Abstract
Background Experimental evidence suggests that anesthetic preconditioning and postconditioning could effectively attenuate myocardial ischemia/reperfusion (I/R) injury. In this study, we aimed at investigating whether there are age-associated differences in response to sevoflurane postconditioning during myocardial I/R injury in young and old rats, and explore the underlying molecular mechanisms. Methods Young and old rats were subjected to 30 min myocardial ischemia, followed by 2 h of reperfusion, with or without sevoflurane postconditioning. Results Both 1 and 2 minimal aveolar concentration (MAC) sevoflurane postconditioning reduced infarct size (IS) (34 ± 3% and 32 ± 2% vs. 58 ± 5%, p < 0.05) and apoptotic index (8 ± 1% and 7 ± 1% vs. 15 ± 2%, p < 0.05) in young rats, compared to young control group. In contrast, they could not reduce IS (45 ± 3% and 43 ± 3% vs. 47 ± 3%, p > 0.05) and apoptotic index (28 ± 3% and 25 ± 2%, vs. 26 ± 2%, p > 0.05) in old rats, compared to old control group. Mechanistically, we found that the phosphorylation of both Akt and ERK1/2 but not STAT3 was substantially enhanced after sevoflurane postconditioning in young rats, compared to young control group, but not in old rats, relative to old control group. Conclusion There are age-related differences after exposure to sevoflurane postconditioning that protects young, but not old rat hearts against I/R injury, which may be at least associated with the inability to activate Akt and ERK1/2.
Introduction
Ischemic preconditioning and postconditioning is one of the most effective strategies for providing cardioprotection against myocardial ischemia/reperfusion (I/R) injury.[Citation1,Citation2] In particular, experimental evidence suggests that anesthetic preconditioning and postconditioning could effectively attenuate myocardial I/R injury.[Citation3–6] However, the majority of these experimental data have been obtained in young animals and only a few studies have examined the effects of age on myocardial I/R injury and on the therapeutic effects of preconditioning and postconditioning.[Citation7,Citation8] Furthermore, some small-scale clinical trials on ischemic preconditioning and postconditioning on patients over 65 years old showed that the extent of cardioprotection in these trials was much less than that observed in experimental models.[Citation9,Citation10] Therefore, we speculated that cardiac I/R insult may cause more severe damage to the heart in aged cohorts than in young counterparts, and stronger pharmacological preconditioning and postconditioning regimens are needed to elicit cardioprotection in aged cohorts.
In this study, we aimed at investigating whether there are age-associated differences in response to sevoflurane postconditioning during myocardial I/R injury in young and old rats, and explore the underlying molecular mechanisms.
Methods and materials
Animal models
This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals and all experimental procedures and protocols were approved by the Care of Experimental Animals Committee of Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Our institution is one of the foreign institutions with a PHS approved animal welfare assurance, whose assurance number was A5519-01.
Total 130 male Sprague–Dawley rats, including 64 young rats (3–4 months; 330 ± 8 g) and 66 old rats (20–24 months; 520 ± 11 g), were maintained on the same laboratory animal diet with free water access. Anesthesia was induced with an intraperitoneal injection of sodium pentobarbital (30–40 mg/kg), with additional maintenance doses given when necessary. Animals were tested for the absence of pedal reflexes throughout the experimental protocols to ensure adequate anesthesia. Mechanical ventilation was achieved using tidal volume of 2–3 ml for young rats or 5–6 ml for old rats (model Inspira 557058, Harvard Apparatus, Holliston, MA), with 50% of final oxygen concentration, at a rate of 60–80 breaths/min to keep blood gas partial pressure within a normal range. Body temperature was maintained at 37 °C using a heating lamp. Sevoflurane was administered with calibrated sevoflurane vaporizer. End-tidal concentrations of sevoflurane, O2 and CO2 were measured using a monitor (Datex Ultima, Helsinki, Finland). One minimal alveolar concentration (MAC) of sevoflurane was 1.8% and 1.2% in young and old rats, respectively. A left thoracotomy was performed between the fourth and fifth intercostal space after local infiltrating anesthesia with 0.5% lidocaine, and the pericardium was opened. A 7-0 Prolene suture was placed around the left anterior descending (LAD) coronary artery in the area just below the left atrial appendage. Regional myocardial ischemia for 30 min by LAD ligation with slip knot was confirmed under direct vision with the demonstration of epicardial cyanosis and changes in electrocardiographic tracing. Reflow was verified by observing an epicardial hyperemic response.
