Abstract
Objectives. To evaluate the long-term efficacy and safety of certolizumab pegol (CZP) treatment and to assess the efficacy of two CZP maintenance dosing schedules in Japanese rheumatoid arthritis (RA) patients who could not receive methotrexate (MTX).
Methods. HIKARI double-blind (DB) patients were entered into an open-label extension (OLE) study. Patients withdrawn at 16 weeks due to lack of efficacy and DB completers without a 24-week American College of Rheumatology (ACR)20 response received CZP 200 mg every 2 weeks (Q2W). DB completers with 24-week ACR20 responses were randomized to CZP 200 mg Q2W or CZP 400 mg every 4 weeks.
Results. The ACR20/ACR50/ACR70 response rates of DB completers (n = 98) were 82.7%/56.1%/34.7% at OLE entry, and 83.7%/65.3%/48.0% at 52 weeks, respectively. Other clinical, functional, and radiographic outcomes were sustained during long-term administration of CZP, even without MTX. No new unexpected adverse events were observed during long-term CZP treatment. The efficacy and safety of CZP treatment were similar between the two dosing schedules.
Conclusions. Long-term CZP administration is efficacious and safe for RA patients. No obvious differences in clinical efficacy and safety were observed between the two dosing schedules. The choice between two maintenace regimens adds flexibility in administration schedules for RA patients and physicians.
Introduction
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease characterized by persistent and chronic joint inflammation [Citation1]. A critical factor in this inflammatory process is the production of TNFα, which causes immune cell activation and chronic inflammation [Citation2]. Introduction of TNF inhibitors in clinical practice has brought significant changes to the treatment of RA. These agents lead to improved signs and symptoms of RA and inhibit further structural joint damage, which restore the physical function and the quality of life in RA patients [Citation3–7].
Certolizumab pegol (CZP) is a polyethylene glycol (PEG)ylated Fc-free anti-TNFα agent [Citation8,Citation9]. The efficacy of CZP with concomitant methotrexate (MTX) treatment has previously been demonstrated in patients with active RA, who did not respond adequately to MTX alone. These studies include the RAPID1 and RAPID2 studies conducted internationally [Citation10,Citation11] and the J-RAPID study conducted in Japan [Citation12]. However, as MTX is not tolerated by all patients due to side effects related to its anti-metabolite activity [Citation13,Citation14], additional studies were performed to test the ability of CZP to improve disease in RA patients without concomitant MTX treatment. In a 24-week multicenter, double-blind (DB), placebo-controlled study (FAST4WARD), administration of CZP 400 mg every 4 weeks (Q4W) given as monotherapy significantly reduced the signs and symptoms of active RA in patients who had failed at least one prior disease-modifying antirheumatic drug (DMARD) [Citation15]. Moreover, a similar 24-week DB, placebo-controlled HIKARI study, which targeted Japanese RA patients in whom MTX could not be administered, demonstrated the efficacy and safety of CZP 200 mg administered every 2 weeks (Q2W) without MTX [Citation16]. Additionally, the HIKARI study demonstrated that administration of CZP results in rapid, sustained reductions in signs and symptoms of RA, both as monotherapy and with non-MTX DMARDs. Notably, CZP monotherapy showed significant inhibition of radiographic progression [Citation16].
CZP has been shown to be safe and efficacious in short-term treatment studies [Citation10–12,Citation16]; however, whether the beneficial effects of CZP are maintained during long-term treatment is unclear in Japanese RA patients, especially in the absence of co-treatment with MTX. To this end, we conducted an open-label extension (OLE) study of the HIKARI trial to evaluate the long-term efficacy and safety of CZP treatment in Japanese RA patients who could not be treated with MTX. In this OLE study, we also aimed to compare the efficacy of two maintenance regimens, CZP 200 mg Q2W and CZP 400 mg Q4W. We hereby report the 52-week interim results and post-hoc analysis from the ongoing HIKARI-OLE study.
Material and methods
HIKARI and HIKARI-OLE study design
The HIKARI-OLE study (NCT00850343) is an OLE study of the HIKARI study (NCT00791921). In brief, the HIKARI study (hereinafter referred to as the “DB phase”) was a 24-week, phase III, DB study conducted in 65 centers across Japan in patients with active RA, who could not receive MTX due to insufficient efficacy, safety concerns, or previous discontinuation for safety reasons [Citation16]. Eligible patients were aged 20–74 years and had a diagnosis of adult-onset RA as defined by American College of Rheumatology (ACR) criteria [Citation17] of 0.5–15 years’ disease duration. The subjects were randomized 1:1 to a CZP or placebo group. In the CZP group, 400 mg of CZP was subcutaneously administered at Weeks 0, 2, and 4. Subsequently, CZP was subcutaneously administered at a maintenance dose of 200 mg Q2W. The primary endpoint of this study was an ACR20 response at week 12 [Citation16]. In this study, the concomitant use of DMARDs other than MTX and leflunomide (hereinafter referred to as non-MTX DMARDs) was permitted if the drug combination and dosage were maintained. The non-MTX DMARDs used included Salazosulfapyridine (n = 58), Tacrolimus Hydrate (n = 34), Bucillamine (n = 32), Mizoribine (n = 8), Sodium Aurothiomalate (n = 4), Actarit (n = 1), and Auranofin (n = 1).
