = | permanent atrial fibrillation |
| SR | = | sinus rhythm |
| CV | = | cardioversion |
| CHF | = | congestive heart failure |
| IHD | = | ischemic heart disease |
Acknowledgements
The authors are grateful for the expert statistical assistance provided by Head of the Statistics Function at the Clinical Research Centre, Steen Ladelund.
Declaration of interest
The work was supported by: The Beckett-Foundation, Hvidovre Hospital Executive’s Research Foundation, Snedkermester Sophus Jacobsen and Hustru Astrid Jacobsen’s Foundation, The A.P. Møller Foundation, Holger Rabitz and Hustru Doris Mary born Phillipp’s Memorial Foundation, The Family Hede Nielsen’s Foundation, Torben and Alice Frimodt’s Foundation, The Spar Nord Foundation, Dagmar Marshall’s Foundation, The TOYOTA Foundation and Thorkild Steenbeck’s Scholarship.
References
- Bromage DI, Davidson SM, Yellon DM. Stromal derived factor 1alpha: a chemokine that delivers a two-pronged defence of the myocardium. Pharmacol Ther. 2014;143:305–315.
- Penn MS, Pastore J, Miller T, et al. SDF-1 in myocardial repair. Gene Ther. 2012;19:583–587.
- Zaruba MM, Franz WM. Role of the SDF-1-CXCR4 axis in stem cell-based therapies for ischemic cardiomyopathy. Expert Opin Biol Ther. 2010;10:321–335.
- Wojakowski W, Tendera M, Zebzda A, et al. Mobilization of CD34(+), CD117(+), CXCR4(+), c-met(+) stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. Eur Heart J. 2006;27:283–289.
- Stellos K, Rahmann A, Kilias A, et al. Expression of platelet-bound stromal cell-derived factor-1 in patients with non-valvular atrial fibrillation and ischemic heart disease. J Thromb Haemost. 2012;10:49–55.
- Goette A, Jentsch-Ullrich K, Lendeckel U, et al. Effect of atrial fibrillation on hematopoietic progenitor cells: a novel pathophysiological role of the atrial natriuretic peptide? Circulation. 2003;108:2446–2449.
- Burstein B, Qi XY, Yeh YH, et al. Atrial cardiomyocyte tachycardia alters cardiac fibroblast function: a novel consideration in atrial remodeling. Cardiovasc Res. 2007;76:442–452.
- Kim SK, Pak HN, Park JH, et al. Serological predictors for the recurrence of atrial fibrillation after electrical cardioversion. Korean Circ J. 2010;40:185–190.
- Subramanian S, Liu C, Aviv A, et al. Stromal cell-derived factor 1 as a biomarker of heart failure and mortality risk. Arterioscler Thromb Vasc Biol. 2014;34:2100–2105.
- Pompei P, Charlson ME, Ales K, et al. Relating patient characteristics at the time of admission to outcomes of hospitalization. J Clin Epidemiol. 1991;44:1063–1069.
- Zoni-Berisso M, Lercari F, Carazza T, et al. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220.
- Qvist JF, Sorensen PH, Dixen U. Hospitalisation patterns change over time in patients with atrial fibrillation. Dan Med J. 2014;61:A4765.
- Steinberg BA, Kim S, Fonarow GC, et al. Drivers of hospitalization for patients with atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2014;167:735–742.
- Jorbenadze R, Schleicher E, Bigalke B, et al. Expression of platelet-bound stromal-cell derived factor-1 (SDF-1) and number of CD34 progenitor cells in patients with congestive heart failure. Platelets. 2014;25:409–415.
- Kim SK, Pak HN, Park JH, et al. Non-ischaemic titrated cardiac injury caused by radiofrequency catheter ablation of atrial fibrillation mobilizes CD34-positive mononuclear cells by non-stromal cell-derived factor-1alpha mechanism. Europace. 2009;11:1024–1031.
- Popple A, Durrant LG, Spendlove I, Rolland P, et al. The chemokine, CXCL12, is an independent predictor of poor survival in ovarian cancer. Br J Cancer. 2012;106:1306–1313.
- Kryczek I, Lange A, Mottram P, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472.
- Sasaki T, Fukazawa R, Ogawa S, et al. Stromal cell-derived factor-1alpha improves infarcted heart function through angiogenesis in mice. Pediatr Int. 2007;49:966–671.