Abstract
Objective The aim of this study was to investigate the role of thiol disulfide homeostasis in the presence of slow coronary flow. Material and methods In this cross-sectional study, a total of 110 patients who admitted to our hospital between March 2014 and December 2015 were included in the study. There were 65 patients in the slow coronary flow, and 45 patients in the normal flow groups. Results We found significant differences between slow coronary flow and the normal flow groups for thiol disulfide homeostasis, and the results of our study indicated that hsCRP, and thiol disulfide ratio were independently associated with slow coronary flow. Conclusion Our study showed that thiol disulfide homeostasis was significantly and independently related to the presence of slow coronary flow.
Introduction
Slow coronary flow (SCF) is an angiographic finding, and it is characterized by delayed opacification of the coronary epicardial arteries in the absence of any coronary artery stenosis.[Citation1] Even though the exact etiopathogenesis of SCF remains unclear, several mechanisms have been proposed including inflammation, oxidative stress, small vessel disease, microvascular dysfunction, diffuse atherosclerosis and endothelial dysfunction.[Citation2–5] In fact, oxidative stress, inflammation, and endothelial dysfunction are well recognized in the pathogenesis of SCF.[Citation6,Citation7]
The plasma thiol pool is largely formed by albumin and protein thiols, with a considerably smaller amount from the low-molecular weight thiols as cysteinylglycine, homocysteine, cysteine denoted (Cys), γ-glutamylcysteine and glutathione.[Citation8] Thiols can undergo oxidation reaction with oxidants, and form disulfide bonds.[Citation9] Oxidation of Cys residues can lead to reversible formation of mixed disulfides between low-molecular-mass thiols and protein thiol groups when oxidative stress increases. Formed disulfide bonds can again be reduced to thiol groups, therefore thiol disulfide homeostasis is maintained.[Citation10] Previous studies demonstrated that thiol disulfide homeostasis plays critical roles in antioxidant protection, signal transduction, detoxification, apoptosis and cellular signaling mechanisms.[Citation11,Citation12] The role of thiol disulfide homeostasis was also shown in cardiovascular diseases.[Citation13–15]
We aimed to investigate the relationship between thiol disulfide homeostasis and SCF. To the best of our knowledge, no studies up to date investigated thiol disulfide homeostasis as a novel oxidative stress marker in patients with SCF, and compared the results with the normal flow group.
Materials and methods
Study population
The laboratory, clinical and angiographic data of consecutive patients who had underwent coronary angiography for stable angina pectoris (identified by the European Society of Cardiology guidelines),[Citation16] and had normal coronary arteries without any atherosclerotic lesions on angiography between March 2014 and December 2015 were analyzed. A total of 110 patients were included in this cross-sectional study. There were 65 patients (42 males, 64.6%) in SCF, and 45 patients (28 males, 62.2%) in normal flow groups. The mean ages were 58 ± 17, and 55 ± 15 years in SCF and normal flow groups, respectively (p = 0.270). The demographic characteristics of the patients are presented in .
Table 1. Clinical, laboratory and angiographic characteristics of the study population.
Patients with acute coronary syndromes [ST segmentation elevation myocardial infarction (STEMI) and non-STEMI] were excluded. We also excluded patients with left ventricular systolic dysfunction [left ventricular ejection fraction (LVEF) < 40%], kidney and liver problems, malignancy, or any other acute or chronic inflammatory disease, current therapy with non-steroidal anti-inflammatory and corticosteroids drugs, and hematologic diseases including anemia, as well as the ones that had coronary artery bypass grafting before and underwent percutaneous coronary intervention.
Arterial hypertension was considered in patients with repeated blood pressure measurements >140/90 mmHg, or current use of antihypertensive drugs. Diabetes mellitus was defined as fasting plasma glucose levels > 7 mmol/L on multiple measurements, or current use of antidiabetic medications.
Our hospital’s Local Ethics Committee approved the study protocol, and all participants provided their written informed consents.