The HIKARI-OLE study was conducted between March 25, 2009 and August 12, 2011. In the OLE phase, we divided HIKARI study patients into four groups based on the clinical responses during the DB phase. Patients who did not achieve an ACR20 response both at Weeks 12 and 14 were withdrawn from the DB phase at Week 16, assigned to Group I (n = 110), and treated with CZP 200 mg Q2W thereafter. Patients who exhibited an ACR20 response at Week 12 or 14 but failed to achieve an ACR20 response at Week 24 were assigned to Group II (n = 12) and also received CZP 200 mg Q2W. Patients who achieved an ACR20 response at Week 12 or 14 as well as at Week 24 were randomized 1:1 to either CZP 200 mg Q2W (Group III, n = 43) or CZP 400 mg Q4W (Group IV, n = 43) (). Of importance, we established this dosing schedule so that the total dose received by patients in Groups III and IV over a 1-month period was the same.
Figure 1. HIKARI-OLE study design. The diagram depicts the breakdown of HIKARI DB study patients into four groups for the OLE phase of the study. *Regardless of their initial DB phase group assignment, patients who achieved an ACR20 response at Week 12 or 14 as well as at Week 24 were randomized (1:1) to either CZP 200 mg Q2W (Group III, n = 43) or CZP 400 mg Q4W (Group IV, n = 43).
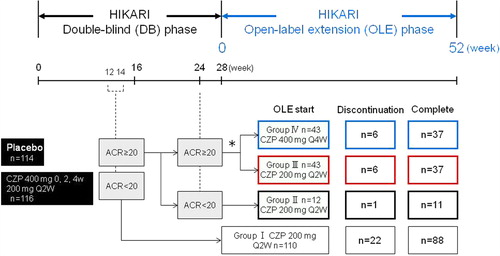
Week 0 of the OLE phase of Groups II, III, and IV (HIKARI DB phase completers: hereinafter referred to as DB completers) corresponds to Week 28 of the DB phase, and Week 0 of the OLE phase of Group I (early escape) corresponds to Week 16 of the DB phase. Patients assigned to the placebo group during the DB phase were also included in this OLE study. A change in dosage or the discontinuation of concomitant DMARDs was permitted after Week 24 of the OLE phase. However, any new addition of concomitant DMARDs or readministration of previously discontinued drugs was not permitted.
The outcome of the study was measurement of continuous efficacy and safety monitoring during the long-term treatment with CZP without MTX. Efficacy outcomes included ACR20 response rates, and changes in Health Assessment Questionnaire Disability Index (HAQ-DI), Disease Activity Score in 28 Joints-Erythrocyte Sedimentation Rate (DAS28-ESR), Short Form-36 Health Survey (SF-36), and Pain Visual Analog Scale (VAS) from HIKARI pre-study baseline. In addition, to measure radiographic disease progression, changes in modified Total Sharp Score (mTSS) from OLE study entry was assessed by linear extrapolation. Comprehensive disease control (CDC) was defined by simultaneous triple criteria: that is, low disease activity (LDA) (DAS28-ESR: ≤ 3.2), functional remission (HAQ-DI: ≤ 0.5), and radiographic nonprogression (yearly ΔmTSS: ≤ 0.5). Comprehensive disease remission (CDR) was also defined by simultaneous triple remission criteria: clinical remission (DAS28-ESR: < 2.6), functional remission (HAQ-DI: ≤ 0.5), and radiographic nonprogression (yearly ΔmTSS: ≤ 0.5). To calculate CDC and CDR for overall DB completers (n = 98), yearly ΔmTSS from HIKARI pre-study baseline (linear extrapolation, with nonresponder imputation for patients with no data) was used. Safety outcomes were reported for all patients who received at least one dose of CZP in the OLE study (n = 208).
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and with the Pharmaceutical Affairs Law Standards for the Conduct of Clinical Trials on Drugs (Ministry of Health, Labour and Welfare Ordinance no. 28, 27 March 1997) and related notifications. Institutional review board approval was obtained at all centers and all patients provided written informed consent.
Post-hoc analyses
Since the OLE study included patients who received placebo during the DB phase, an additional post-hoc analysis of efficacy was performed on patients who received CZP in the DB phase, to observe the effects of continuous CZP treatment during the combined DB and OLE phases of the study. This data set includes patients who were originally assigned to CZP 200 mg treatment groups in the DB phase and completed the DB phase with an ACR20 response at Week 12 or 14 (CZP-DB completers; n = 81). Of note, this data set excluded all patients who previously received placebo in the DB phase, even if they completed the phase. We focused on ACR20/ACR50/ACR70 response rates, DAS28-ESR scores, HAQ-DI scores, and disease activity state (high: DAS28-ESR: > 5.1, moderate: > 3.2 and ≤ 5.1, LDA: ≤ 3.2, and remission: < 2.6) in this post-hoc analysis.
Statistical analyses
The efficacy analysis was performed on the full-analysis set (FAS) using the last observation carried forward (LOCF) to impute missing data. We used HIKARI pre-study baseline as baseline values. Safety analyses were performed on all subjects who received at least one dose of CZP during the OLE study. Because the objective of the study was to evaluate the long-term efficacy and safety of CZP treatment, inferential analyses were not performed.
Results
Patient characteristics and disposition of the HIKARI-OLE study
HIKARI DB phase patients were consented to enter the OLE study (n = 210). Two hundred and eight patients were included in the efficacy and safety analyses, because two patients withdrew from the OLE study before receiving CZP treatment. During the 52-week treatment, an additional 35 patients withdrew from the study. A few patients withdrew from the study due to an inadequate response (5.8% in total, ). Other reasons for withdrawal are shown in . A total of 173 patients (83%) completed the 52-week interim period of the OLE phase of the study.