Experimental protocols
Our experimental design is demonstrated in . Young or old rats were randomly assigned to the following four groups: (1) control group (rats were subjected to 30 min of LAD occlusion, followed by 2 h of reperfusion without any intervention); (2) 1 MAC sevoflurane postconditioning group (1 MAC sevoflurane was administered at 1 min before and for the first 5 min after reflow); (3) 2 MAC sevoflurane postconditioning group (2 MAC sevoflurane was administered at 1 min before and for the first 5 min after reflow); (4) sham control group (animals underwent thoracotomy without LAD occlusion). Protocol A was designed for the measurements of infarct size (IS) and apoptosis. Protocol B was designed for the detection of protein phosphorylation.
Figure 1. Experimental protocol A for the measurements of infarct size and apoptosis. Protocol B for the detection of protein phosphorylation. I/R: ischemia/reperfusion; SC: sham control; Y: young; MAC: minimum alveolar concentration; 1 MAC: 1 MAC sevoflurane postconditioning; 2 MAC: 2 MAC sevoflurane postconditioning; O: old.
Measurement of myocardial IS
Myocardial IS was measured as previously described.[Citation11] Briefly, the LAD coronary artery was re-ligated after 2 h of reperfusion, and 2% Evans blue was injected intravenously to demarcate the left ventricle (LV) area at risk (AAR). The heart was rapidly excised and the LV was isolated and stored at −20 °C for 2 h, then cut into transverse slices about 3 mm after a freeze/thaw cycle. Then the LV slices were incubated at 37 °C for 15 min in 1% 2,3,5-triphenyltetrazolium chloride (TTC) in 0.1 M phosphate buffer adjusted to a pH of 7.4. After fixed in 10% formalin overnight, the viable myocardium within AAR was stained deep red, and the infarcted was not stained and became pale. LV slices were observed under IXUS 800 (Canon, Tokyo, Japan), IS and AAR were determined by planimetry (Image J 1.37 software, Bethesda, MD). AAR/LV was about 40% in all groups, except for sham control groups. Infarction area was expressed as IS/AAR (n = 6/group).
TUNEL assay
The apoptosis of cardiomyocyte was assessed by Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) assay. The apoptotic cells were identified in formalin-fixed paraffin-embedded heart tissue sections from AAR using an in situ cell death detection kit (Roche, Basel, Germany) according to the manufacturer’s protocol. Apoptosis quantification was performed by counting TUNEL-positive cells per high-power field (magnification, ×400). A total of 10 fields per heart were analyzed (n = 6/group).
Western blot analysis
After 15 min reperfusion, the heart samples harvested from AAR (n = 4/group) were collected and immediately frozen in liquid nitrogen. Frozen heart tissue samples were homogenized using a Heidolph Homogenizer (SilentCrusher M, Schwabach, Germany) in ice-cold lysis buffer. The samples were then centrifuged at 12 000g for 10 min at 4 °C. Protein concentrations of the supernatants were determined using BCA method. Equal amounts of proteins were separated on a 10% polyacrylamide gel and transferred (Trans-Blot SD Semi-Dry Transfer Cell; Bio-Rad, Hercules, CA) to nitrocellulose membrane. The membranes were incubated with primary antibodies to p-STAT3 (Ser727; dilution 1:500; Cell Signaling Technology, Danvers, MA), p-Akt (Ser473; dilution 1:2000; Cell Signaling Technology, Danvers, MA), and p-ERK1/2 (Thr202/Tyr 204; dilution 1:1000; Cell Signaling Technology, Danvers, MA). Specific protein bands on the blots were visualized with SuperSignal West Femto Maximum Sensitivity Substrate (34095, Thermo, Waltham, MA). To determine total STAT3, Akt and ERK1/2, the membranes were stripped and reprobed with STAT3 (1:500; Cell Signaling Technology Danvers, MA), Akt (1:2000; Cell Signaling Technology Danvers, MA), and ERK1/2 antibody (1:1000; Cell Signaling Technology Danvers, MA), respectively. The stripping buffer was BestBio (Shanghai, China). Quantitative analysis of the band densities was performed using GelDoc imaging system and Quantity One software (Bio-Rad, Hercules, CA).
