Abstract
Objective: To evaluate the safety and efficacy of golimumab + methotrexate (MTX) in Japanese patients with active rheumatoid arthritis (RA).
Methods: Japanese patients with active RA despite MTX were randomized to placebo + MTX (Group 1, n = 88), golimumab 50 mg + MTX (Group 2, n = 86), or golimumab 100 mg + MTX (Group 3, n = 87). Patients with <20% improvement in swollen/tender joint counts entered early escape at week 16. At week 24, all remaining placebo patients crossed over to golimumab 50 mg. Efficacy assessments included ACR20, DAS28-ESR, and HAQ-DI. Radiographic progression was assessed with the van der Heijde-modified Sharp (vdH-S) score.
Results: ACR20 response rates in Group 1, Group 2, and Group 3 were 67.9, 86.1, and 82.4%, respectively, at week 52 and were maintained through week 104 (87.1, 94.0, and 88.7%) and week 156 (97.1, 94.1, and 89.5%). Proportions of patients with good/moderate DAS28-ESR response or clinically meaningful improvement in HAQ-DI were also maintained through week 156. The majority of patients did not experience radiographic progression through week 156. Among 257 golimumab-treated patients, 251 (97.7%) had ≥1 AE; 54 (21.0%) had ≥1 serious AE through week 156. Infections were the most common type of AE.
Conclusions: Response to golimumab + MTX was maintained over 3 years in Japanese patients with active RA despite MTX. Safety results were consistent with the known safety profile of golimumab.
Introduction
It is estimated that approximately 0.6–1.0% of the Japanese population has rheumatoid arthritis (RA), a chronic inflammatory joint disease [Citation1]. The joint destruction that is characteristic of RA can lead to a substantial loss of physical function [Citation2]. In addition, patients with RA often experience extra-articular manifestations including pulmonary, kidney, and cardiovascular disease [Citation3,Citation4]. Timely and adequate treatment for RA with the goal of low disease activity or remission may prevent long-term radiographic damage and disability [Citation5–7].
Golimumab is a human monoclonal antibody that binds to and blocks the effects of tumor necrosis factor α (TNFα), one of the key inflammatory cytokines involved in the pathogenesis of RA. The safety and efficacy of subcutaneous (SC) golimumab with and without methotrexate (MTX) were evaluated in three large global trials of patients with RA who were MTX-naïve (GO-BEFORE) [Citation8], MTX-experienced (GO-FORWARD) [Citation9], or had been previously treated with anti-TNF agents (GO-AFTER) [Citation10]. The Phase 2/3 GO-FORTH trial further evaluated the safety and efficacy of SC golimumab plus MTX in Japanese patients with active RA despite treatment with MTX [Citation11]. Through 24 weeks of the GO-FORTH trial, patients treated with golimumab plus MTX had significantly greater improvements in the signs and symptoms of RA and less radiographic progression than did patients who received MTX monotherapy [Citation11]. We now report the final safety and efficacy results from the GO-FORTH trial through 156 weeks.
Materials and methods
Patients
The patient eligibility criteria have been previously reported in detail [Citation11]. Briefly, Japanese patients with active RA despite prior MTX therapy were eligible. Active RA was defined as at least four swollen joints (0–66) and at least four tender joints (0–68) and at least two of the following criteria: (1) C-reactive protein (CRP) > 1.5 mg/dL or erythrocyte sedimentation rate (ESR) by Westergren method > 28 mm/h, (2) morning stiffness ≥ 30 min, (3) radiographic evidence of bone erosion, or (4) positive test for anti-cyclic citrullinated peptide antibody or rheumatoid factor. Patients had to have been receiving a stable dose of MTX (6–8 mg/week) for at least 4 weeks prior to the first study agent administration.
Study design
The GO-FORTH trial was a Phase 2/3, multicenter, randomized, placebo-controlled trial (ClinicalTrials.gov NCT00727987). Details of the trial design have been previously reported [Citation11]. Briefly, eligible patients were randomly assigned (1:1:1) to receive placebo plus MTX (Group 1), golimumab 50 mg plus MTX (Group 2), or golimumab 100 mg plus MTX (Group 3). Subcutaneous placebo and golimumab injections were administered at baseline and every 4 weeks thereafter. All patients were to continue receiving concomitant MTX at a dose of 6–8 mg/week, with dose adjustments permitted only when required for cases of MTX toxicity. At week 16, patients with <20% improvement from baseline in swollen and tender joint counts entered early escape in a double-blinded fashion such that patients in Group 1 switched from placebo to golimumab 50 mg, and patients in Group 2 had their golimumab dose increased to 100 mg. No changes in golimumab dose were permitted for patients in Group 3 regardless of early escape status. At week 24, all patients in Group 1 who were still receiving placebo crossed over to golimumab 50 mg. After week 52, patients who were receiving golimumab 100 mg could have their dose reduced to 50 mg at the investigator’s discretion. The final golimumab administration was at week 152.