Table 1. Reasons for discontinuation of therapy.
Based on their response during the DB phase, patients were separated into four groups in the OLE phase. Patients with an ACR20 response at Week 24 of the DB phase (DB responders: Groups III and IV) and DB non-responders (Groups I and II) were distinguished in order to evaluate the sustained efficacy of continued long-term CZP treatment. As shown in , all of these groups included patients who were on placebo during the DB phase. The fraction of patients that received placebo during the DB phase were 78.2% (86 patients), 41.7% (5 patients), 18.6% (8 patients), and 9.3% (4 patients) in Groups I, II, III, and IV, respectively. DB responders were further randomized into two groups to evaluate the efficacy of two different dosing schedules. DB responder patients (n = 86) were randomized to either a CZP 200 mg Q2W (Group III, n = 43: 35 patients from the CZP group and 8 patients from the placebo group) or a CZP 400 mg Q4W (Group IV, n = 43: 39 patients from the CZP group and 4 patients from the placebo group) treatment group as shown in . At OLE study entry (OLE Week 0), the mean DAS28-ESR scores of Groups I, II, III, and IV were 6.16, 5.26, 3.33, and 3.58, respectively.
Table 2. Patient demographics and disease status at the HIKARI pre-study baseline (FAS population).
Patient demographics and HIKARI pre-study baseline characteristics are summarized in . Patients who withdrew from the DB phase at Week 16 (Group I) and overall DB completers (Groups II + III + IV) had mean DAS28-ESR scores of 6.27 and 6.11, respectively, at HIKARI pre-study baseline. 44.7% of patients did not receive any DMARDs at the initiation of the DB phase, and remained untreated with DMARDs during the OLE phase of up to 52 weeks.
Long-term CZP treatment sustains the clinical efficacy of CZP
We conducted the HIKARI-OLE study to assess the clinical response obtained after prolonged treatment with CZP without MTX. In Groups I, II, III, IV, and overall DB completers (Groups II + III + IV), the ACR20/ACR50/ACR70 response rates, as calculated from HIKARI pre-study baseline, were increased or sustained for up to 52 weeks of CZP treatment in the OLE phase.
At OLE study entry and at 52 weeks of the OLE phase, the ACR20 response rates were 4.5% and 70.0% for Group I, 8.3% and 83.3% for Group II, 90.7% and 76.7% for Group III, and 95.3% and 90.7% for Group IV, respectively (). The ACR50 response rates were 0.9% and 40.9% for Group I, 0.0% and 58.3% for Group II, 65.1% and 62.8% for Group III, and 62.8% and 69.8% for Group IV, respectively (). The ACR70 response rates were 0.9% and 22.7% for Group I, 0.0% and 16.7% for Group II, 39.5% and 58.1% for Group III, and 39.5% and 46.5% for Group IV, respectively ().
Figure 2. The ACR20/ACR50/ACR70 rates in patients from each treatment group. The percentages of patients in Groups I (n = 110), II (n = 12), III (n = 43), IV (n = 43), and patients in Groups II + III + IV combined (DB completers, n = 98) who achieved an (a) ACR20, (b) ACR50, or (c) ACR70 response were plotted over time for the DB and the OLE phase of the study (FAS population and LOCF imputation). Of note, Week 0 of the OLE phase of Group I (early escape) corresponds to Week 16 of the DB phase. There are no points in the missing section of the graph for Group I.
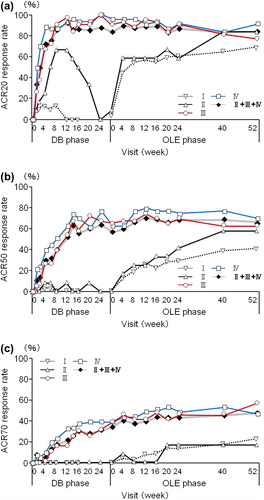
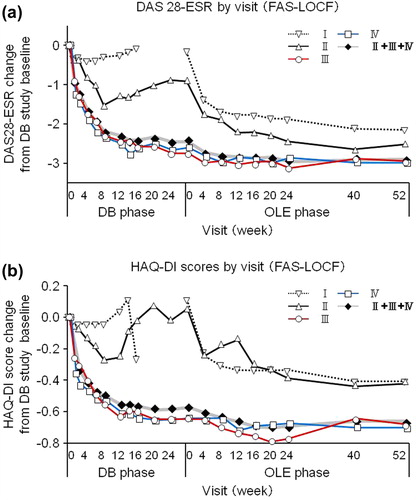
For overall DB completers (Groups II + III + IV), at OLE study entry and at 52 weeks of the OLE phase, the ACR20 response rates were 82.7% and 83.7%, the ACR50 response rates were 56.1% and 65.3%, and the ACR70 response rates were 34.7% and 48.0%, respectively (). A marked improvement in DAS28-ESR was also sustained for up to 52 weeks of the OLE phase (). The DAS28-ESR remission rates (defined as DAS28-ESR < 2.6) for overall DB completers were 23.5% and 35.7% at OLE entry and at 52 weeks of the OLE phase, respectively. Improvements in HAQ-DI (), pain VAS, and SF-36 scores were also sustained. The HAQ-DI remission rates (defined as HAQ-DI ≤ 0.5) for overall DB completers were 58.2% and 68.4% at OLE entry and at 52 weeks of the OLE phase, respectively, indicating that most of patients achieved functional remission. The mean ± SD in 100 mm pain VAS improvement from the HIKARI pre-study baseline was − 32.0 ± 23.1 at OLE study entry and maintained at − 35.4 ± 27.0 at 52 weeks of the OLE phase. Moreover, the mean ± SD changes of SF-36 scores from HIKARI pre-study baseline at OLE study entry and at Week 52 were 10.3 ± 8.7 and 12.6 ± 12.2 in physical component summary scores and 7.2 ± 11.7 and 5.6 ± 13.4 in mental component summary scores, respectively. Similar to the summary scores, the change in each of the individual eight domains of the SF-36 score was all maintained, indicating sustained improvement in the quality of life of RA patients (data not shown).