Coronary angiography
Baseline angiography was performed through radial or femoral arteries, using standard Judkin’s technique and 6F catheters, and Siemens Axiom Sensis XP device. All the angiograms were recorded 30 frames per second. We used Iopamiro (Iopamidol-300) as the contrast agent in all participants. The flow rates of the patients were determined with TIMI frame count technique, as illustrated by Gibson et al. [Citation17] Two experienced cardiologists blinded to the clinical information of the patients evaluated each coronary vessel using TIMI frame count. Intra- and inter-observer coefficients were 3.0 and 5.0%, respectively. Here, the first frame was noted to be at >70% mark human opacification, with proper antegrade filling. Then, the final frames were analyzed when the contrast material reached to a certain distal landmark in every vessel. The distal bifurcation was used (whale’s tail) for left anterior descending artery (LAD). The most distal bifurcation of the obtuse marginal branch furthest from the coronary ostium was used as the distal landmark for the left circumflex artery (LCx). The first branch of the posterolateral segment was used for the right coronary artery (RCA). Normal visualization of the arteries for the standard mean values are described as 36.2 + 2.6 frames for LAD, 22.2 + 4.1 frames for LCx and 20.4 + 3 frames for RCA. Since LAD is usually longer than the other major coronary arteries, the TIMI frame count for this vessel is often higher. Therefore, TIMI frame count was divided by 1.7 to obtain the corrected TIMI frame count for LAD. The standard corrected mean TIMI frame count for LAD is 21.1 + 1.5 frames. The participants who had TIMI frame counts greater than two standard deviations of previously published range were considered to have SCF for that particular vessel.[Citation17]
Data collection
The blood samples of the study population were obtained during on their admission to the inpatient ward. Automated Beckman Coulter analyzer (Beckman Coulter, Brea, CA) was used to measure complete blood count parameters. Total cholesterol, low- and high-density lipoprotein cholesterol (LDL-C, HDL-C), creatinine and the total bilirubin were measured as blood biochemistry parameters. High sensitivity C-reactive protein (hsCRP) was measured with an automatized analyzer (Beckman Coulter), using nephelometric method, after or during coronary angiography. Blood samples for thiol disulfide homeostasis were collected into plain tubes after or during the coronary angiography, and serum was separated after centrifugation at 1500 g for 10 min. The sera were stored at −80 °C until analysis. Determination of thiol disulfide homeostasis was described previously.[Citation18] Briefly, reducible disulfide bonds were first reduced to form free functional thiol groups. Unused reductant sodium borohydride was consumed and removed with formaldehyde, and all thiol groups including reduced and native ones were detected after reaction with DTNB [5,5’-dithiobis-(2-nitrobenzoic) acid]. Half of the difference between total and native thiols provided the dynamic disulfide amount (–S–S). After determination of native thiol (–SH) and disulfide (–S–S) amount, native thiol/disulfide ratio (–S–S–/– S–S) was calculated. Transthoracic echocardiography was performed with Simpson’s method, and left ventricular ejection fraction was analyzed in all patients.
Statistical analysis
SPSS 22.0 Statistical Package Program for Windows (SPSS Inc, Chicago, IL) was used for statistical analysis of data. The distribution patterns of the variables were analyzed with Kolmogorov-Smirnov test. Numerical variables with a normal distribution were presented as mean ± standard deviation. They were presented as median and interquartile range they were not distributed normally. Categorical variables were presented as number and percentage. Student’s t-test was used to compare parametric continuous variables. Mann-Whitney U test was used to compare nonparametric continuous variables, and Chi-square test was used to compare categorical variables. Comparison of multiple means was carried out using Kruskal-Wallis tests or analysis of variance, whenever appropriate. Spearman rank test was performed to define the correlation of thiol disulfide homeostasis and corrected TIMI frame count. Logistic regression analysis was performed to determine independent predictors for SCF. The variables with an unadjusted p value < 0.20 in logistic regression analysis were identified as potential risk markers, and included in the multivariate model. We eliminated potential risk markers with reduced model using multivariate logistic regression analysis. A p value < 0.05 was considered as statistically significant, with a confidence interval of 95%.
Results
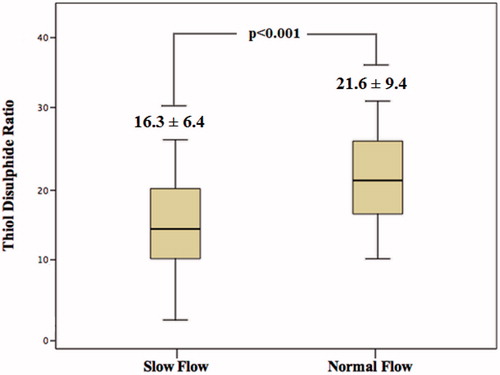
A total of 110 patients were enrolled in this study (65 patients in SCF group, and 45 patients in normal flow group). Clinical, laboratory, and angiographic features of the study groups are presented in . The rate of hypertension and the mean corrected TIMI frame count were significantly higher in the SCF group as compared to the normal flow group. Total white blood cell (WBC), hsCRP, and free thiol and disulfide levels were significantly higher; on the contrary, thiol disulfide ratio was significantly lower in the SCF group ().
Correlation analysis revealed a significant and negative correlation between thiol disulfide ratio and corrected TIMI frame count (p <0.001; ). As shown in , the SCF group had significantly lower thiol disulfide ratios when compared to the normal flow group. In multivariate logistic regression analysis, thiol disulfide ratio was found to have an independent association with the presence of SCF (p < 0.001). Moreover, hsCRP was also independently associated with SCF ().
Figure 1. Graph showing a significant and negative correlation between thiol disulfide ratio and corrected TIMI frame count.