This study was performed in accordance with the principles in the Declaration of Helsinki and Good Clinical Practice guidelines in Japan. The protocol was reviewed by the institutional review board or ethics committee at each site. All patients gave written informed consent before any study-related procedures were performed.
Assessments
The primary endpoint was the proportion of patients with ≥20% improvement from baseline in the American College of Rheumatology criteria (ACR20) at week 14. Other clinical efficacy assessments included the ACR-N Index of Improvement and the proportions of patients with ACR50 and ACR70 responses, a good or moderate response [Citation12] using the 28-joint count Disease Activity Score using ESR (DAS28-ESR), or DAS28-ESR remission (<2.6). Physical function was assessed using the Health Assessment Questionnaire-Disability Index (HAQ-DI) [Citation13], with a minimal clinically important difference defined as an improvement of at least 0.25 [Citation14]. Efficacy assessments were collected through week 156.
Radiographs of the hands and feet were collected at weeks 0, 52, 104, and 156 and were scored using the van der Heijde modification of the Sharp (vdH-S) score [Citation15]. The smallest detectable change (SDC) was determined using the standard deviation for the difference in absolute change in the vdH-S scores between the readers. Readers were blinded to patient identity, treatment group, and time point at which the radiograph was obtained. An exploratory post-hoc analysis was performed to compare baseline characteristics of patients who did and did not have a clinically relevant progression in total vdH-S score (i.e., change in vdH-S ≥ 3) [Citation16] from baseline to week 104. This analysis was not performed at week 156 due to the small patient numbers in each group at that time point.
In a post-hoc analysis, remission rates were determined through week 156 using the simplified disease activity index (SDAI ≤ 3.3) [Citation17], clinical disease activity index (CDAI ≤ 2.8) [Citation18], and Boolean criteria [Citation17]. Comprehensive remission (defined as DAS28-ESR < 2.6, HAQ-DI < 0.5, and change in vdH-S ≤ 0) was also evaluated as a post-hoc analysis. In addition, a Pearson correlation analysis was performed to examine the relationship between clinical response at week 12 (DAS28-ESR, DAS28-CRP, SDAI, and CDAI) and response at week 104.
Safety assessments were performed through week 156 and included adverse event (AE) reporting and laboratory tests. Blood samples were collected at weeks 0, 24, 52, 104, and 156 for evaluation of the presence of antibodies to golimumab [Citation19]; if patients discontinued treatment, samples were collected at the final visit and at 8 and 12 weeks after the last golimumab administration. On days when study agent was administered, blood samples were collected before the golimumab administration.
Statistical analysis
Clinical efficacy, including the post-hoc remission analyses, and radiographic results from week 52 to week 156 were summarized using descriptive statistics according to randomized treatment group. For clinical efficacy and radiographic outcomes, observed data were reported with no imputation for missing data, and no adjustments were made for early escape status at week 16. As all patients were receiving golimumab + MTX after week 24, no statistical comparisons were performed among the treatment groups.
Adverse events were summarized by actual treatment received (i.e., placebo plus MTX, golimumab 50 mg plus MTX, or golimumab 100 mg plus MTX). Due to the planned crossover from placebo to golimumab and the early escape design, some patients may be included in more than one group if they experienced AEs with more than one treatment regimen.
Results
Baseline characteristics and patient disposition
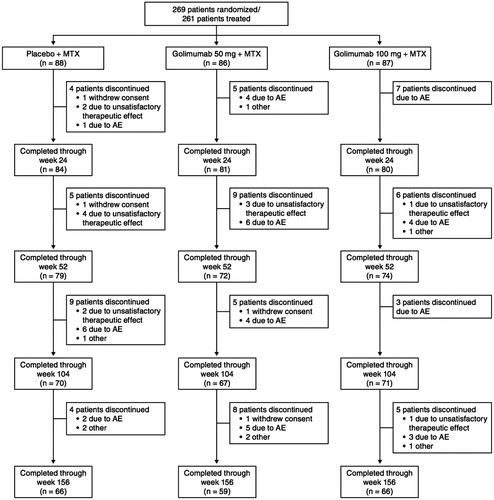
Baseline demographics and disease characteristics were generally well balanced among the three treatment groups and have been previously reported [Citation11]. Of the 269 patients who were randomized, 261 (Group 1, n = 88; Group 2, n = 86; Group 3, n = 87) received at least one dose of study agent. Patient disposition through week 24 has been previously described in detail [Citation11]. At week 16, 28 patients in Group 1 and 9 patients in Group 2 entered early escape. A total of 245 patients (Group 1, n = 84; Group 2, n = 81; Group 3, n = 80) completed study treatment through week 24 [Citation11], and 225, 208, and 191 completed the study through weeks 52, 104, and 156, respectively (). Due to local regulations, the trial was discontinued when golimumab was approved by the Ministry of Health, Labor and Welfare in Japan. Patients for whom the study was terminated before the week 156 visit (Group 1, n = 32; Group 2, n = 25; Group 3, n = 28) were counted as having completed the study; however, they did not have available observed data at the final time point. Thus, at week 156, 66 patients in Group 1, 59 patients in Group 2, and 66 patients in Group 3 were counted as having completed the study, and among these, 34, 34, and 38 patients, respectively, had available data at the final visit (week 156).
