Abstract
Systemic Lupus Erythematosus (SLE) is an autoimmune rheumatic disease which predominantly affects women of reproductive age. Despite significant progress in recent years to elucidate many potential mechanisms involved in the generation of autoimmunity the factors behind the high incidence among women, the relapsing–remitting clinical course and pregnancy-related complications in SLE remain unclear. In this review, we hypothesize a potential role for uterine endometrium through its production of relaxin, a peptide hormone, as a “missing-link” to explain this female predominance, variable clinical course and obstetric complications operating in SLE.
Background
Systemic Lupus Erythematosus (SLE) is an autoimmune disease which can affect any part of the body leading to a myriad of clinical manifestations. Characteristic features include the generation of autoantibodies against host antigens, such as double stranded DNA, and a predilection for women of reproductive age with a relapsing–remitting clinical course [Citation1]. A number of different triggers for autoimmunity in SLE have been proposed including impaired apoptotic cell clearance, genetic, hormonal and environmental factors. Not all of these factors, however, readily explain the fact that 90% of patients are women and attention has focused upon the role of sex hormones. The mechanisms however, linking these hormones with generation of autoimmunity and disease exacerbations, remain unclear. Therefore, other factors should be considered and in this article we will discuss the potential effects of a hormone called relaxin upon uterine endometrium leading to generation of recurrent antigenic load and thus disease pathogenesis.
Physiological factors influencing autoimmunity
Apoptosis is a physiological process of programed cell death occurring in multi-cellular organisms throughout their lifetime to ensure the orderly removal of cells from the body. Macrophages are the major scavengers of dying cells and it is increasingly recognized that they can acquire extremes of functional phenotypes broadly divided as M1 and M2 [Citation2]. M1 macrophages are pro-inflammatory cells which combat infections, express high levels of Interleukin 1β (IL1β), generate inflammatory reactions against apoptotic cells and present host antigens to adaptive immune cells, which may serve as the initial trigger of autoimmunity [Citation3].
In contrast, M2 macrophages are important in tissue repair and wound healing. They express high levels of anti-inflammatory cytokines (such as IL10) as well as growth factors such as vascular endothelial growth factor (VEGF) and basic Fibroblast growth factor (bFGF). M2 macrophages also express high levels of mannose-binding lectins (MBL) receptor and C1q complement receptors which help in the efficient and safe clearance of apoptotic cells [Citation4]. Therefore, factors which regulate the balance of production of these different functional phenotypes of these innate immune cells may influence the generation of autoimmune diseases [Citation5]. A similar process operates in cancer cells which generate a variety of factors to evade host immune response by promoting production of the M2 macrophage phenotype. Many cancers express immunomodulatory factors such as relaxin peptides to regulate the function of innate immune cells [Citation6]. These immunomodulatory effects may also be important in tissues with physiological high cellular turnover such as the uterine endometrium and thus relevant to the pathogenesis of SLE.
Relaxin peptides
Relaxin peptides are insulin-like growth factors expressed by three genes in humans [Citation7]. H1 relaxin and H2 relaxin share 95% sequence homology and are expressed in the uterine decidua, placenta, corpus luteum, endometrium and mammary glands in women and by prostate and seminal vesicles in men. They are believed to have similar physiological effects [Citation8, Citation9], which are described below. Other major sites of H1 and H2 expression are skin, myocardium, kidney and lung. H3 relaxin is predominantly expressed in the brain and it is considered as a neuropeptide associated with stress response [Citation10].
Four G-protein coupled receptors (GPCR) have been described as targets for these peptides [Citation11]. Leucine-rich GPCR, Relaxin Family Peptide Receptors 1 (RXFP1) (also known as LGR7) and RXFP2 (also known as LGR8) are widely expressed throughout the body especially by immune cells. These GPCRs show high affinity for H2 relaxin. Small peptide-associated GPCR RXFP3 (also known as GPCR135) and RXFP4 (also known as GPCR142), are expressed in the brain and are the main target receptors for H3 relaxin. Since H2 relaxin is the main member of this family of peptides in the female reproductive tract and its physiological functions are extensively studied, all further reference to relaxin in this article will be synonymous with H2 relaxin.
Immune modulation by relaxin
In humans, relaxin peptides act mainly in paracrine or autocrine fashion and circulating levels are negligible. Findings from cancer research [Citation12] suggest that relaxin has three crucial effects on the innate immune cells: to recruit monocytes, to promote differentiation into the M2 macrophage phenotype and to inhibit migration of differentiated macrophages ().
