Abstract
Objectives. Atherosclerosis is an inflammatory disease of multifactorial origin, in which immune cells together with metabolic risk factors may initiate, propagate, and activate lesions in the arterial tree. We investigated the role of auto-antibodies against endothelial cells in patients with previous myocardial infarction. Design. One hundred and four patients were studied four to five years after acute myocardial infarction (aged 36–84 years) and 83 sex-matched healthy controls (aged 32–70 years). Anti-endothelial cells IgM and IgG auto-antibodies (AECA) were quantified in plasma using flow cytometry. Results. IgM and IgG AECA were significantly higher (23.08 ± 0.81 and 10.63 ± 0.31 channel shifts, respectively; p<0.001) in patients as compared to controls (13.40 ± 0.95 and 3.53 ± 0.54 channel shifts, respectively). Further, patients who got an invasive treatment had significantly higher levels of AECA as compared to patients with only medical treatment. Plasma concentration of IgG was positively (p<0.05) correlated to the levels of high-sensitivity C-reactive protein (hsCRP). Conclusions. We report for the first time evidence that AECA are related to signs of inflammation and are increased in patients with atherosclerosis and previous myocardial infarction and with further increase with severity of disease.
Cardiovascular disease is a major cause of morbidity and mortality in the western world (WHO Statistical Information System, www.who.int/whosis). The endothelium and endothelial dysfunction have been shown to play crucial roles in cardiovascular disease (Citation1). Endothelial damage can result in increased vascular permeability followed by migration of monocytes and vascular smooth muscle cell proliferation in the vascular wall, causing an atherosclerotic lesion (Citation2). An atherothrombotic event such as a myocardial infarction or a stroke is believed frequently to be initiated by a plaque rupture, exposing e.g. tissue factors to circulating platelets and coagulation factors that can initiate thrombosis.
Autoimmunity has been suggested as a causative factor for atherosclerosis, especially in severe forms of atherosclerosis. Thus it is known that patients suffering from autoimmune diseases such as systemic lupus erythematosus and the primary anti-phospholipid syndrome have more aggressive forms of atherosclerosis (Citation3).
Anti-endothelial cell antibodies (AECA) signify a heterogeneous group of auto-antibodies detected in a variety of disorders connected with endothelial damage (Citation4). They bind to different endothelial structures, mainly via the F(ab)2 portion of the IgG immunoglobulin with low avidity (Citation5).
The aim and hypothesis of this study were to determine that AECA have a role in patients with previous myocardial infarction. A cohort of patients suffering from an acute myocardial infarction was studied. Presence of these antibodies was related to clinical characteristics and to hsCRP, a marker of inflammation.
Material and methods
Subjects
One hundred and four patients, men and women aged between 36–84 years with a history of previous acute myocardial infarction were included. The index acute myocardial infarction occurred at an average of 4.5 years before the study of AECA. The patients were recruited from the Department of Cardiology at Karolinska University Hospital Huddinge, Sweden. The exclusion criteria were known diabetes mellitus and chronic inflammatory disease such as Rheumatoid arthritis, Wegener's Granulomatosis, Vasculitis, Systemic Lupus Erythematosus, Sjögren's Syndrome, Polymyalgia Rheumatica and Giant Cell Arteritis. Coronary revascularisation procedures (e.g., percutaneous coronary angioplasty (PCI) and coronary artery bypass graft surgery (CABG) were registered.
The control group comprised 83 sex-matched healthy controls, aged between 32–70 years (blood bank, Karolinska University Hospital Huddinge, Sweden). All controls were healthy, non-smokers with no previous history of cardiovascular disease and none were on any form of medication for any other type of illness. All patents gave informed consent after written and oral information. The ethics committee of the Karolinska Institute at Karolinska University Hospital approved the study.
Acute myocardial infarction was defined using the criteria of the European Society of Cardiology and the American College of Cardiology (Citation6). Thus, patients were diagnosed as having an acute myocardial infarction if they had two values of serum troponin T greater than 0.05 g/l together with either typical symptoms or new Q-waves in at least two of the 12 standard electrocardiographic leads, or electrocardiogram changes indicating acute ischemia (ST-elevation, ST-depression, or T-wave inversion).
Measurements
Venous blood was drawn after an overnight fast and 5 minutes of supine rest for determination of serum levels of cholesterol, triglycerides and fasting plasma glucose using established methods. An immunophelometric assay, N High Sensitivity C-reactive protein (CRP) (DadeBehring), was used to estimate CRP levels (Citation7). A standardised oral glucose tolerance test with 75 g of glucose (dissolved in 250 ml water) was performed after an overnight fast. Fasting plasma glucose and measurement of plasma glucose 120 min following ingestion of 75 g glucose were analysed, using glucose oxidase technique on a Hitachi 917 system.