Figure 3. The change of DAS28-ESR and HAQ-DI over HIKARI pre-study baseline in patients from each treatment group. Changes in (a) DAS28-ESR and (b) HAQ-DI from HIKARI pre-study baseline of Groups I (n = 110), II (n = 12), III (n = 43), IV (n = 43), and patients in Groups II + III + IV combined (DB completers, n = 98) were plotted against time for the DB and the OLE phase of the study (FAS population and LOCF imputation). Of note, Week 0 of the OLE phase of Group I (early escape) corresponds to Week 16 of the DB phase. There are no points in the missing section of the graph for Group I.
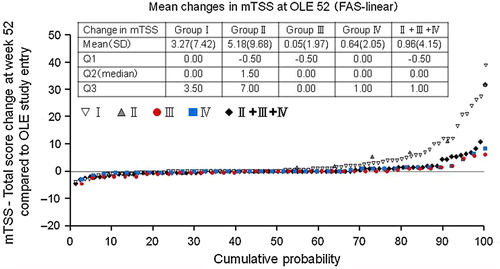
In addition to signs and symptoms, and patient-reported outcome indicators, changes in mTSS (ΔmTSS) at Week 52 from OLE study entry were assessed. The mean ± SD and median ΔmTSS were 0.96 ± 4.15 and 0.00 in DB completers, respectively (). 69.8% of overall DB completers had a ΔmTSS ≤ 0.5 at Week 52, suggesting that continued CZP treatment was beneficial in attenuating further joint destruction.
Figure 4. Inhibition of progression of structural damage: cumulative probability plot representing the change from OLE study entry in mTSS at Week 52 (FAS population and linear extrapolation). The graph depicts the cumulative probability of patients displaying a particular change in mTSS from OLE study entry in Groups I (n = 89), II (n = 11), III (n = 38), IV (n = 37), and patients in Groups II + III + IV combined (DB completers, n = 86).
The proportion of DB completers achieving CDC (i.e., DAS28-ESR ≤ 3.2, HAQ-DI ≤ 0.5, and ΔmTSS ≤ 0.5) at OLE study entry and at 52 weeks were 19.4% and 30.6%, respectively. The proportion of DB completers achieving CDR (i.e., DAS28-ESR < 2.6, HAQ-DI ≤ 0.5, and ΔmTSS ≤ 0.5) at OLE study entry and at 52 weeks were 15.3% and 23.5%, respectively. Together, these data suggest that the clinical, functional, and radiographic benefits obtained after short-term CZP treatment is sustained by long-term treatment with CZP.
Comparable clinical benefit is achieved by the two different maintenance regimens (CZP at 200 mg Q2W vs. 400 mg Q4W)
As a subsidiary objective, we evaluated the clinical efficacy of two different maintenance dosing schedules by randomly assigning patients who achieved an ACR20 response in HIKARI study DB phase into either CZP 200 mg Q2W (Group III) or CZP 400 mg Q4W (Group IV). The ACR20/ACR50/ACR70 rates and changes in DAS28-ESR scores and HAQ-DI scores from HIKARI pre-study baseline were sustained similarly well in both Groups III and IV through 52 weeks of the OLE study phase ( and ). For example, the ACR20 response rates were 90.7% and 76.7% for Group III and 95.3% and 90.7% for Group IV at OLE study entry and at 52 weeks of the OLE phase, respectively (). In addition to clinical parameters, the mean ± SD of ΔmTSS from OLE study entry between Groups III and IV were similar at 0.05 ± 1.97 with a median of 0.00 compared to 0.64 ± 2.05 with a median of 0.00 at Week 52, respectively (). At Week 52, 78.9% and 67.6% of patients had a ΔmTSS ≤ 0.5 in Groups III and IV, respectively. These data suggest that both regimens are similarly effective at inhibiting radiographic progression. Thus, both CZP maintenance regimens can be used to sustain the clinical efficacy of CZP for long-term treatment.
Assessment of sustained clinical efficacy of long-term CZP treatment by a post-hoc analysis through the DB and OLE phase
All groups (I–IV) of the OLE study protocol included patients who were originally randomized to the placebo group during the 24-week DB phase of HIKARI study (). In order to observe the effects of continuous CZP treatment during the combined DB and OLE phases of the study, conducting analyses in the original groups that include placebo-treated patients during the DB phase, was thought to be inadequate. Thus, we performed a post-hoc analysis that only includes patients who were originally assigned to the CZP treatment group in the DB phase and completed the DB phase with an ACR20 response at Week 12 or 14 (CZP-DB completers; n = 81). In further analyses, CZP-DB completers included in this post-hoc analysis described above were divided into two subgroups: Patients who were on CZP monotherapy (n = 34) and those who were treated with CZP plus non-MTX DMARDs (n = 47).