Figure 1. Immune modulation by relaxin. Summary of potential immunonodulatory effects of relaxin upon monocytes leading to differentiation into M2 macrophages and Th cells. VEGF, Vascular-induced growth factor; bFGF, basic fibroblast growth factor; MMP, Matrix metalloproteinase.
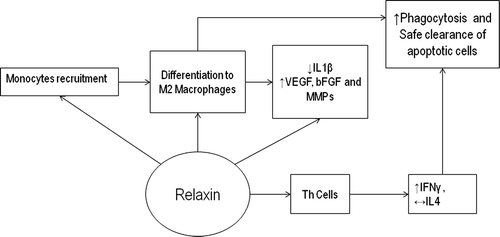
Recruitment of monocytes by relaxin depends on monocyte chemo attractant peptide (MCP-1) levels. In vivo, MCP-1 is expressed by monocytes, macrophages and endothelial cells leading to marginalization of monocytes before migration into the tissues [Citation13]. It up-regulates expression of cell surface adhesion molecules in monocytes to form cell clusters and differentiation to macrophages. It inhibits IL1β expression and promotes M2 phenotype acquisition. Furthermore, IL1β has been shown to be suppressed in monocytes pre-treated with relaxin even after exposure to pro-inflammatory stimuli such as Lipopolysacchride (LPS) [Citation12]. In addition, relaxin inhibits migration of differentiated macrophages. Relaxin also induces expression of VEGF, bFGF and matrix metallo proteinases (MMPs) by the monocytes and macrophages [Citation14].
Few studies have addressed the effect of relaxin on other immune cells. On T helper (Th) cells, it has been shown to promote expression of interferon γ without altering IL4 expression [Citation15] and suppress the release of proteases from neutrophils and basophils [Citation16,Citation17]. Overall, relaxin has been shown to generate a potential anti-inflammatory immune environment and promote apoptotic cell-clearance capacity by recruiting and modulating monocytes’ function in cancer cells. Therefore, it is possible that relaxin may have significant immunomodulatory effects upon apoptotic cell clearance in healthy cells with high physiological turnover such as the uterine endometrium.
Relaxin and the endometrium
High levels of auto antibodies may be generated in patients with SLE through exposure to increased amounts of potentially more immunogenic antigen from impaired clearance of apoptotic cells. Therefore, regulation of this process is particularly important in endometrial tissue with high cyclical cellular turnover in women of reproductive age. Sex hormones regulate the cellular proliferation and survival of the endometrial cells during menstrual cycle and pregnancy. They also influence expression of relaxin and its receptor RXFP1 at the uterine endometrium. Estrogens promote proliferation of endometrial cells during the first half of the menstrual cycle and up-regulate expression of uterine RXFP1 receptors [Citation18]. Ovulation is then followed by formation of the corpus luteum which secretes progesterone during the luteal phase. Progesterone promotes endometrial cell maturation, secretion as well as survival, and up-regulates relaxin expression by the uterine endometrium [Citation19], ().
Figure 2. Alterations of sex hormone and relaxin levels regulating menstrual cycle. Graphical display of changes in sex hormone and relaxin levels as well as the endometrium during the menstrual cycle. VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; IFNγ, Interferon gamma; MMPs, Matrix metalloproteinases.
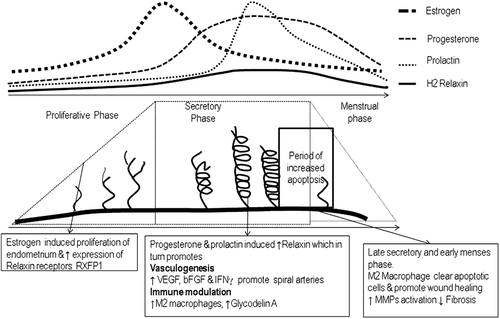
In the absence of fertilization, prolactin levels peak during the latter half of luteal phase and in conjunction with progesterone promote relaxin expression [Citation20]. Prolactin also increases MCP-1 expression in the endothelial cells [Citation21]. Relaxin levels peak on the 8th day after ovulation – in conjunction with a prolactin surge – and may then enhance endometrial recruitment and differentiation into the M2 macrophage phenotype to aid clearance of the increased number of apoptotic endometrial cells present at the end of the luteal phase. By this mechanism, relaxin helps to replenish the tissue resident scavenger macrophage system during each menstrual cycle to clear apoptotic cells.