Statistical analysis
Data were expressed as mean ± SD and analyzed using PASW statistics 18.0 (SPSS Inc., IBM, Armonk, NY). Two-way analysis of variance (ANOVA) with post hoc Student–Newman–Keuls test was performed for comparison. p < 0.05 was considered significant.
Results
Evaluation of the animal models
A total of 64 young rats were used in this study, among which six died of ventricular fibrillation, and two were excluded due to the failure of TTC staining, thus 56 young rats were included in the final analysis. Total 66 old rats were used, among which eight died of ventricular fibrillation, and two were excluded due to the failure of TTC and TUNEL staining, thus 56 old rats were included in the final analysis. AAR/LV was similar within young and old groups (). There were young/old sham control (YSC, OSC), young/old I/R (YC, OC), young/old 1 MAC sevoflurane postconditioning (Y 1 MAC, O 1 MAC), young/old 2 MAC sevoflurane postconditioning (Y 2 MAC, O 2 MAC) groups.
Table 1. AAR/LV for all groups.
Systemic hemodynamics
No differences in the baseline hemodynamics were observed among all groups (). In protocol A, the mean blood pressure and heart rate significantly (p < 0.05) decreased in the YC, Y 1 MAC, Y 2 MAC, OC, O 1 MAC and O 2 MAC groups during the first and second hour of reflow when compared with their respective baseline period.
Table 2. Hemodynamics.
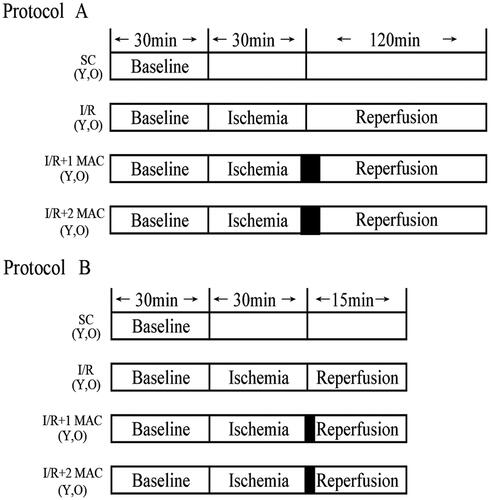
Sevoflurane postconditioning relieves myocardial infarct in young but not old rats. Either 1 or 2 MAC sevoflurane postconditioning significantly attenuated IS in young rats, compared with YC group (34 ± 3% and 32 ± 2% vs. 58 ± 5%, p < 0.05, ), but there was no difference in IS between the two sevoflurane postconditioning groups. However, neither 1 nor 2 MAC sevoflurane postconditioning could elicit the infarct-relieving effect in old rats, compared with OC group (45 ± 3% and 43 ± 3% vs. 47 ± 3%, p > 0.05, ). In fact, myocardial I/R caused larger IS in the hearts of young rats than in those of old rats (58 ± 5% vs. 47 ± 3%, p < 0.05, ). These data suggest that sevoflurane postconditioning relieves myocardial infarct in young but not old rats.
Figure 2. Histogram of myocardial infarct size expressed as the percentage of the area at risk. Data were shown as mean ± SD (n = 6/group). AAR: area at risk; YC: young control; Y 1 MAC: young 1 MAC sevoflurane postconditioning; Y 2 MAC: young 2 MAC sevoflurane postconditioning; OC: old control; O 1 MAC: old 1 MAC sevoflurane postconditioning; O 2 MAC: old 2 MAC sevoflurane postconditioning; MAC: minimum alveolar concentration. *p < 0.05 vs. YC. †p < 0.05 vs. OC, O 1 MAC or O 2 MAC.
