Abstract
Objectives: The crucial role of twisting motion on both left ventricular (LV) contraction and relaxation has been clearly identified. However, the reports studying the association between LV torsion and loading conditions have revealed conflicting outcomes. Previously normal saline infusion was shown to increase LV rotation. Our aim was to test this phenomenon after volume depletion in healthy volunteer blood donors. Design: A total of 26 healthy male volunteers were included in the study. LV end-diastolic and end-systolic diameter, LV ejection fraction, LV diastolic parameters, LV apical and basal rotation and peak systolic LV torsion were measured by speckle-tracking echocardiography before and after 450 mL blood donation. Results: Blood donation led to a significant decrease in end-diastolic LV internal diameter (48.7 ± 0.4 versus 46.4 ± 0.4 mm; p < 0.001) and cardiac output (6.2 ± 1.0 versus 5.1 ± 0.7 L/min; p < 0.001). There was a significant decrease in the magnitude of peak systolic apical rotation (4.4 ± 1.9° versus 2.9 ± 1.5°; p < 0.001) but no change in basal rotation (2.6 ± 1.4° versus 2.7 ± 1.6°; p = 0.81). Peak systolic LV Torsion decreased after blood donation (6.9 ± 1.9° versus 5.7 ± 2.1°; p = 0.028). Conclusions: LV apical rotation and peak systolic LV torsion seem to be preload dependent. Preload reduction provided by 450-mL blood donation decreased LV torsion in healthy male volunteers. Volume dynamics should be taken into account in the evaluation of LV torsion.
Introduction
Rotation of the left ventricle (LV) is crucial for both contraction and relaxation of the heart.[Citation1] Rotational movement of the LV, which is determined by speckle tracking echocardiography is a more recent and sophisticated parameter in the evaluation of myocardial systolic function. In addition to the contribution of the torsional motion to the systole, the muscle fibers that revert back secondary to contrary movement in early diastole also contribute significantly to filling of LV.[Citation2]
In fact, torsion of the LV is the wringing motion of the ventricle around its long axis. It occurs as a result of contraction of the oblique located muscle fibers. The normal left ventricle presents an opposite rotation between apex and base. As viewed from the apex, it shows a counter-clockwise rotation at the apex and a clockwise rotation at the base. The difference in rotation angles between the base and apex is called net twist angle or net torsion angle and expressed in degrees. Briefly, the result of this rotational movement in the opposite directions is defined as LV torsion, which is essential for proper myocardial function.[Citation3–5]
Speckle tracking technique enabled further evaluation of left ventricular (LV) deformation.[Citation6] Deterioration in the LV torsion has already been demonstrated in various cardiovascular disorders, such as ischemic heart disease, diastolic dysfunction, hypertrophic and dilated cardiomyopathy, coronary slow flow and heart valve diseases.[Citation7–13] All these findings show that LV torsion may be considered as a marker for cardiovascular diseases. Additionally, quantification of LV torsion might be helpful in clinical decision-making.[Citation14]
The impact of cardiac volume overload on LV torsion in healthy human heart has previously been studied and it was suggested that peak LV torsion and peak early diastolic untwisting rate are preload dependent.[Citation15] However, impact of volume depletion on LV torsion has not been studied and we hypothesized that volume decrease provided by blood donation would result in significant decrease in LV torsion.
Material and methods
Study population
Study was performed on randomly selected healthy male volunteer blood donors at the blood donation unit of our hospital in January 2012. Subjects were eligible if they were between 18 and 45 years old. Exclusion criteria included hypertension, diabetes mellitus, heart failure, known coronary artery disease, prior myocardial infarction, congenial heart disease, heart valve disease, atrial fibrillation or any cardiac arrhythmia, chronic kidney disease, chronic obstructive lung disease, anemia, thyroid dysfunction and any continued medication for any reasons. Written informed consent was obtained from all the participants and the study was approved by the local ethical committee.