In addition, relaxin also promotes expression of Glycodelin, a lipocalin and immune regulator in the uterine endometrium [Citation22]. Glycodelin induces apoptosis of pro-inflammatory monocytes and natural killer cells and cytotoxic T cells. In addition, it also suppresses B cell differentiation [Citation23]. Therefore, loss of the anti-inflammatory immunosuppressive effects of relaxin may conceivably contribute to disease pathogenesis in women of reproductive age.
Endometrium and female predilection of SLE
Much interest has focused upon whether sex hormone fluctuations contribute to SLE pathogenesis and disease activity [Citation24]. The frequency of disease flares have been shown to decrease after menopause or ovarian failure [Citation25,Citation26]. Clinical trials however, which have examined whether estrogen (Hormone replacement or combined oral contraceptive) therapy exacerbates disease activity in patients with SLE, have not found such a direct relationship. These studies did not find any difference in severe disease flare rates between the estrogen and placebo-treated groups, although mild/moderate disease flares were significantly increased in the hormone-replacement group [Citation27,Citation28].
Given that uterine endometrium is a site of physiological cellular turnover, under the influence of sex hormones it may provide a potential link between sex hormones and SLE. In a retrospective case control study, the effect of hysterectomy among 1006 premenopausal women with SLE were assessed for onset, disease activity and incidence of nephritis compared with age and ethnically matched controls with lupus who had not undergone hysterectomy. Patients who had undergone hysterectomy had significantly decreased disease activity, reduced ANA and improved clinical progression compared with those who had not had a hysterectomy. Interestingly, 90% of these women were also reported to be taking hormone replacement therapy, yet had reduced disease activity [Citation29].
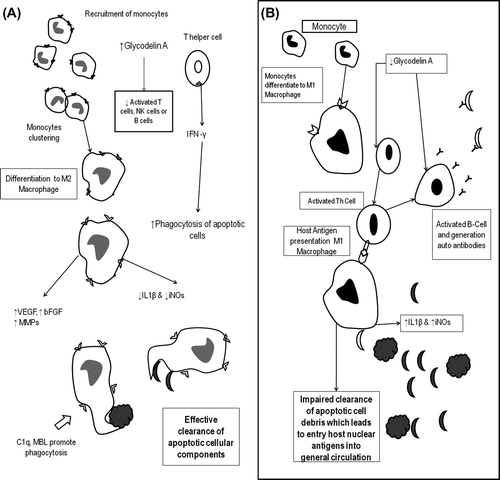
Therefore, dysregulation of relaxin, mediated immune modulation in patients with SLE may serve to increase apoptotic load and M1 macrophage formation may () in the uterine endometrium.
Figure 3. Relaxin immune regulations, apoptotic cell clearance and autoimmunity. Summary of monocyte differentiation and apoptotic clearance in the presence (A) and absence (B) of relaxin. C1q, complement component; MBL, Mannose-binding lectin; iNOs, inflammatory Nitric oxide synthase; NK cells, Natural Killer Cells; VEGF, Vascular endothelial growth factor; bFGF, basic Fibroblast Growth Factor; MMPs, Matrix metalloproteinases.
Relaxin: a missing link in SLE?
To date, no studies have been performed examining the potential relationship between relaxin and pathogenesis of SLE, to conclusively answer the question of whether dysregulation of relaxin provides a missing link to lupus pathogenesis. Indirect evidence, however, exists that reduced levels of relaxin may contribute to pregnancy morbidity, gram-negative sepsis and reduced cancer risk which are all recognized complications of SLE.
Pre-eclampsia is increased in patients with SLE [Citation30] which occurs due to abnormalities of trophoblastic invasion and spiral artery development [Citation31]. In many mammals, relaxin has been found to be important in normal trophoblastic invasion [Citation32] and its pro-angiogenic effects would be expected to promote spiral artery formation and improve placental circulation. Reduced placental expression of RXFP1 has been reported in otherwise healthy patients who develop pre-eclampsia [Citation33]. Direct evidence, however, to link the development of pre-eclampsia in patients with SLE with reduced RXFP1 and/or dysregulation of relaxin is lacking.
During pregnancy, relaxin is expressed at the feto–maternal interface where it influences immune cells and suppresses MHC-1 expression [Citation34]. These immunomodulatory effects of relaxin may promote maternal tolerance and prevent generation of immunity against fetal antigens (35). In the post-partum period, under the influence of estrogen and prolactin, relaxin aids in uterine involution and healing of endometrium [Citation36]. Therefore, loss of these relaxin-mediated effects may contribute to a break in maternal tolerance during pregnancy and increase antigen load post-partum leading to increased pregnancy morbidity and disease relapse.