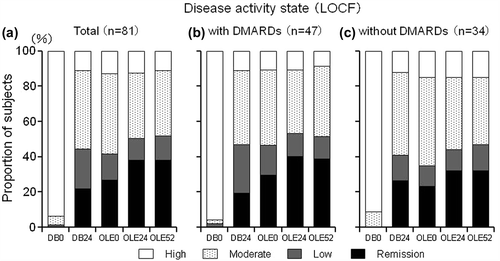
Compared to OLE study entry, the ACR20, ACR50, and ACR70 response rates in CZP-DB completers were all maintained up to Week 52 of the OLE phase of HIKARI study (). 86.4% (70/81) of CZP-DB completers receiving 200 mg CZP during the DB phase continued treatment with CZP to 52 weeks of the OLE phase, with the ACR20/ACR50/ACR70 responses rates of 81.5%/63.0%/48.1% at Week 52, respectively (). Moreover, compared to OLE study entry, the mean changes in DAS28-ESR scores and HAQ-DI scores from HIKARI pre-study baseline in CZP-DB completers were also sustained up to 52 weeks of the OLE phase (). Furthermore, achievement of LDA and remission rates (defined as DAS28-ESR ≤ 3.2 and < 2.6, respectively) in CZP-DB completers was sustained during the 52-week period of the OLE phase. In CZP-DB completers receiving CZP with or without non-MTX DMARDs, the combined rates of LDA and remission (DAS28-ESR ≤ 3.2) were 46.8% and 35.3%, respectively, at OLE entry, and 53.2% and 47.1%, respectively, at Week 52 (). The remission rates (DAS28-ESR < 2.6) were 29.8% and 23.5%, respectively, at OLE entry, and 40.4% and 32.4%, respectively, at Week 52 (). Therefore, this post-hoc analysis demonstrates that long-term CZP treatment, regardless of the concomitant use of non-MTX DMARDs, sustains clinical efficacy, even when the analysis set is restricted to patients who have achieved an ACR20 clinical response after 12–14 weeks of CZP treatment.
Figure 5. Post-hoc analysis of ACR20/50/80 response rates in patients from Groups II, III, and IV excluding those who were in the placebo group during the DB phase (CZP-DB completers). The percentages of patients who achieved an ACR20, ACR50, and ACR70 response of (a) all CZP-DB completers (n = 81), (b) CZP-DB completers treated with additional non-MTX DMARDs (n = 47), and (c) CZP-DB completers treated without additional non-MTX DMARDs (n = 34) were plotted against time for the DB and the OLE phase of the study (LOCF imputation).
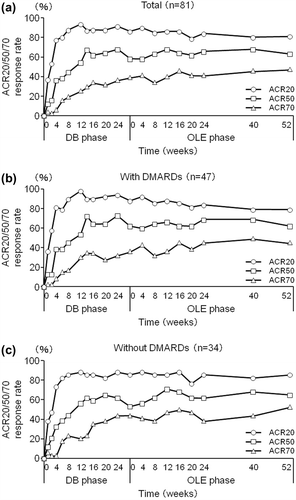
Figure 6. Post-hoc analysis of changes in (a) DAS28-ESR and (b) HAQ-DI scores from HIKARI pre-study baseline in patients from Groups II, III, and IV excluding those who were in the placebo group during the DB phase (CZP-DB completers). The DAS28-ESR and HAQ-DI scores of all CZP-DB completers (n = 81), CZP-DB completers treated with additional non-MTX DMARDs (n = 47), and CZP-DB completers treated without additional non-MTX DMARDs (n = 34) were plotted against time for the DB and the OLE phase of the study (LOCF imputation).
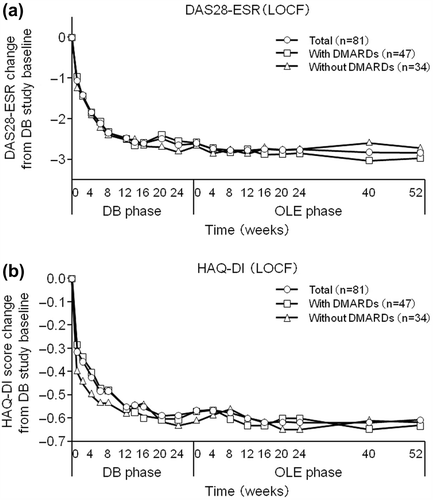
Figure 7. Post-hoc analysis of disease activity states in patients from Groups II, III, and IV excluding those who were in the placebo group during the DB phase (CZP-DB completers). The proportions of patients with high (defined as DAS28-ESR > 5.1), moderate (> 3.2 and ≤ 5.1), low (≤ 3.2), or remission (< 2.6) disease activity states among (a) all CZP-DB completers (n = 81), (b) CZP-DB completers treated with additional non-MTX DMARDs (n = 47), and (c) CZP-DB completers treated without additional non-MTX DMARDs (n = 34) at DB Week 0 (DB0), DB Week 24 (DB24), OLE Week 0 (OLE0), OLE Week 24 (OLE24), and OLE Week 52 (OLE52) are shown (LOCF imputation).