Resting blood pressure was measured in the right arm after about 10 min supine rest. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). The same blood samples were taken at inclusion and closing session.
Smoking status was assessed by a questionnaire.
Detection of AECA
Blood samples were collected in 5 ml sodium citrate vacutainer tubes. Plasma was obtained by centrifuging the samples at 1800xG for 10 min at room temperature, frozen in small aliquots and stored at −80°C until testing.
Human Umbilical Vein Endothelial Cells (HUVEC, Clonetics) were used for detection of AECA. HUVEC were first cultured in medium recommended by the supplier, and stimulated with recombinant tumour necrosis factor-alpha and interferon-gamma (20 ng/ml and 200 ng/ml respectively; R&D; 210-TA and 285-IF) overnight (Citation8). After trypsinisation, cells were distributed to several tubes (5×105 cells/tube) and were incubated with 50 μl plasma of control or patient for 30 min at room temperature, then washed twice with phosphate-buffered saline. Negative and positive controls (Department of Clinical Immunology, Karolinska University Hospital Huddinge, Stockholm, Sweden) were used in each test. Goat anti-human immunoglobulin M (IgM, FITC, 2:100. Sigma; F 5384) and goat anti-human immunoglobulin G (IgG, Cy3, 1:100, Jackson Immunoresearch; 109-166-098) were added and incubated at 4°C for another 30 min. Cells were washed and analysed on a Becton Dickinson flow cytometer (FACSorter; Becton Dickinson,). Fluorescence signals from 10 000 cells were counted and the FITC or Cy3 positive cells were recorded as a channel shift value after subtraction of the negative control. This method was modified from Westphal et al. (Citation9).
Statistical analysis
Statistical inhomogeneity between groups was tested with two-tailed unpaired Student's t-test. Chi square test (χ2) was used to test for differences in prevalence of AECA between the groups (age were selected ≤70 years). Correlation between CRP and AECA was analysed with the Pearson Correlation test. Statistical analysis was performed with the SPSS software version 17.0, p<0.05 was considered significant. Data are presented as mean ± standard error of mean (SE).
Results
Clinical characteristics of the patients are given in . Percutaneous intervention had been performed in almost 60% of the patients and bypass surgery in about one fourth.
Table I. Clinical characteristics of the patients (n=104).
Levels of AECA in patients and controls
The patients had AECA levels of both IgM and IgG that were significantly higher (23.08 ± 0.81 and 10.63 ± 0.31 channel shifts, respectively; p<0.001) than the control group (13.40 ± 0.95 and 3.53 ± 0.54 channel shifts, respectively) (). shows that patients had higher prevalence of AECA than healthy controls (p<0.05). The IgM levels of AECA tended to be higher in women than in men (25.19 ± 1.59 and 22.36 ± 0.82 channel shifts, respectively; p=0.085) while AECA levels did not vary between age groups in patients.
Figure 1. Levels of anti-endothelial cell antibodies (AECA) in atherosclerotic patients and controls.
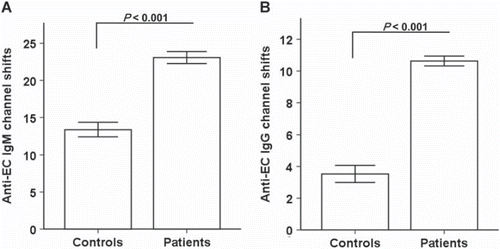
Table II. Number and percentage of AECA positive patients and healthy controls.
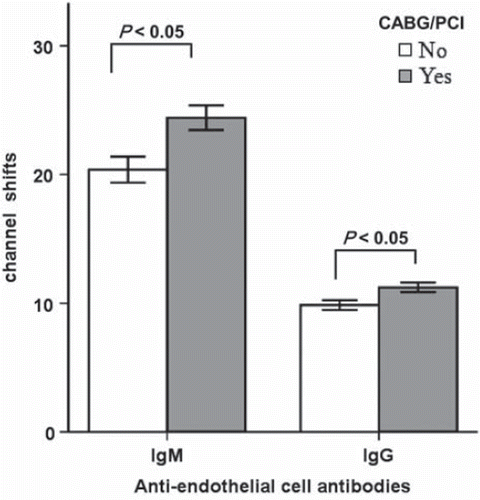
Previous treatment procedures and AECA
Patients with previous coronary interventions (percutaneous coronary intervention or coronary bypass surgery) had higher levels of AECA of both IgM and IgG type (24.41 ± 0.95 and 11.23 ± 0.36 channel shifts, respectively; p<0.05) compared to non-treated (20.38 ± 1.01 and 9.85 ± 0.38 channel shifts, respectively) ().