Gram negative bacterial sepsis has been reported as a major cause of mortality among patients with SLE [Citation37]. Activation of TLR4 on monocytes by bacterial LPS to release IL1β [Citation38] is a critical step leading to uncontrolled immune activation observed in sepsis. Relaxin has been shown in-vitro to inhibit LPS-mediated IL1β expression by monocytes [Citation12]. Given that the female genital tract is a potential site for gram negative bacterial infections, immunoregulatory effects of relaxin at this anatomical site may be protective and the increased risk of sepsis in SLE could be a result of reduced relaxin expression or function.
Interestingly patients with SLE have been reported to have significantly lower incidence of breast, ovarian and endometrial carcinoma [Citation39]. Despite an increased incidence of human papiloma viral infection and cervical dysplasia in women with lupus, the incidence of cervical cancer is not higher than in the general population [Citation40]. In addition, men with SLE have a reduced incidence of prostatic carcinoma compared with the general population [Citation41], in whom the prostate gland is the most common site for cancer growth in the male reproductive tract. Interestingly, in non-lupus patients the prostate is known to express high levels of relaxin [Citation42] which has immunomodulatory effects to enable cancer cells to evade host immune response and pro-angiogenic properties to promote cellular proliferation. Therefore, it is theoretically possible that the reduced cancer risk observed in patients with SLE may be partly explained by early detection of abnormal cells by host immune system due to relaxin dysfunction.
Conclusion
The female predominance and relapsing–remitting clinical course observed in patients with SLE remains unexplained. In this article we hypothesize that relaxin-mediated effects on the uterine endometrium may partly explain these clinical features. Despite a lack of direct evidence the known immunoregulatory effects of relaxin put it a critical junction in the network of known disease mechanisms and complications and may represent a missing link in our understanding of pathomechanisms in SLE. Further studies, however, are required to explore the potential contribution of this peptide to SLE pathogenesis and disease progression.
Acknowledgements
Authors are immensely grateful to Dr Ian Giles, University College London for his guidance and insights.
Conflict of interest
None.
References
- Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929–39.
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23(4):344–6.
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–37.
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35.
- Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. Phenotypic and functional switch of macrophages induced by regulatory CD4+ CD25+ T cells in mice. Immunol Cell Biol. 2011;89(1):130–42.
- Silvertown1 JD, Summerlee AJ, Klonisch T. Relaxin-like peptides in cancer. Int J Cancer. 2003;107:513–9.
- Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev. 2004;25(2):205–34.
- Bryant-Greenwood GD, Schwabe C. Human relaxins: chemistry and biology. Endocr Rev. 1994;15(1):5–26.
- Evans BA, Fu P, Tregear GW. Characterization of two relaxin genes in the chimpanzee. J Endocrinol. 1994;140(3):385–92.
- Tanaka M. Relaxin-3/insulin-like peptide 7, a neuropeptide involved in the stress response and food intake. FEBS J. 2010;277(24):4990–7.
- Van Der Westhuizen1 ET, Summers1 RJ, Halls1 ML, Bathgate RAD, Sexton PM. Relaxin receptors–new drug targets for multiple disease states. Curr Drug Targets. 2007;8(1):91–104.
- Figueiredo KA, Rossi G, Cox ME. Relaxin promotes clustering, migration, and activation states of mononuclear myelocytic cells. Ann N Y Acad Sci. 2009;1160:353–60.
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–74.
- Unemori EN, Lewis M, Constant J, Arnold G, Grove BH, Normand J, et al. Relaxin induces vascular endothelial growth factor expression and angiogenesis selectively at wound sites. Wound Repair Regen. 2000;8(5):361–70.
- Piccinni MP, Bani D, Beloni L, Manuelli C, Mavilia C, Vocioni F, et al. Relaxin favors the development of activated human T cells into Th1-like effectors. Eur J Immunol. 1999;29(7):2241–7.
- Masini E, Nistri S, Vannacci A, Bani Sacchi T, Novelli A, Bani D. Relaxin inhibits the activation of human neutrophils: involvement of the nitric oxide pathway. Endocrinology. 2004;145(3):1106–12.
- Bani D, Baronti R, Vannacci A, Bigazzi M, Sacchi TB, Mannaioni PF, Masini E. Inhibitory effects of relaxin on human basophils activated by stimulation of the Fc epsilon receptor. The role of nitric oxide. Int Immunopharmacol. 2002;2(8):1195–204.
- Bond CP, Parry LJ, Samuel CS, Gehring HM, Lederman FL, Rogers PA, Summers RJ. Increased expression of the relaxin receptor (LGR7) in human endometrium during the secretory phase of the menstrual cycle. Ann N Y Acad Sci. 2005;1041:136–43.