Echocardiographic assessments
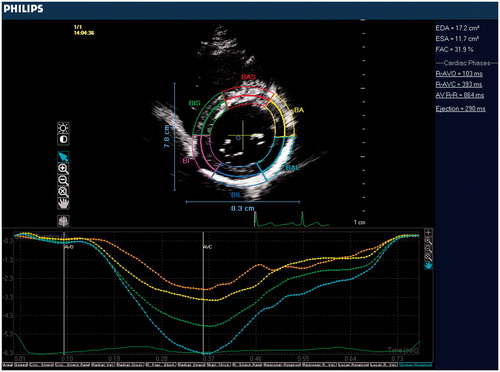
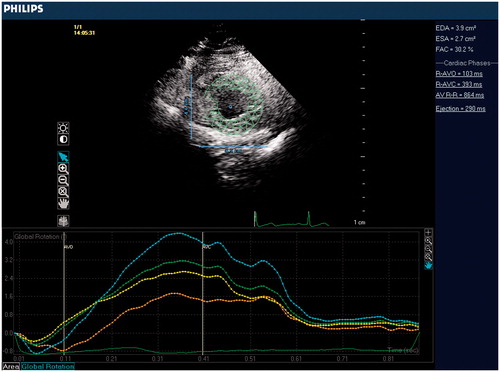
Transthoracic echocardiography was performed using Philips iE33 xMATRIX Echocardiography system (Philips Healthcare, Andover, MA). Subjects were studied at baseline and again immediately after the blood donation. Blood donation procedure was applied in 5-10 min, post-donation measurements were performed just after the end of procedure, any time delay was avoided to get the accurate results of acute volume change. Two-dimensional, pulse Doppler, continuous Doppler, tissue Doppler imaging were performed from standard parasternal and apical positions. Cardiac structural measurements were made according to current guidelines. LV ejection fraction was calculated using the Teicholz technique. Left ventricular outflow track (LVOT) was measured from parasternal long axis view. Stroke volume (SV) was calculated as LVOT cross-sectional area × LVOT velocity time integral (LVOT VTI). Cardiac output was calculated as SV × heart rate. Peak early (E) and late (A) diastolic velocities, and tissue Doppler (E') measurements were quantified from apical four-chamber view via standard technique.On the purpose of LV torsion measurement, the basal level was defined as the highest basal imaging plane at which uniform full thickness myocardium was observed surrounding the mitral valve at end-systole (). The apical level was defined as the imaging plane with no visible papillary muscles (). Speckle-tracking analysis was used to measure LV rotation and LV torsion as previously described.[Citation16] The highest-quality digital 2D basal and apical images were selected and the endocardium was traced. A full-thickness myocardial region of interest was selected, and suitable stable objects within this region were tracked. LV rotation at the basal and apical short-axis planes was determined as the average angular displacement of six myocardial segments. Curves of basal and apical LV rotation were automatically generated by the software. Peak systolic LV torsion was calculated as the maximum difference between peak systolic apical and basal rotation.
Statistical analysis
All statistical analyses were performed by using statistical software (SPSS 19 for Windows, SPSS, Chicago, IL). The Kolmogorov-Smirnov test was used to define whether variables were normally distributed or not. The paired Student’s t-test was used to compare the mean values before and after the blood donation. A p-value <0.05 was considered as statistically significant.
Results
Twenty-six subjects with a mean age of 35 ± 8.7 years completed the full protocol. All individuals had vital signs that were within normal limits and had structurally normal hearts. Age, heart rate and echocardiographic measurements of the subjects are shown in . A blood donation of 450 mL was successfully completed in all subjects.
Table I. Baseline characteristics of the subjects.
The echocardiographic parameters of the subjects before and after blood donation are given in . There was a significant decrease in LV end-diastolic diameter (48.7 ± 4.8 and 46.4 ± 4.3 mm, p < 0.001) before and after blood donation. Blood donation significantly decreased both stroke volume (45.6 ± 7.4 and 37 ± 5.8 ml, p < 0.001) and cardiac output (6.2 ± 1.0 and 5.1 ± 0.7 L/min, p < 0.001). The change in the cardiac output was due to the decrease in the stroke volume because there was no significant change in the heart rate (79.6 ± 9.7 versus 80.2 ± 8.3/min, p = 0.6). There was a significant change in LV ejection fraction after blood donation (67.4 ± 5.0 versus 65.1 ± 6.3%, p = 0.015). Decrease in LV ejection fraction was due to decrease in the LV end diastolic diameter because there was no change in the LV end-systolic diameter ().