Throughout the study, 70 patients (Group 1, n = 22; Group 2, n = 27; Group 3, n = 21) discontinued golimumab therapy. The most common reasons for discontinuation were AEs (n = 45/261 [17.2%]) and unsatisfactory therapeutic effect (n = 13/261 [5.0%]).
Efficacy
As previously reported, the primary endpoint of the study (ACR20 response at week 14) was met, and patients in Groups 2 and 3 also had significantly greater ACR50 and ACR70 response rates than did patients in Group 1 [Citation11]. ACR response rates in Group 1 increased following crossover from placebo to golimumab, and were similar to those in Groups 2 and 3 at weeks 52, 104, and 156 (). ACR20 response rates in Group 1, Group 2, and Group 3 were 67.9, 86.1, and 82.4%, respectively, at week 52, and responses were maintained through week 104 (87.1, 94.0, and 88.7%, respectively) and week 156 (97.1, 94.1, and 89.5%, respectively; ). A similar result was observed for ACR50 and ACR70 response rates through week 156 (). Similar trends were also observed for ACR-N scores and changes from baseline in DAS28-ESR scores as well as the proportions of patients with a moderate or good DAS28-ESR response and DAS28-ESR remission (). The proportions of patients in remission through week 24, using the SDAI, CDAI, and Boolean criteria, were numerically greater in Groups 2 and 3 than in Group 1 (). The proportions of patients in remission were similar among the treatment groups at week 52 and were maintained through week 156. In addition, comprehensive remission was achieved at week 52 by 16 (19.8%) patients in Group 1, 18 (25.0%) patients in Group 2, and 18 (24.3%) patients in Group 3 (). Among these patients, 11 in Group 1, 13 in Group 2, and 10 in Group 3 maintained comprehensive remission at week 104 (data not shown). No significant correlations were observed between clinical response at week 12 and response at week 104 (data not shown).
Figure 2. The proportions of patients* with an ACR20 (A), ACR50 (B), and ACR70 (C) response through week 156. *Observed data without imputation. ACR20/50/70, ≥ 20%/50%/70% improvement in American College of Rheumatology criteria; MTX, methotrexate.
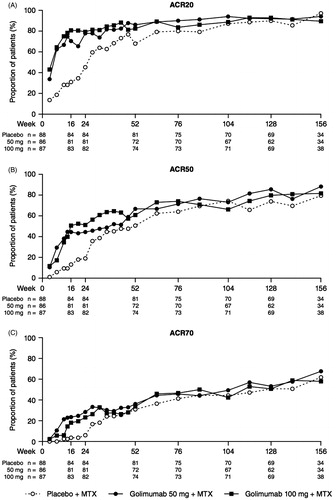
Figure 3. The proportions of patients* with remission as defined by the CDAI (A), SDAI (B), and Boolean (C) criteria through week 156. *Observed data without imputation. CDAI, clinical disease activity index; MTX, methotrexate; SDAI, simplified disease activity index.
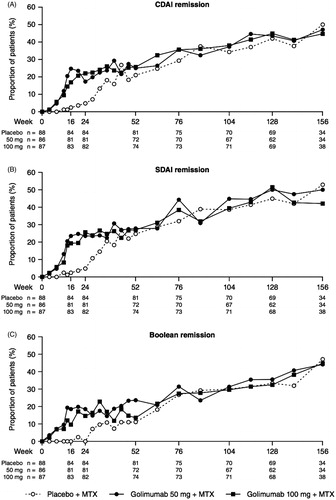
Table 1. Clinical efficacy results at weeks 52, 104, and 156.