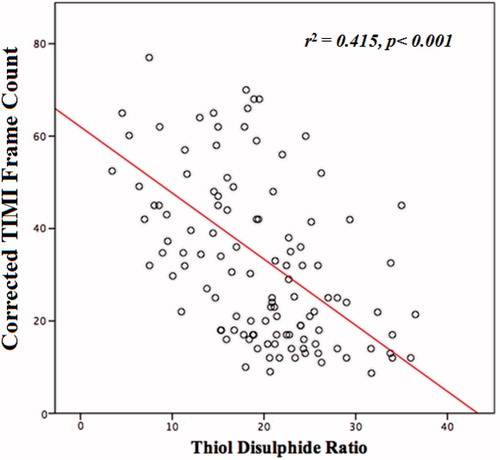
Table 2. Multivariate logistic regression analysis showing independent predictors of slow coronary flow.
Discussion
In this study, we found significant differences between SCF and the normal flow groups for thiol disulfide homeostasis. The results of our study indicated that hsCRP and thiol disulfide ratio were independently associated with SCF. To the best of our knowledge, this is the first study that investigated thiol disulfide homeostasis as a novel marker of oxidative stress in patients with SCF, and compared the results with a normal flow group.
Slow coronary flow is well defined by interventional cardiologists as a situation where opacification of the main epicardial coronary arteries have gone through a delay without atherosclerotic stenosis.[Citation1] Besides its simple definition, the exact etiopathogenesis is still unclear. Various mechanisms have been suggested in the pathogenesis of SCF including early form of atherosclerosis, coronary vasomotor dysfunction, diffuse atherosclerosis, small vessel disease, and endothelial dysfunction.[Citation2,Citation5,Citation6] Systemic inflammation and oxidative stress have also been accused for SCF.[Citation7,Citation19–23] Li and colleagues [Citation24] demonstrated that CRP and interleukin six levels were significantly higher in patients with SCF. Similarly, Barutcu and colleagues [Citation3] reported that hsCRP, a well-known systemic inflammation marker, could be related to occurrence of SCF, or could contribute to the pathogenesis of SCF. Furthermore, it was hypothesized that SCF phenomenon was a systemic vascular disturbance rather than a localized epicardial coronary artery pathology, and develops as an interaction between systemic and local factors.[Citation25]
Oxidative stress markers have been found to increase in patients with SCF. Enli et al. [Citation4] investigated the role of free-radical damage in the pathogenesis SCF, and found that serum malondialdehyde and erythrocyte superoxide dismutase levels increased while erythrocyte reduced glutathione levels decreased significantly in patients with SCF when compared to normal flow patients, and concluded that free-radical damage might play a role in the pathogenesis of SCF. Similarly, Yucel et al. [Citation23] analyzed plasma oxidative status in patients with SCF, and found that plasma total oxidative status and oxidative stress index were significantly higher in patients with SCF compared to the normal flow group, and plasma total oxidative status levels were independently associated with mean TIMI frame count. However, no studies up to date have investigated oxidative status in patients with SCF using thiol disulfide homeostasis as a marker for oxidative stress.
Non-enzymatic or enzymatic antioxidant mechanisms known as catalase, glutathione S-trans enzyme systems, superoxide dismutase and important biological thiols such as cysteine, glutathione, N-acetyl cysteine, homocysteine and gamma glutamine prevent injury due to reactive oxygen species (ROS). Thiol contains an –SH group and an organic compound, and it plays a critical role in prevention of oxidative stress in cells. The –SH groups of sulfur-containing amino acids in proteins such as methionine, cysteine, etc. are the primary targets of ROS. When in the same environment with ROS, –SH groups are oxidized, and form reversible disulfide bonds. This is the first sign of radical-mediated protein oxidation.[Citation26] Loss of thiol groups is the main molecular mechanism resulting in structural and functional alterations in proteins.[Citation27] Antioxidants, and particularly thiol groups attempting to prevent devastating effects of free radicals, may not preserve their plasma and tissue levels during those interactions.[Citation28] However, formed disulfide bonds may again be reduced to thiol groups by the cellular reducing effects of some antioxidants, and thiol disulfide homeostasis is maintained in this way. Various in vitro studies showed that abnormal thiol disulfide homeostasis resulted in proliferation or apoptosis at the cellular level.[Citation29,Citation30] Thiol disulfide homeostasis could be measured unilaterally in the past, however the levels of both substances may be measured separately and additively, and may be evaluated both individually and in total with the method developed by Erel and Neselioglu.[Citation18]
We hypothesized that thiol disulfide homeostasis, in association with oxidative stress, might play a role in SCF. Our findings showed that thiol disulfide ratio was significantly lower in the SCF group as compared to the normal flow group. In addition, we found a negative correlation between thiol disulfide ratio and TIMI frame count, which supports the role of thiol disulfide ratio in oxidative stress status in SCF. As in line with the previous studies, we also confirmed that hsCRP, a biomarker for systemic inflammation, was significantly higher in patients with SCF as compared to normal flow group. Therefore, the findings of all those studies as well as our study empower the knowledge that inflammation and oxidative stress play key roles in the etiopathogenesis of SCF.