Adverse events reported in patients with long-term CZP treatment
During the 52-week OLE phase, 179 patients (86.1%) experienced adverse events (AEs) and 29 patients (13.9%) experienced serious AEs (SAEs) (). Among SAEs, five patients (2.4%) reported joint-related events, two patients (1.0%) reported infections, and two patients (1.0%) developed colonic polyps. Two patients (1.0%) developed a malignancy (gastric cancer and non-Hodgkin's lymphoma). Nasopharyngitis, eczema, and upper respiratory tract infection represented the most common AEs, which were mostly mild to moderate (76.9%). No tuberculosis infections or deaths were reported. The overall AE rate was similar among all groups (Groups I–IV). None of these AEs were unanticipated. Together, these data suggest that long-term CZP treatment for 52 weeks of the OLE phase was well-tolerated by patients ().
Table 3. Treatment-emergent adverse events.
Discussion
Previous clinical studies have demonstrated the benefits of CZP in improving RA disease parameters after short-term treatment of 24 weeks duration [Citation10–12,Citation15]. Similar data were obtained in the DB placebo-controlled HIKARI study, which was designed to investigate the short-term efficacy of CZP in patients who could not receive MTX [Citation16]. Since the clinical efficacy and safety of long-term CZP treatment without MTX is unknown in Japanese RA patients, we conducted an OLE study of the HIKARI study. This OLE study was designed to evaluate the safety of long-term CZP treatment and to assess whether the clinical benefit obtained from the 24-week treatment period in the HIKARI study could be sustained by extending the treatment for another 52 weeks. As a subsidiary objective, we utilized the OLE study to evaluate the standard dosing schedule (CZP 200 mg Q2W) compared to another optional dosing schedule (CZP 400 mg Q4W).
Relating to the primary objective of our study, our data demonstrate that long-term CZP treatment continues to maintain the clinical benefit of CZP obtained after 24 weeks of treatment. All outcome parameters including high ACR response rates, and changes in DAS28-ESR scores, SF-36 scores, and pain VAS were sustained by long-term CZP treatment in DB completers. In addition, clinical remission was observed in 35.7% of patients with long-term treatment at 52 weeks of the OLE study. Functional remission was also observed in 68.4% at 52 weeks of the OLE phase, indicating that most of patients achieved functional remission. Furthermore, patients treated with long-term CZP did not incur further joint destruction, since 69.8% of the patients had a ΔmTSS ≤ 0.5. Therefore, long-term CZP treatment appears to be effective at controlling RA disease progression. The low withdrawal rate (5.8%) of patients from the OLE study due to insufficient response further supports this notion. Importantly, long-term CZP treatment was well-tolerated by patients. No unexpected new AEs were detected in patients treated with long-term CZP compared to those observed in previous clinical studies involving short-term CZP treatment.
Anti-TNFα antibodies other than CZP that are currently in clinical use are full antibodies consisting of an Fc region and an antigen-binding Fab region [Citation18]. In contrast, CZP is a humanized Fab’ fragment fused to a 40-kD PEG moiety without an Fc region. One disadvantage of Fab’ fragments relates to their shorter in vivo half-life due to the hastened clearance of Fab’ fragments in the absence of an Fc region. However, the attachment of a PEG moiety to the Fab’ fragment has overcome this instability of Fab’ fragments and has extended the plasma half-life of CZP to about 2 weeks. Due to the extended half-life, a CZP maintenance dosing schedule with a longer interval is possible. Our data demonstrate that patients treated at Q2W (CZP 200 mg) or Q4W (CZP 400 mg) intervals (Group III vs. Group IV) exhibited similar clinical responsiveness and safety to long-term CZP treatment. This is important as patients and physicians gain the flexibility of choosing between the two different dosing schedules based on their needs. In some patients, a Q4W schedule might decrease the number of doctor visits. In others, a Q2W schedule might be favorable to allow closer monitoring of disease symptoms.
The design of HIKARI-OLE study included patients who were previously on placebo during the DB phase of HIKARI study. To observe the effects of continuous CZP treatment throughout the combined DB and OLE phases of the study (80 weeks), an additional post-hoc analysis was performed on CZP-DB completers who received CZP during the DB phase. Restricting our data analysis to this population clearly showed that long-term CZP treatment sustained the clinical, functional, and radiographic efficacy of CZP against disease, even in the absence of non-MTX DMARDs (CZP monotherapy). Thus, we conclude that long-term treatment for up to 80 weeks is beneficial for a sustained positive response to CZP even when used as monotherapy.
MTX is a critical therapeutic component in the treatment of RA [Citation14,Citation19]. MTX co-treatment is recommended in patients who receive CZP therapy because the development of anti-CZP antibodies is lower in patients that are treated with CZP plus MTX compared to those treated with CZP as monotherapy (data not shown). However, it is not uncommon for patients to be intolerant to MTX therapy. In fact, it is said that ˜30% of patients receive biologics as monotherapy in the United States [Citation20,Citation21] and in European countries such as the UK [Citation22]. A recently reported meta-analysis demonstrated that a number of biologics including etanercept, adalimumab, and tocilizumab were effective as monotherapy in improving ACR20/ACR50/ACR70 response rates [Citation23]. With regards to CZP, data from the FAST4WARD study conducted in Europe and the United States showed that CZP monotherapy for 24 weeks effectively reduced the signs and symptoms of active RA in patients [Citation15]. Similarly, the HIKARI DB study conducted on Japanese patients showed that CZP administration without MTX (both as monotherapy and in combination with non-MTX DMARDs) significantly relieved RA symptoms and radiographic progression of disease [Citation16]. Our current post-hoc analysis investigated the clinical efficacy of CZP without MTX treatment over a ˜80-week period (28 weeks during the DB phase + > 52 weeks during the OLE phase) in CZP-DB completers. Our data support the long-term use of CZP for treatment of RA for sustaining clinical efficacy either as monotherapy or in combination with non-MTX DMARDs.