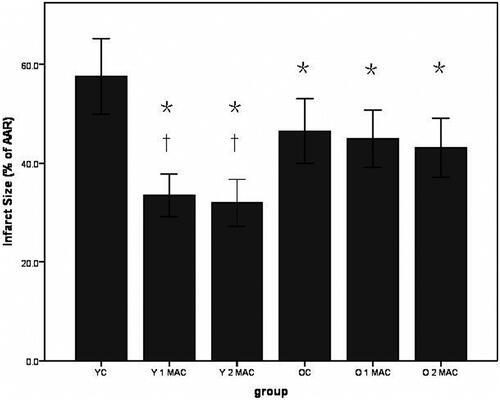
Sevoflurane postconditioning reduces cardiomyocyte apoptosis in young but not old rats. The apoptosis of cardiomyocytes in different experimental groups was evaluated by TUNEL staining (). Apoptotic index (%) was calculated as the number of apoptotic cardiomyocytes/the number of total cardiomyocytes. Apoptotic index was significantly decreased in the hearts of young rats postconditioned with 1 or 2 MAC sevoflurane, compared to YC group (8 ± 1% and 7 ± 1% vs. 15 ± 2%, p < 0.05, ). In contrast, there was no difference in apoptotic index after exposure to 1 or 2 MAC sevoflurane, compared to OC group in old rats (28 ± 3% and 25 ± 2%, vs. 26 ± 2%, p > 0.05, ). In addition, more apoptotic cardiomyocytes induced by I/R were observed in old rats than in young rats (26 ± 2% vs. 15 ± 2%, p < 0.05, ). Taken together, these results suggest that sevoflurane postconditioning reduces cardiomyocyte apoptosis in young but not old rats.
Figure 3. Representative images of TUNEL-stained apoptotic nuclei in brown and non-apoptotic nuclei in blue in different groups. In each group, six hearts were used. YC: young control; Y 1 MAC: young 1 MAC sevoflurane postconditioning; Y 2 MAC: young 2 MAC sevoflurane postconditioning; OC: old control; O 1 MAC: old 1 MAC sevoflurane postconditioning; O 2 MAC: old 2 MAC sevoflurane postconditioning; MAC: minimum alveolar concentration.
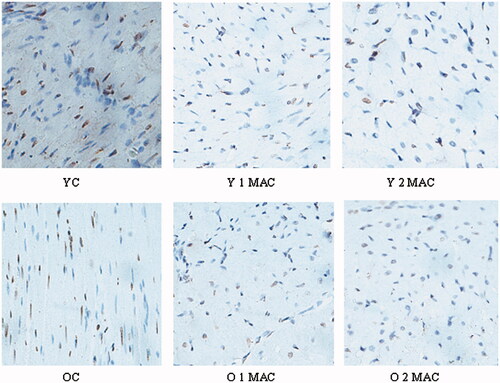
Figure 4. Quantification of apoptosis of cardiomyocyte in different groups. Apoptosis index was calculated by counting TUNEL-positive cells (magnification, ×400, 10 fields per heart) in each group, and shown as mean ± SD. YC: young control; Y 1 MAC: young 1 MAC sevoflurane postconditioning; Y 2 MAC: young 2 MAC sevoflurane postconditioning; OC: old control; O 1 MAC: old 1 MAC sevoflurane postconditioning; O 2 MAC: old 2 MAC sevoflurane postconditioning; MAC: minimum alveolar concentration. †p < 0.05 vs. YC.
Sevoflurane postconditioning differentially modulates the activation of Akt and ERK1/2 in the hearts of young and old rats. To explore the underlying molecular mechanisms by which sevoflurane postconditioning provides cardioprotection in young but not old rats, we compared the activation of Akt, ERK1/2 and STAT3 in the hearts of young and old rats. Western blot analysis showed that either 1 or 2 MAC sevoflurane postconditioning significantly enhanced the phosphorylation levels of both Akt (0.72 ± 0.03% and 0.72 ± 0.03% vs. 0.29 ± 0.03%, p < 0.05, ) and ERK1/2 (0.86 ± 0.03% and 0.88 ± 0.03% vs. 0.27 ± 0.02%, p < 0.05, ), compared with YC group. In contrast, there was no difference in the phosphorylation levels of both Akt (0.48 ± 0.03% and 0.46 ± 0.03% vs. 0.43 ± 0.03%, p > 0.05, ) and ERK1/2 (0.48 ± 0.03% and 0.46 ± 0.03% vs. 0.44 ± 0.03%, p > 0.05, ) in response to either 1 or 2 MAC sevoflurane postconditioning, compared with OC group in old rats. In addition, the phosphorylation levels of both Akt (0.46 ± 0.03% vs. 0.23 ± 0.02%, p < 0.05, ) and ERK1/2 (0.45 ± 0.03% vs. 0.26 ± 0.03%, p < 0.05, ) were significantly elevated in OSC group, compared to YSC group. Furthermore, we examined the activation of STAT3 and found that the phosphorylation level of STAT3 in the hearts was unaltered after exposure to 1 or 2 MAC sevoflurane postconditioning, compared to YC group in young rats (0.42 ± 0.03% and 0.43 ± 0.03% vs. 0.41 ± 0.03%, p > 0.05, ). Similarly, neither 1 nor 2 MAC sevoflurane postconditioning had significant effects on the phosphorylation level of STAT3 compared with OC group in old rats (0.41 ± 0.02% and 0.44 ± 0.02% vs. 0.43 ± 0.04%, p > 0.05, ). Collectively, these results suggest that sevoflurane postconditioning differentially modulates the activation of Akt and ERK1/2 in the hearts of young and old rats.