- Garibay-Tupas JL, Okazaki KJ, Tashima LS, Yamamoto S, Bryant-Greenwood GD. Regulation of the human relaxin genes H1 and H2 by steroid hormones. Mol Cell Endocrinol. 2004;30: 219(1–2):115–25.
- Peters CA, Maizels ET, Robertson MC, Shiu RPC, Soloff MS, Hunzicker-Dunn M. Induction of relaxin messenger RNA expression in response to prolactin receptor activation requires protein kinase C δ signaling. Mol Endocrinol. 2000;14(4):576–90.
- Arici A, Senturk LM, Seli E, Bahtiyar MO, Kim G. Regulation of monocyte chemotactic protein-1 expression in human endometrial stromal cells by estrogen and progesterone. Biol Reprod. 1999; 61(1):85–90.
- Tseng L, Zhu HH, Mazella J, Koistinen H, Seppälä M. Relaxin stimulates glycodelin mRNA and protein concentrations in human endometrial glandular epithelial cells. Mol Hum Reprod. 1999;5(4):372–5.
- Alok A, Karande AA. The role of glycodelin as an immune-modulating agent at the feto-maternal interface. J Reprod Immunol. 2009; 83(1–2):124–7.
- McMurray RW, May W. Sex hormones and systemic lupus erythematosus: review and meta-analysis. Arthritis Rheum. 2003; 48(8):2100–10.
- Urowitz MB, Ibañez D, Jerome D, Gladman DD. The effect of menopause on disease activity in systemic lupus erythematosus. J Rheumatol. 2006;33(11):2192–8.
- Mok CC, Wong RW, Lau CS. Ovarian failure and flares of systemic lupus erythematosus. Arthritis Rheum. 1999;42(6):1274–80.
- Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al;OC-SELENA Trial. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–8.
- Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142(12 Pt 1):953–62.
- Namjou B, Scofield RH, Kelly JA, Goodmon E, Aberle T, Bruner GR, Harley JB. The effects of previous hysterectomy on lupus. Lupus. 2009;18(11):1000–5.
- Mok CC, Wong RW. Pregnancy in systemic lupus erythematosus. Postgrad Med J. 2001;77(905):157–65.
- Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179(5):1359–75.
- Klonisch T, Hombach-Klonisch S. Review: Relaxin expressed at the feto–maternal interface. Reprod Dom Anim. 2000;35(3–5): 149–52.
- Wang YQ, Li J, Yang Z. [Expression of relaxin receptor in placental tissues of normal pregnancy and pre-eclampsia]. Zhonghua Fu Chan Ke Za Zhi. 2008;43(4):269–7
- Klonisch T, Mathias S, Cambridge G, Hombach-Klonisch S, Ryan PL, Allen WR. Placental localization of relaxin in the pregnant mare. Placenta. 1997;18(2–3):121–8.
- Linker-Israeli M, Quismorio FP Jr, Wong DK, Friou GJ. Serum antibodies to human fetal antigens in patients with systemic lupus erythematosus (SLE). J Immunol. 1980;124(3):1154–9.
- Adams WC, Frieden EH. Inhibition of postpartum uterine involution in the rat by relaxin. Biol Reprod. 1985;33(5):1168–75.
- Kim WU, Min JK, Lee SH, Park SH, Cho CS, Kim HY. Causes of death in Korean patients with systemic lupus erythematosus: a single centre retrospective study. Clin Exp Rheumatol. 1999; 17(5):539–45.
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. 1999;274(16):10689–92.
- Bernatsky S, Ramsey-Goldman R, Foulkes WD, Gordon C, Clarke AE. Breast, ovarian, and endometrial malignancies in systemic lupus erythematosus: a meta-analysis. Br J Cancer. 2011;104(9):1478–81.
- Santana IU, Gomes Ado N, Lyrio LD, Rios Grassi MF, Santiago MB. Systemic lupus erythematosus, human papillomavirus infection, cervical pre-malignant and malignant lesions: a systematic review. Clin Rheumatol. 2011;30(5):665–72.
- Bernatsky S, Ramsey-Goldman R, Gordon C, Clarke AE. Prostate cancer in systemic lupus erythematosus. Int J Cancer. 2011;129(12):2966–9.
- Thompson VC, Morris TG, Cochrane DR, Cavanagh J, Wafa LA, Hamilton T, et al. Relaxin becomes upregulated during prostate cancer progression to androgen independence and is negatively regulated by androgens. Prostate. 2006;66(16):1698–709.