Table II. Echocardiographic and hemodynamic parameters before and after blood donation.
Trans-mitral Doppler-derived diastolic measurements changed significantly after blood donation. Early diastolic blood flow velocity (E-wave) values were significantly higher after blood donation when compared to basal values (75 ± 13 versus 63 ± 11 cm/s, p < 0.001). Late diastolic blood flow velocity (A-wave) values also increased significantly when compared to basal values before the procedure (63 ± 10 versus 58 ± 9 cm/s, p = 0.012). Finally, early diastolic peak tissue velocity (Em) values were significantly higher when compared to basal values (9 ± 1.9 versus 7 ± 1.7 cm/s, p < 0.001) ().
Table III. Left ventricular systolic and diastolic parameters before and after blood donation.
Evaluation of the LV torsion is given in . There was a significant decrease in peak systolic apical rotation after blood donation. Before and after values of apical rotation were 4.3 ± 1.9° and 2.9 ± 1.5°, respectively (p < 0.001). In contrast, basal rotation was unchanged after protocol. Before and after values of basal rotation were −2.6 ± 1.4° and −2.7 ± 1.6°, respectively (p = 0.81). This resulted in an 18% increase in peak systolic LV torsion. Basal and post-procedural values were 5.7 ± 2.1° and 6.9 ± 1.9°, respectively (p = 0.02).
Table IV. Evaluation of LV torsion before and after blood donation.
Discussion
The association between acute volume depletion and LV torsion was evaluated in our study. Blood donation was used to induce acute volume depletion. A significant decrease was observed in LV end-diastolic diameter, cardiac output and stroke volume secondary to 450 mL blood donation. The deterioration in diastolic function was also prominent. The primary aim of our study was to investigate the rotation dynamics of the LV. We observed significant decrease in LV apical rotation and peak systolic LV torsion. These results demonstrated that LV torsion is significantly altered with acute volume depletion.
The significant changes in mitral inflow velocities and mitral annular velocity measurements might be either due to a decrease in preload or an acute change in diastolic functions. Since the LV filling pressures seems to be constant according to E/Em measurements, it is more likely that the decrease in preload subsequently altered these measurements. LV torsion plays an important role in relaxation of the heart as well as contraction. Potential energy is stored in the subendocardial fiber matrix secondary to twist during ejection. Subsequent recoil of twist deformation leads to release of the restoring forces and contributes to LV diastolic relaxation and early diastolic filling. Thus, a causative association can exist between alteration of LV torsion and alterations of diastolic parameters. Although the change in the diastolic parameters can be attributed to the alteration in LV torsion since untwisting motion following LV torsion plays an important role in relaxation of the heart, such relationship between diastolic parameters and LV torsion should be documented via specific measurements of untwisting rate. This topic could be a subject for another study.
Torsion of the LV develops secondary to shortening of oblique LV fibers. Therefore, to assume an association between twisting motion of the heart and volume status can be reasonable. However, the effects of loading condition on LV rotation dynamics differ a little from theoretical view. The literature includes conflicting reports regarding the effects of loading conditions on the twisting motion of the heart. Considering the outcomes of the previous animal studies, it was concluded that loading conditions significantly alter the twisting motion of the heart. In these experimental models, preload was manipulated by volume loading. The increase in preload was supported by the increase in stroke volume and the increase in preload resulted in increase in LV torsion. It was concluded that LV torsion is load dependent.[Citation17,Citation18]
On the other hand, some clinical reports studying on human hearts have revealed controversial outcomes suggesting that LV torsion may not be preload dependent.[Citation19,Citation20] Reports demonstrating the exact opposite are also present. In a prior study, glyceryl trinitrate (GTN) administration was used to manipulate the loading conditions and both preload and afterload reduction were achieved. It was suggested that LV torsion parameters are sensitive to acute changes in volume status and therefore need to be interpreted in the context of current loading conditions.[Citation21] In contrast to our results, GTN administration augmented peak LV torsion. However, it can be concluded that pure preload reduction could not have been achieved in the mentioned study and results may have been altered because of drug-induced manipulation of both preload and afterload. Laser et al. [Citation22] also revealed a study quantifying LV torsion in patients undergoing interventional closure of patent arterial ducts and atrial septal defects, which demonstrated a decrease in LV torsion due to volume overload. In this way, it is not clear whether behavior of torsion is volume dependent. Furthermore, the effects of load on this deformation remain controversial.