Figure 5. Phosphorylation of Akt in different groups. Data were shown as mean ± SD (n = 4/group). GAPDH was used as loading control. a.u.: arbitrary units; YSC: young sham control; YC: young control; Y: young; MAC: minimal alveolar concentration; 1 or 2 MAC: 1 or 2 MAC sevoflurane postconditioning; OSC: old sham control; OC: old control; O: old. *p < 0.05 vs. YC. †p < 0.05 vs. Y 1 MAC or Y 2 MAC. ‡p < 0.05 vs. YSC.
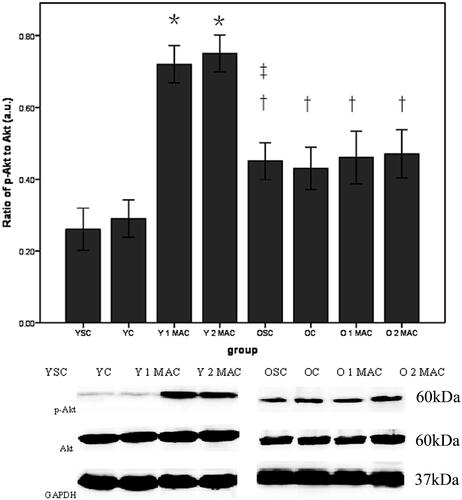
Figure 6. Phosphorylation of ERK1/2 in different groups. Data are given as mean ± SD (n = 4/group). GAPDH was used as loading control. a.u.: arbitrary units; YSC: young sham control; YC: young control; Y: young; MAC: minimal alveolar concentration; 1 or 2 MAC: 1 or 2 MAC sevoflurane postconditioning; OSC: old sham control; OC: old control; O: old. *p < 0.05 vs. YC. †p < 0.05 vs. Y 1 MAC or Y 2 MAC. ‡p < 0.05 vs. YSC.
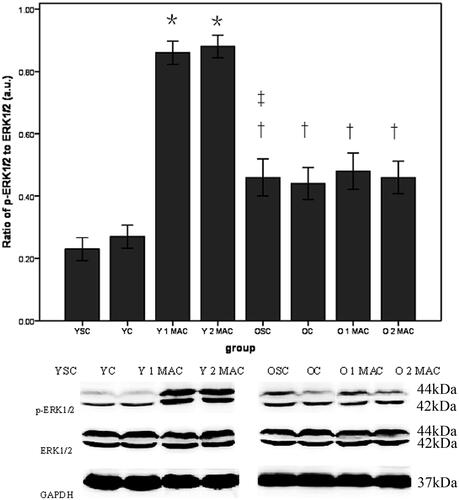
Figure 7. Phosphorylation of STAT3 in different groups. Data are given as mean ± SD (= 4/group). GAPDH was used as loading control. a.u.: arbitrary units; YSC: young sham control; YC: young control; Y: young; MAC: minimal alveolar concentration; 1 or 2 MAC: 1 or 2 MAC sevoflurane postconditioning; OSC: old sham control; OC: old control; O: old.
Discussion
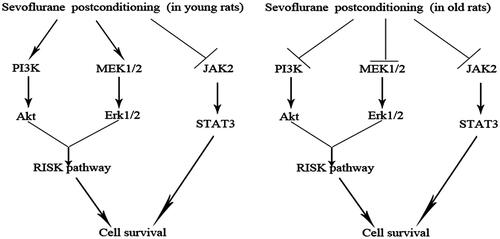
In this study, we reported several interesting findings (summarized in ). First, myocardial I/R resulted smaller IS and more apoptotic cardiomyocytes in old rats, compared with young rats. Second, postconditioning with 1 or 2 MAC sevoflurane is equally effective to attenuate IS and apoptosis in young but not old rats. Third, the phosphorylation levels of both Akt and ERK1/2 are dramatically augmented in response to either 1 or 2 MAC sevoflurane postconditioning in young but not old rats. Fourth, phosphorylated Akt and ERK1/2 are constitutively higher in the hearts of old rats than in those of young rats. Last, neither 1 nor 2 MAC sevoflurane postconditioning has impact on the phosphorylation of STAT3 in the hearts of both young and old rats.