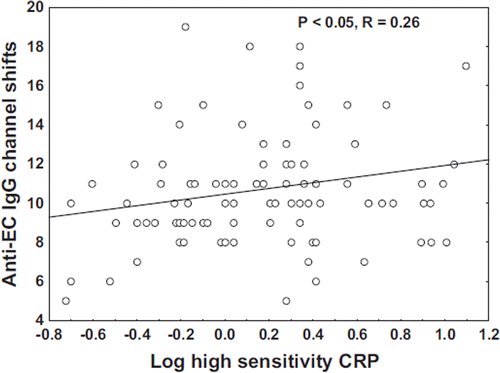
Correlation between CRP and AECA
Since inflammation is known to play a key role in atherosclerosis, we investigated the relation between the inflammatory marker hsCRP and AECA. Interestingly, there was a positive correlation between CRP and AECA (p<0.05; R=0.26) (). We did not find any correlation between AECA levels and the following risk factors; total cholesterol, triglycerides and smoking.
Discussion
Anti-endothelial cells antibodies have been detected in several autoimmune and inflammatory diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis and vasculitis (Citation10,Citation11). Also AECA were considered to be a risk factor for premature atherosclerosis in SLE patients (Citation12).
In this study, we investigated the levels of AECA in patients four to five years after myocardial infarction. Apart from patients with a history of chronic inflammatory disease, patients with diagnosed diabetes mellitus were excluded as this disorder has also been reported to be associated with increased expression of AECA (Citation13). Thus in this study with a well defined group of patients with ischemic heart disease, the main finding is that circulating levels of AECA of both IgM and IgG types are higher in patients with myocardial infarction compared to healthy controls. This is in agreement with Farsi et al. (Citation14) who found that of patients with ischemic heart disease, about 13% were IgG-AECA positive at two instants, three months apart.
On the contrary, George et al. (Citation15) reported no difference between controls and patients with one, two or three vessel disease as regards IgG as well as IgM AECA. A variety of factors, in particular the group of patients selected and differing methodology employed, may account for these discrepant findings. The selected group of patients by George et al. (Citation15) had no previous history of myocardial infarction. To detect the presence of membrane-specific AECA in patient sera we used flow cytometry, which has been reported to be a more reliable method than ELISA (Citation9) that was used in the cited study.
We report a significant positive correlation between hsCRP and AECA. HsCRP is an inflammatory mediator that might play major roles in the formation of atherosclerotic lesions and atheroma instability (Citation16). Ridker et al. (Citation17) showed that there is a correlation between CRP, and the risk for cardiovascular disease in healthy individuals from the general population or in cardiology patient cohorts. Our findings thus extend the role of CRP as being related also to the appearance of AECA in patients with atherosclerosis and previous myocardial infarction.
Patients with a history of acute myocardial infarction treated invasively had higher circulating levels of AECA. On one hand, the mechanism for the increased levels may be that these patients have more advanced atherosclerosis with more opportunities to induce AECA than that the group of patients with only medical treatment. On the other hand, the invasive treatment itself may cause local inflammation and damage to the endothelial layer, which my lead to exposure or translocation of sequestered antigens to the cell surface with further activation upon binding by AECA. Such a mechanism has been proposed in systemic vasculitis. The auto-antigen becomes available on the neutrophil surface for binding of auto-antibodies (anti-neutrophil cytoplasmic antibody (ANCA)) under the influence of pro-inflammatory cytokines, such as tumour necrosis factor-a (Citation18) or interleukin-8 (Citation19).
Anti-endothelial cells antibodies bind to endothelial cell antigens and induce endothelial damage. One suggested mechanism is AECA-induced endothelial cell apoptosis (Citation18). There is evidence of apoptosis induction in diverse autoimmune and infectious diseases. The AECA antigenic targets are not completely deciphered. Heat Shock Proteins may be an important antigen (Citation20).
AECA effects on the endothelial cell have been considered responsible, at least in part, for the vascular injury which occurs in autoimmune rheumatic diseases (Citation21). In keeping with our results, AECA may contribute to the instability of angina pectoris and post angioplasty intimal proliferation, associated with the high restenosis rate after percutaneous transluminal coronary angioplasty (Citation14).