In a recent study about loading dependency of LV torsion, IV saline infusion was used to increase preload.[Citation15] Administration led to a significant increase in LV end-diastolic diameter, LV end-diastolic volume, stroke volume and cardiac output. There was a significant increase in the magnitude of peak systolic apical rotation but no change in basal rotation. This saline-induced increase in LV torsion was associated with a marked increase in peak early diastolic untwisting rate. Consequently, it was suggested that peak systolic LV torsion and peak early diastolic untwisting rate are preload-dependent.
This study is novel in terms of investigating the impact of actual volume decrease on LV torsion in healthy human hearts. Results of our study demonstrate that LV torsion is preload dependent. In addition, our results showed that decrease in LV torsion is driven by peak systolic apical rotation. These results support the findings of the previous studies, which concluded that the magnitude of systolic apical counterclockwise rotation was the primary determinant of peak systolic LV torsion.[Citation23] The current literature supports that recoiling of cardiac muscle fibers has an important role in cardiac filling and this recoiling is mainly driven by the potential energy stored during counter directional rotation process of cardiac muscle fibers in systole.[Citation24] It should be noted that another prominent finding of our study was deterioration of diastolic function as well as LV torsion. Concurrent decrease in LV torsion and diastolic parameters (both early diastolic trans-mitral Doppler and early diastolic trans-mitral tissue Doppler velocities) may indicate the specific role of LV torsion in interdependence between systole and diastole.
The way to reduce preload was drug administration in the previous studies. Unlike them, in our study the volume depletion was provided actually by blood donation. The observed decrease in LV end-diastolic diameter was consistent with the notion that blood donation was an effective method of reducing LV preload. This might enable to observe the pure effects of real and acute intravascular volume depletion on LV torsion properly without manipulation of systemic vascular tonus. On the other hand, the pre-donation blood pressure (BP) measurements were recorded and hypotensive or hypertensive patients excluded from study, but post-donation recordings were not taken unless patients develop hypotensive symptoms or tachycardia. Since none of the patients had such symptoms and the heart rate measurements did not differ significantly before and after the procedure, the post-donation BP measurements were not recorded. Although the primary goal of the study was to evaluate the effect of preload reduction on LV torsion, this is a limitation of the study in terms of documenting the hemodynamic stability and afterload situation of the patients. In addition, relationship between diastolic parameters and LV torsion should be documented via specific measurements of untwisting rate, which was not applied. This topic could be a subject for another study. Evaluation of diastolic function and the relevancy between LV torsion and diastolic parameters were also significant. On the other hand, only healthy male subjects were included in this study. This can be a handicap to generalize our findings to specific cardiac disorders as well as female subjects. We used only diameters for calculation of LV volumes and EF, use of two perpendicular image planes for this purpose would be more illuminative. Lack of a confirmative invasive technique to measure the LV end-diastolic pressure can also be considered as a limitation. LV apical rotation and peak systolic LV torsion seem to be preload dependent. Preload reduction provided by 450 mL blood donation decreased LV torsion in healthy male volunteers. Volume dynamics should be taken into account in the evaluation of LV torsion.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Sengupta PP, Tajik AJ, Chandrasekaran K, et al. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008;1:366–376.
- Notomi Y, Popovic ZB, Yamada H, et al. Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol. 2008;294:505–513.
- Bloechlinger S, Grander W, Bryner J, et al. Left ventricular rotation: a neglected aspect of the cardiac cycle. Intensive Care Med. 2011;37:156–163.