Figure 8. Diagram illustrating cardioprotective pathways of sevoflurane postconditioning in young and old rats in our model.
It is known that the activation of pro-survival signaling pathways such as Akt and ERK1/2 pathways plays a critical role in various preconditioning and/or postconditioning.[Citation12–14] In this study, we demonstrate that either 1 or 2 MAC sevoflurane postconditioning substantially reduced myocardial IS, in conjunction with significant augmentation of the phosphorylation of both Akt and ERK1/2 in young rats. However, we observed no dose-dependent effect of sevoflurane on IS because 2 MAC sevoflurane failed to induce smaller myocardial IS, compared with 1 MAC anesthetic, which is in agreement with our previous study.[Citation15]
However, either 1 or 2 MAC sevoflurane postconditioning failed to protect old rats against myocardial I/R injury. Compared to young rat hearts, both phosphorylated Akt and ERK1/2 were constitutively elevated to some extent, and neither 1 nor 2 MAC sevoflurane postconditioning could further increase the phosphorylation of Akt and ERK1/2 in old rat hearts. Because adequate activation of both Akt and ERK1/2 seems to be necessary for the infarct-limiting effect in young rats, failure to mediate cardioprotection in old rats may be the result of inability to activate both Akt and ERK1/2. The 20–24 months old rats used in this study are commonly considered aged cohorts because these ages correspond to approximately 65–75 years in human.[Citation16] Our results in old rats are in agreement with previous observations that the efficacy of ischemic preconditioning and postconditioning was attenuated with advanced age.[Citation17,Citation18]
It is well documented that JAK2-STAT3 signaling pathway plays an essential role in ischemic preconditioning and postconditioning.[Citation19–21] However, in this study we found that neither 1 nor 2 MAC sevoflurane postconditioning could enhance the phosphorylation of STAT3 in young rats. In addition, either 1 or 2 MAC sevoflurane postconditioning has no significant effects on the phosphorylation of STAT3 in old rats. These results suggest that STAT3 may not be implicated in the cardioprotection induced by sevoflurane postconditioning. However, it is unclear whether JAK2-STAT3 pathway is activated in other models or activated by other anesthetic postconditioning algorithm, such as isoflurane postconditioning, or recruited by other anesthetic preconditioning, which needs further investigation.[Citation22]
Interestingly, our results demonstrate that the magnitude of myocardial I/R injury was different between young and old rats. Although AAR heart tissue was about 40% in both young and old rats, myocardial I/R led to larger IS in young rats compared to old rats, which is somewhat against the intuition because it is well recognized that old patient hearts are more vulnerable and less resistant to I/R insult in clinic situation. One possible explanation is that phosphorylated Akt and ERK1/2 have been constitutively enhanced in old rat hearts, which implies that these two kinases are partially activated, thereby providing some beneficial effects.
Necrosis and apoptosis are two main types of cell death following I/R injury.[Citation23] In this study, we demonstrate that myocardial I/R caused more cardiac myocyte apoptosis in old rats than in young rats. One explanation is that 2 h of reperfusion may not be long enough to activate caspases such as caspase-3 and -9 in young rats because it is reported that complete activation of caspases could require 3 h of reperfusion in young mice.[Citation24]
Several limitations of this study should be mentioned. First, we did not provide evidence that the infarct-relieving effect in response to sevoflurane postconditioning in young rats was abolished by Akt or ERK1/2 inhibitor, because the specificity of these inhibitors is questioned.[Citation13] Second, we did not elucidate the mechanisms responsible for the activation of cardiomyocyte apoptosis in old rats, such as the activation of caspases. Further studies are needed to address these limitations.
Conclusion
Our results provide evidence that sevoflurane postconditioning reduces IS following myocardial I/R injury in young rats, which may involve the activation of Akt and ERK1/2 but not STAT3. However, sevoflurane postconditioning fails to protect old rat hearts against I/R injury, which may be at least associated with the inability to activate Akt and ERK1/2. In addition, age has an important impact on the magnitude of myocardial I/R injury, which may have significant clinical implications.