In summary, our data suggest that continuous CZP treatment provides long-term clinical, functional, and radiographic benefits either as monotherapy or in conjunction with non-MTX DMARDs in Japanese RA patients, who could not receive MTX. Remarkably, the efficacy of CZP is maintained as monotherapy until 80 weeks. Long-term treatment was well-tolerated with no new unexpected AEs observed. Both the Q2W and Q4W dosing schedules of CZP were similarly effective at sustaining the clinical response to CZP. One limitation of our study was that this was an OLE study and therefore was not blinded. However, we still believe that our data still suggest that long-term CZP treatment is beneficial for continued suppression of RA. Thus, we propose that patients who could not receive MTX should undergo continuous long-term treatment of CZP at either a Q2W or Q4W schedule to obtain long-lasting relief from RA symptoms.
Acknowledgments
This manuscript was funded by UCB. The authors acknowledge Tadao Okamoto, PhD, UCB, Tokyo, Japan, for publication management and Taku Kambayashi, MD, PhD, for editorial services. The authors also acknowledge all investigators from HIKARI-OLE study: Hokkaido University Hospital (T. Atsumi), Tomakomai City Hospital (A. Fujisaku), Oki Medical Clinic (I. Oki), Tohoku University Hospital (T. Ishii), Taga General Hospital (S. Ota), Inoue Hospital (H. Inoue), Saitama Medical Center (K. Amano), Jichi Medical University Hospital (T. Yoshio), Tsukuba University Hospital (T. Sumida), Chiba University Hospital (N. Watanabe), Yonemoto Orthopedic Clinic (K. Yonemoto), The University of Tokyo Hospital (K. Kawahata), Tokyo Medical and Dental University Hospital Faculty of Medicine (H. Kohsaka), Institute of Rheumatology Tokyo Women's Medical University (H. Yamanaka), Keio University Hospital (M. Kuwana), Juntendo University Hospital (Y. Takasaki), Toho University Ohashi Medical Center (T. Ogawa), Showa University Hospital (T. Kasama), Kyorin University Hospital (Y. Arimura), Fukuhara Hospital (A. Yamaguchi), Tokyo Metropolitan Ohtsuka Hospital (T. Yamada), Yokohama Rosai Hospital (Y. Kita), Tokai University Hospital (Y. Suzuki), Kitasato University Hospital (S. Hirohata), Gunma University Hospital (Y. Nojima), Nagoya University Hospital (T. Kojima), Nagoya Medical Center (A. Kaneko), Kondo Orthopedic and Rheumatology Clinic (K. Kondo), Ito Orthopedic Clinic (T. Ito), Fujita Health University Hospital (S. Yoshida), Nagoya City university Hospital (Y. Hayami), Toyama University Hospital (H. Taki), Saiseikai Takaoka Hospital (S. Honjo), Niigata Rheumatic Center (A. Murasawa), Tonami General Hospital (H. Yamada), Matsuno Clinic for Rheumatic Diseases (H. Matsuno), Kyoto University Hospital (T. Nojima), Osaka Minami Medical Center (Y. Saeki), Matsubara Mayflower Hospital (T. Matsubara), Sanin Rosai Hospital (H. Kishimoto), Higashi-Hiroshima Memorial Hospital (S. Yamana), Kurashiki Medical Clinic (Y. Yoshinaga), Hiroshima Clinic (K. Amano), Kagawa University Hospital (H. Dobashi), Tokushima University Hospital (J. Kishi), University of Occupational and Environmental Health, Hospital (Y. Tanaka), Kitakyushu Municipal Medical Center (H. Nishizaka), Kyushu University Hospital (T. Horiuchi), Kyushu Medical Center (H. Miyahara), Shono Rheumatism Clinic (E. Shono), Kondo Rheumatology and Orthopedic Clinic (M. Kondo), Kurume University Medical Center (T. Fukuda), Nagasaki University Hospital of Medicine and Dentistry (A. Kawakami), Nagasaki Medical Center (K. Migita), Sasebo Chuo Hospital (Y. Ueki), Nagasaki Medical Hospital of Rheumatology (M. Tsuboi), Nagasaki Atomic Bomb Hospital (M. Nakashima), Saga University Hospital (K. Nagasawa), Oribe Clinic of Rheumatism and Medicine (M. Oribe), Otsuka Clinic of Medicine and Rheumatism (E. Otsuka), Kumamoto Saishunso National Hospital (S. Mori), Kumamoto Orthopaedic Hospital (M. Sakaguchi), Center for Arthritis and Clinical Rheumatology Matsubara Clinic (S. Matsubara), Shimin-No-Mori Hospital (T. Hidaka), and Kagoshima Red Cross Hospital (T. Matsuda).
Conflict of interest
The competing interests of all authors are provided below.