In conclusion, these data suggest that AECA are increased in patients with previous myocardial infarction and are related to inflammation. Large scale follow-up study is required to monitor the levels of AECA at different time points, and with correlation to clinical data and risk factors. Mechanisms for appearance and pathogencity of these antibodies need to be further investigated.
Acknowledgements
This study was supported by the Karolinska Institutets Stiftelser and the Swedish Heart and Lung Foundation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ross R, Glomset J, Harker L. Response to injury and atherogenesis. Am J Pathol. 1977;86:675–84.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
- Farhey Y, Hess EV. Accelerated atherosclerosis and coronary disease in SLE. Lupus. 1997;6:572–7.
- Del Papa N, Gambini D, Meroni PL. Anti-endothelial cell antibodies and autoimmune diseases. Clin Rev Allergy. 1994;12:275–86.
- Praprotnik S, Blank M, Meroni PL, Rozman B, Eldor A, Shoenfeld Y. Classification of anti-endothelial cell antibodies into antibodies against microvascular and macrovascular endothelial cells: The pathogenic and diagnostic implications. Arthritis Rheum. 2001;44:1484–94.
- Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, . Universal definition of myocardial infarction. Circulation. 2007;116:2634–53.
- Chenillot O, Henny J, Steinmetz J, Herbeth B, Wagner C, Siest G. High sensitivity C-reactive protein: Biological variations and reference limits. Clin Chem Lab Med. 2000;38: 1003–11.
- Ge X, Uzunel M, Ericzon BG, Sumitran-Holgersson S. Biliary epithelial cell antibodies induce expression of toll-like receptors 2 and 3: A mechanism for post-liver transplantation cholangitis? Liver Transpl. 2005;11:911–21.
- Westphal JR, Boerbooms AM, Schalwijk CJ, Kwast H, De Weijert M, Jacobs C, . Anti-endothelial cell antibodies in sera of patients with autoimmune diseases: Comparison between ELISA and FACS analysis. Clin Exp Immunol. 1994;96:444–9.
- Cines DB, Lyss AP, Reeber M, Bina M, DeHoratius RJ. Presence of complement-fixing anti-endothelial cell antibodies in systemic lupus erythematosus. J Clin Invest. 1984;73:611–25.
- Meroni P, Ronda N, Raschi E, Borghi MO. Humoral autoimmunity against endothelium: Theory or reality? Trends Immunol. 2005;26:275–81.
- Fischer K, Brzosko M, Walecka A, Ostanek L, Sawicki M. [Antiendothelial cell antibodies as a risk factor of atherosclerosis in systemic lupus erythematosus]. Ann Acad Med Stetin. 2006;52(Suppl 2):95–9.
- Triolo G, Accardo-Palumbo A, Carbone MC, Ferrante A, Casiglia D, Giardina E. IgG anti-endothelial cell antibodies (AECA) in type I diabetes mellitus; induction of adhesion molecule expression in cultured endothelial cells. Clin Exp Immunol. 1998;111:491–6.
- Farsi A, Domeneghetti MP, Brunelli T, Gori AM, Fedi S, Gensini GF, . Activation of the immune system and coronary artery disease: The role of anti-endothelial cell antibodies. Atherosclerosis. 2001;154:429–36.
- George J, Meroni PL, Gilburd B, Raschi E, Harats D, Shoenfeld Y. Anti-endothelial cell antibodies in patients with coronary atherosclerosis. Immunol Lett. 2000;73:23–7.
- Hu CL, Xiang JZ, Hu FF, Huang CX. Adventitial inflammation: A possible pathogenic link to the instability of atherosclerotic plaque. Med Hypotheses. 2007;68:1262–4.
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5.
- Csernok E, Ernst M, Schmitt W, Bainton DF, Gross WL. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 1994;95:244–50.
- Rainger GE, Fisher AC, Nash GB. Endothelial-borne platelet-activating factor and interleukin-8 rapidly immobilize rolling neutrophils. Am J Physiol. 1997;272:H114–22.
- Tobon GJ, Alard JE, Youinou P, Jamin C. Are autoantibodies triggering endothelial cell apoptosis really pathogenic? Autoimmun Rev. 2009;8:605–10.
- Domiciano DS, Carvalho JF, Shoenfeld Y. Pathogenic role of anti-endothelial cell antibodies in autoimmune rheumatic diseases. Lupus. 2009;18:1233–8.