Given the relatively low similarity between rat myocardium and clinical cardiac patients, it is necessary to further investigate the impact of age on anesthetic postconditioning.
Acknowledgements
The study was performed at experimental animal center and core laboratory of Fuwai Cardiovascular Hospital.
Disclosure statement
The work is funded by National Natural Science Foundation of China (No. 81070098).
References
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135.
- Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588.
- Raphael J, Zuo Z, Abedat S, et al. Isoflurane preconditioning decreases myocardial infarction in rabbits via up-regulation of hypoxia inducible factor 1 that is mediated by mammalian target of rapamycin. Anesthesiology. 2008;108:415–425.
- Ge ZD, Pravdic D, Bienengraeber M, et al. Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism. Anesthesiology. 2010;112:73–85.
- Fang NX, Yao YT, Shi CX, Li LH. Attenuation of ischemia-reperfusion injury by sevoflurane postconditioning involves protein kinase B and glycogen synthase kinase 3 beta activation in isolated rat hearts. Mol Biol Rep. 2010;37:3763–3769.
- Yao YT, Li LH, Chen L, et al. Sevoflurane postconditioning protects isolated rat hearts against ischemia-reperfusion injury: the role of radical oxygen species, extracellular signal-related kinases 1/2 and mitochondrial permeability transition pore. Mol Biol Rep. 2010;37:2439–2446.
- Deyhimy DI, Fleming NW, Brodkin IG, Liu H. Anesthetic preconditioning combined with postconditioning offers no additional benefit over preconditioning or postconditioning alone. Anesth Analg. 2007;105:316–324.
- Zhong C, Fleming NW, Lu X, et al. Age-associated differences in gene expression in response to delayed anesthetic preconditioning. Age. 2012;34:1459–1472.
- Peart JN, Headrick JP. Clinical cardioprotection and the value of conditioning responses. Am J Physiol Heart Circ Physiol. 2009;296:H1705–H1720.
- Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261.
- Zhu J, Rebecchi MJ, Tan M, et al. Age-associated differences in activation of Akt/GSK-3 singaling pathways and inhibition of mitochondrial permeability transition pore opening in the rat heart. J Gerontol A Biol Sci Med Sci. 2010;65:611–619.
- Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976.
- Yao Y, Li L, Li L, et al. Sevoflurane postconditioning protects chronically-infarcted rat hearts against ischemia-reperfusion injury by activation of pro-survival kinases and inhibition of mitochondrial permeability transition pore opening upon reperfusion. Biol Pharm Bull. 2009;32:1854–1861.
- Lu HS, Chen HP, Wang S, et al. Hypoxic preconditioning up-regulates DJ-1 protein expression in rat heart-derived H9c2 cells through the activation of extracellular-regulated kinase 1/2 pathway. Mol Cell Biochem. 2012;370:231–240.
- Chen D, Cheng B, Zhou HY, Li LH. The effect of sevoflurane postconditioning on cardioprotection against ischemia-reperfusion injury in rabbits. Mol Biol Rep. 2012;39:6049–6057.
- Nguyen LT, Rebecchi MJ, Moore LC, et al. Attenuation of isoflurane-induced preconditioning and reactive oxygen species production in the senescent rat heart. Anesth Analg. 2008;107:776–782.
- Boengler K, Konietzka I, Buechert A, et al. Loss of ischemic preconditioning’s cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochonfrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2007;292:H1764–H1769.
- Przyklenk K, Maynard M, Darling CE, Whittaker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol. 2008;51:1393–1398.
- Boengler K, Hilfiker-Kleiner D, Drexler H, et al. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Therapeut. 2008;120:172–185.
- Boengler K, Buechert A, Heinen Y, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–135.
- Zhuo C, Wang Y, Wang X, et al. Cardioprotection by ischemic postconditioning is abolished in depressed rats: role of Akt and signal transducer and activator of transcription-3. Mol Cell Biochem. 2011;346:39–47.
- Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury:does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40.
- Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609.
- Souktani R, Pons S, Guegan C, et al. Cardioprotection against myocardial infarction with PTD-BIR3/RING, a XIAP mimicking protein. J Mol Cell Cardiol. 2009;46:713–718.