Y. Tanaka has received research funding from BMS, MSD, Chugai, Mitsubishi-Tanabe, Astellas, Abbvie and Daiichi- Sankyo and has served on speaker bureaus for UCB, Mitsubishi-Tanabe, Abbott, Abbvie, Eisai, Chugai, Janssen, Pfizer, Takeda, Astellas, Daiichi-Sankyo, GSK, AstraZeneca, Eli Lilly, Quintiles, MSD and Asahi Kasei.
K. Yamamoto has served as a consultant for UCB, Pfizer, Abbott, BMS, Roche, Chugai, Mitsubishi-Tanabe and Eisai and has received research funding from UCB, Pfizer, Abbott, Santen, Mitsubishi-Tanabe and Eisai.
T. Takeuchi has served as a consultant for AstraZeneca, Eli Lilly, Novartis, Mitsubishi-Tanabe and Asahi Kasei, has received research support from Abott, Astellas, BMS, Chugai, Daiichi-Sankyo, Eisai, Janssen, Mitsubishi-Tanabe, Nippon Shinyaku, Otsuka, Pfizer, Sanofi-Aventis, Santen, Takeda and Teijin, and has served on speaker bureaus for Abbott, BMS, Chugai, Eisai, Janssen, Mitsubishi-Tanabe, Pfizer and Takeda.
H. Yamanaka has served as a consultant for, and received research funding from, UCB, Abbott, Astellas, BMS, Chugai, Eisai, Janssen, Mitsubishi-Tanabe, Pfizer and Takeda.
N. Ishiguro has received research funding from Takeda, Mitsubishi-Tanabe, Astellas, Chugai, Abbott, BMS, Eisai, Janssen, Kaken and Pfizer and has served on speaker bureaus for Takeda, Mitsubishi-Tanabe, Astellas, Chugai, Abbott, BMS, Eisai, Janssen, Kaken, Pfizer, Taisho-Toyama and Otsuka.
K. Eguchi has served as a consultant for UCB.
A. Watanabe has received research support from Daiichi-Sankyo, Kyorin, Shionogi, Taisho, Dainippon-Sumitomo, Taiho, Toyama Chemical and Meiji Seika and has served on speaker bureaus for MSD, GSK, Shionogi, Daiichi-Sankyo, Taisho-Toyama, Dainippon-Sumitomo, Mitsubishi-Tanabe and Pfizer.
H. Origasa has served as a consultant for UCB and Astellas.
T. Shoji is an employee of UCB.
N. Miyasaka has received research support from Pfizer, Takeda, Mitsubishi-Tanabe, Chugai, Abbott, Eisai and Astellas.
T. Koike has served on speaker bureaus for UCB, Pfizer, Chugai, Abbott, Mitsubishi-Tanabe, Takeda, Eisai, Santen, Astellas, Taisho-Toyama, BMS, Teijin and Daiichi-Sankyo.
References
- Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8(11):656–64.
- Maini RN, Brennan FM, Williams R, Chu CQ, Cope AP, Gibbons D, et al. TNF-alpha in rheumatoid arthritis and prospects of anti-TNF therapy. Clin Exp Rheumatol. 1993;11 (Suppl 8):S173–5.
- Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999; 130(6):478–86.
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35–45.
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50(4):1051–65.
- Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46(6):1443–50.
- Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400–11.
- Nesbitt A, Fossati G, Bergin M, Stephens P, Stephens S, Foulkes R, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007;13(11):1323–32.
- Horton S, Walsh C, Emery P. Certolizumab pegol for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2012;12(2):235–49.
- Keystone E, Heijde D, Mason D Jr, Landewé R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58(11):3319–29.
- Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68(6):797–804.
- Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate. Arthritis Rheum. in press.
- Morgan SL, Baggott JE. Folate supplementation during methotrexate therapy for rheumatoid arthritis. Clin Exp Rheumatol. 2010; 28(5 Suppl 61):S102–9.
- Benedek TG. Methotrexate: from its introduction to non-oncologic therapeutics to anti-TNF-alpha. Clin Exp Rheumatol. 2010;28(5 Suppl 61):S3–8.
- Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68(6):805–11.
- Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and safety of certolizumab pegol without methotrexate co-administration in Japanese patients with active rheumatoid arthritis: the HIKARI randomized, placebo-controlled trial. Mod Rheumatol. 2013; in press.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
- Benucci M, Saviola G, Manfredi M, Sarzi-Puttini P, Atzeni F. Tumor necrosis factors blocking agents: analogies and differences. Acta Biomed. 2012;83(1):72–80.
- Shankar S, Handa R. Biological agents in rheumatoid arthritis. J Postgrad Med. 2004;50(4):293–9.
- Yazici Y, Shi N, John A. Utilization of biologic agents in rheumatoid arthritis in the United States: analysis of prescribing patterns in 16,752 newly diagnosed patients and patients new to biologic therapy. Bull NYU Hosp Jt Dis. 2008;66(2):77–85.
- Lee SJ, Chang H, Yazici Y, Greenberg JD, Kremer JM, Kavanaugh A. Utilization trends of tumor necrosis factor inhibitors among patients with rheumatoid arthritis in a United States observational cohort study. J Rheumatol. 2009;36(8):1611–7.
- Soliman MM, Ashcroft DM, Watson KD, Lunt M, Symmons DP, Hyrich KL. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(4):583–9.
- Orme ME, Macgilchrist KS, Mitchell S, Spurden D, Bird A. Systematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying antirheumatic drug-experienced patients with rheumatoid arthritis: analysis of American College of Rheumatology criteria scores 20, 50, and 70. Biologics. 2012;6:429–64.