At week 24, patients randomized to Groups 2 and 3 had greater mean improvements from baseline in HAQ-DI score when compared with those in Group 1, and greater proportions of these patients had an improvement in HAQ-DI score of at least 0.25 [Citation11]. Physical function continued to improve in all three groups after week 24 when all patients were receiving golimumab plus MTX, and these improvements were maintained through weeks 52, 104, and 156 ().
The mean changes from baseline in total vdH-S score in Groups 1, 2, and 3 at weeks 52, 104, and 156 are shown in . These changes appeared to be smaller in Groups 2 (1.6) and 3 (0.6) than in Group 1 (2.0) at week 52; although no statistical testing between treatment groups was conducted at this time point. The mean changes for Groups 1, 2, and 3 were 1.5, 2.3, and 1.6, respectively, at week 104 and −0.2, 4.1, and 1.7, respectively, at week 156. The median change from baseline in all three groups was 0 at weeks 52, 104, and 156, indicating that half of all patients had minimal radiographic progression. The proportions of patients in each treatment group who had a change from baseline in total vdH-S score greater than the SDC ranged from 9.6% to 17.9% at week 52, from 10.1% to 16.4% at week 104, and from 9.1% to 20.6% at week 156 (), with numerically greater proportions observed in Groups 2 and 3 when compared with Group 1 at weeks 104 and 156. Similar trends among the treatment groups were seen for changes in total vdH-S score from week 52 to week 104 and from week 104 to week 156 ().
Table 2. Radiographic results through 156 weeks.
An exploratory analysis showed that patients with clinically relevant radiographic progression (i.e., a change in total vdH-S ≥ 3) from baseline to week 104 generally had higher disease activity and a higher annual rate of radiographic progression at baseline when compared with patients who had a change in total vdH-S < 3 (Supplemental Table S1).
Safety
Adverse events among patients receiving placebo plus MTX (up to week 24) have been previously reported in detail [Citation11]. A total of 257 patients received at least one administration of golimumab through week 156. Through weeks 52 and 104, 89.1% (n = 229/257) and 96.9% (n = 249/257) of golimumab-treated patients had at least one AE, and 52.1 and 69.3%, respectively, had an AE in the system organ class (SOC) of infections and infestations. The cumulative proportion of patients with at least one AE through week 156 was 95.9% among patients who received the 50-mg dose and 99.0% among those receiving the 100-mg dose (). The most commonly reported AEs among golimumab-treated patients were in the SOC of infections and infestations (). The most common infections among golimumab-treated patients were nasopharyngitis (50 mg, n = 82 [48.2%]; 100 mg, n = 50 [52.1%]) and pharyngitis (50 mg, n = 26 [15.3%]; 100 mg, n = 18 [18.8%]).
Table 3. Adverse events through 156 weeks.
The cumulative proportions of patients with at least one serious AE (SAE) through week 156 were similar for the two golimumab doses (50 mg, n = 36 [21.2%]; 100 mg, n = 19 [19.8%]; all golimumab plus MTX, n = 54 [21.0%]). There was no significant difference in the adjusted incidence of SAEs per 100 patient-years for the two golimumab doses (). Serious infections were reported by 12 (7.1%) and 7 (7.3%) patients receiving golimumab 50 and 100 mg, respectively, with the most common being gastroenteritis (50 mg, n = 2), herpes zoster (100 mg, n = 2), and pneumonia (100 mg, n = 2). There were no cases of tuberculosis.
Six malignancies in six patients were reported. Uterine cancer (n = 1), extranodal marginal zone B-cell lymphoma (n = 1), testicular neoplasm (n = 1), diffuse large B-cell lymphoma (n = 1), and colon cancer (n = 1) occurred in patients receiving golimumab 50 mg, and one case of breast cancer was reported in a patient receiving golimumab 100 mg. One death occurred during the trial (community-acquired pneumonia and amyloidosis) in a patient receiving golimumab 100 mg plus MTX.
Injection site reactions were reported by seven (8.0%) patients receiving placebo plus MTX and 54 (21.0%) patients receiving golimumab plus MTX (50 mg, n = 30 [17.6%]; 100 mg, n = 24 [25.0%]). The most common injection site reaction was erythema (placebo, n = 4 [4.5%]; 50 mg, n = 19 [11.2%]; 100 mg, n = 20 [20.8]). All injection site reactions were considered to be mild, and no patient discontinued the study agent due to an injection site reaction. No anaphylactic reactions or serum sickness-like reactions occurred throughout the trial.