- Takeuchi M, Otsuji Y, Lang RM. Evaluation of left ventricular function using left ventricular twist and torsion parameters. Curr Cardiol Rep. 2009;11:225–230.
- Buckberg G, Hoffman JI, Nanda NC, et al. Ventricular torsion and untwisting: further insights into mechanics and timing interdependence: a viewpoint. Echocardiography. 2011;28:782–804.
- Tadic M, Ilic S, Cuspidi C, et al. Subclinical hyperthyroidism impacts left ventricular deformation: 2D and 3D echocardiographic study. Scand Cardiovasc J. 2015;49:74–81.
- Bertini M, Delgado V, Nucifora G, et al. Left ventricular rotational mechanics in patients with coronary artery disease: differences in subendocardial and subepicardial layers. Heart. 2010;96:1737–1743.
- Bansal M, Leano RL, Marwick TH. Clinical assessment of left ventricular systolic torsion: effects of myocardial infarction and ischemia. J Am Soc Echocardiogr. 2008;21:887–894.
- Asrar ul Haq M, Mutha V, Lin T, et al. Left ventricular torsional dynamics post exercise for LV diastolic function assessment. Cardiovasc Ultrasound. 2014;12:8.
- Prinz C, Faber L, Horstkotte D, et al. Evaluation of left ventricular torsion in children with hypertrophic cardiomyopathy. Cardiol Young. 2014;24:245–252.
- Kanzaki H, Nakatani S, Yamada N, et al. Impaired systolic torsion in dilated cardiomyopathy: reversal of apical rotation at mid-systole characterized with magnetic resonance tagging method. Basic Res Cardiol. 2006;101:465–470.
- Barutçu A, Bekler A, Temiz A, et al. Left ventricular twist mechanics are impaired in patients with coronary slow flow. Echocardiography. 2015;32:1647–1654.
- Nagel E, Stuber M, Burkhard B, et al. Cardiac rotation and relaxation in patients with aortic valve stenosis. Eur Heart J. 2000;21:582–589.
- Chang SA. Ventricular torsion: research tool or novel clinical indicator? J Cardiovasc Ultrasound. 2013;21:163–164.
- Weiner RB, Weyman AE, Khan AM, et al. Preload dependency of left ventricular torsion the impact of normal saline infusion. Circ Cardiovasc Imaging. 2010;3;672–678.
- Notomi Y, Lysyansky P, Setser RM, et al. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol. 2005;45:2034–2041.
- Gibbons Kroeker CA, Tyberg JV, Beyar R. Effects of load manipulations, heart rate, and contractility on left ventricular apical rotation: an experimental study in anesthetized dogs. Circulation. 1995;92:130–141.
- Dong SJ, Hees PS, Huang WM, et al. Independent effects of preload, afterload, and contractility on left ventricular torsion. Am J Physiol. 1999;277:1053–1060.
- Moon MR, Ingels NB Jr, Daughters II GT, et al. Alterations in left ventricular twist mechanics with inotropic stimulation and volume loading in human subjects. Circulation. 1994;89:142–150.
- Hansen DE, Daughters II GT, Alderman EL, et al. Effect of volume loading, pressure loading, and inotropic stimulation on left ventricular torsion in humans. Circulation. 1991;83:1315–1326.
- Burns AT, La Gerche A, Prior DL, et al. Left ventricular torsion parameters are affected by acute changes in load. Echocardiography. 2010;27:407–414.
- Laser KT, Haas NA, Fischer M, et al. Left ventricular rotation and right-left ventricular interaction in congenital heart disease: the acute effects of interventional closure of patent arterial ducts and atrial septal defects. Cardiol Young. 2014;24:661–674.
- Gibbons Kroeker CA, TerKeurs HE, et al. An optical device to measure the dynamics of apex rotation of the left ventricle. Am J Physiol. 1993;265:1444–1449.
- Fukuda N, Granzier H. Role of the giant elastic protein titin in the Frank-Starling mechanism of the heart. Curr Vasc Pharmacol. 2004;2:135–139.