Our study has several limitations. First of all, our sample size is relatively small. Our second limitation may be evaluation method of the normal coronary arteries: although a coronary artery seems normal in the angiogram, one may not be sure that it is normal without using optical coherence tomography and intravascular ultrasound. Finally, we did not compare our findings with other oxidative stress markers.
Conclusion
In conclusion, our study showed that thiol disulfide homeostasis was significantly and independently related to the presence of SCF. Our results suggest that a lower thiol disulfide ratio may represent increased oxidative stress. Decreased thiol disulfide ratio may simply demonstrate increased oxidative stress. However, our results do not imply that thiol disulfide homeostasis affects coronary flow, and it is the responsible factor causing SCF. However, further larger studies are needed to explain the exact mechanistic role of oxidative stress in SCF.
Funding information
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Tambe AA, Demany MA, Zimmerman HA, et al. Angina pectoris and slow flow velocity of dye in coronary arteries – a new angiographic finding. Am Heart J. 1972;84:66–71.
- Erdogan D, Caliskan M, Gullu H, et al. Coronary flow reserve is impaired in patients with slow coronary flow. Atherosclerosis. 2007;191:168–174.
- Barutcu I, Sezgin AT, Sezgin N, et al. Increased high sensitive CRP level and its significance in pathogenesis of slow coronary flow. Angiology. 2007;58:401–407
- Enli Y, Turk M, Akbay R, et al. Oxidative stress parameters in patients with slow coronary flow. Adv Ther. 2008;25:37–44.
- Mosseri M, Yarom R, Gotsman MS, et al. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation. 1986;74:964–972.
- Sezgin AT, Sgrc A, Barutcu I, et al. Vascular endothelial function in patients with slow coronary flow. Coron Artery Dis. 2003;14:155–161.
- Xu Y, Meng HL, Su YM, et al. Serum YKL-40 is increased in patients with slow coronary flow. Coron Artery Dis. 2015;26:121–125.
- Turell L, Radi R, Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med. 2013;65:244–253.
- Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem. 2013;288:26489–26496.
- Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–1338.
- Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564.
- Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762.
- Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50:495–509.
- Kundi H, Ates I, Kiziltunc E, et al. A novel oxidative stress marker in acute myocardial infarction; thiol/disulphide homeostasis. Am J Emerg Med. 2015;33:1567–1571.
- Kundi H, Erel Ö, Balun A, et al. Association of thiol/disulfide ratio with syntax score in patients with NSTEMI. Scand Cardiovasc J. 2015;49:95–100.
- Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003.
- Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326–332.
- Canpolat U, Çetin EH, Cetin S, et al. Association of monocyte-to-HDL cholesterol ratio with slow coronary flow is linked to systemic inflammation. Clin Appl Thromb Hemost. 2015. [Epub ahead of print]. DOI: 10.1177/1076029615594002.
- Kundi H, Gok M, Kiziltunc E, et al. The relationship between serum endocan levels with the presence of slow coronary flow: a cross-sectional study. Clin Appl Thromb Hemost. 2015. [Epub ahead of print]. DOI: 10.1177/1076029615618024.
- Cetin M, Zencir C, Tasolar H, et al. The association of serum albumin with coronary slow flow. Wien Klin Wochenschr. 2014;126:468–473.
- Celik VK, Eken IE, Yildiz G, et al. Vitamin E and antioxidant activity; its role in slow coronary flow. Cardiovasc J Afr. 2013;24: 360–363.
- Yucel H, Ozaydin M, Dogan A, et al. Evaluation of plasma oxidative status in patients with slow coronary flow. Kardiol Pol. 2012;71:588–594.
- Li JJ, Qin XW, Li ZC, et al. Increased plasma C-reactive protein and interleukin-6 concentrations in patients with slow coronary flow. Clin Chim Acta. 2007;385:43–47.
- Wang X, Geng LL, Nie SP. Coronary slow flow phenomenon: a local or systemic disease? Med Hypotheses. 2010;75:334–337.
- Dean R, Fu S, Stocker R, et al. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324:1–18.
- Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985; 54:305–329.
- McCord JM. Human disease, free radicals, and the oxidant/antioxidant balance. Clin Biochem. 1993;26:351–357.
- Kirlin WG, Cai J, Thompson SA, et al. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med. 1999;27:1208–1218.
- Nkabyo YS, Ziegler TR, Gu LH, et al. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am J Physiol Lung Cell Mol Physiol. 2002;283: 1352–1359.