Immunogenicity
A total of 257 patients received at least one administration of golimumab and had at least one post-golimumab treatment serum sample for the analysis of antibodies to golimumab. Two (0.8%) patients (Group 1, n = 1; Group 3, n = 1) tested positive for antibodies to golimumab; both patients had an antibody titer of 1:20.
Discussion
Through 24 weeks of the GO-FORTH trial, Japanese patients with active RA despite prior MTX therapy had significantly greater improvements in the signs and symptoms of RA when treated with golimumab 50 mg or 100 mg plus MTX compared with MTX monotherapy [Citation11]. These improvements were maintained through weeks 52, 104, and 156, the final efficacy evaluation. At week 104, 94% of patients in Group 2 and 89% of patients in Group 3 had an ACR20 response; these rates were further maintained at week 156. Through 3 years, response rates for ACR and DAS28-ESR outcomes and improvements in physical function in Group 2 were generally comparable to or slightly higher than those in Group 3.
Golimumab plus MTX-treated patients also had less radiographic progression through 24 weeks when compared with those receiving MTX monotherapy [Citation11]. Throughout the trial, radiographic progression was inhibited in all treatment groups, with a median change from baseline in total vdH-S score of 0 at weeks 52, 104, and 156. Mean increases in vdH-S scores tended to be smaller in Group 3 than in Group 2; however, no formal statistical comparisons were performed between the two golimumab dose groups. In addition, most patients did not experience progression when assessed using the SDC from baseline to weeks 52, 104, and 156, from week 52 to week 104, or from week 104 to week 156.
At week 52, the proportion of patients with an increase from baseline in vdH-S score that was greater than the SDC was numerically lower among patients who had received golimumab from week 0 (Groups 2 and 3) than in patients initially randomized to placebo (Group 1); however, this trend was reversed at week 104. Although the proportion of patients with radiographic progression greater than the SDC appeared to increase from week 52 to week 104 in Groups 2 and 3, and was numerically greater than that observed in Group 1, the actual number of patients in Groups 2 and 3 who were classified as progressors remained stable during this time period. In contrast, the proportion of patients in Group 1 with progression from baseline greater than the SDC decreased from 17.9% at week 52 to 10.1% at week 104, suggesting that the inhibition of radiographic progression in Group 1 patients was continuing to stabilize following early escape/crossover from placebo plus MTX to golimumab plus MTX. In an exploratory analysis, patients with higher levels of disease activity and radiographic progression at baseline generally had more radiographic progression at week 104 than did patients with less active disease at baseline. This is consistent with a previous analysis of progressors and non-progressors [Citation20] and further supports the concept that earlier treatment of RA may result in prevention of long-term radiographic damage [Citation7].
Adverse events reported during the trial were consistent with those of the known safety profile of golimumab [Citation21–23]. Of the 257 patients included in the safety analysis, 97.7% reported an AE through week 156, the majority of which were considered to be mild, and no disproportionate increase in the cumulative incidence of AEs was observed over time among golimumab-treated patients. Infections were the most commonly reported AE, with nasopharyngitis being the most common. All injection site reactions were considered mild, and none led to discontinuation from treatment. There were no cases of anaphylactic reactions, or serum sickness-like reactions. Approximately 7% of golimumab-treated patients had a serious infection through week 156, which was similar to the cumulative rates reported through 2 years of the global GO-BEFORE [Citation21] and GO-FORWARD [Citation23] golimumab trials and that observed through 3 years in a pooled analysis of golimumab-treated patients with RA, ankylosing spondylitis, and psoriatic arthritis in five phase 3 trials [Citation22]. There were no cases of tuberculosis during the trial. Approximately 21% of golimumab-treated patients experienced at least one SAE through week 156, which was generally consistent with previous studies of golimumab in patients with rheumatic diseases [Citation22]. There was no significant difference between the two golimumab doses in the incidence of SAEs per 100 patient-years. Six malignancies were reported, including breast cancer, colon cancer, extranodal marginal zone B-cell lymphoma, and diffuse large B-cell lymphoma. The observed incidence of malignancies in golimumab-treated patients through 3 years was comparable to that observed in a pooled analysis of golimumab-treated patients with rheumatologic diseases [Citation22]. One death occurred (community-acquired pneumonia and amyloidosis) in a patient receiving golimumab 100 mg plus MTX.
As in other studies, the long-term results of the GO-FORTH study are limited by selection bias over time and the lack of a control group, and the use of observed data may overestimate the proportion of patients in response over time. In addition, changes to golimumab dose after week 52 and the small number of patients with available data at week 156 limit the interpretation of the results. As previously noted [Citation11], although the MTX dose used in the trial was consistent with the approved dose of MTX in Japan at the time the trial was planned, this dose was generally lower than doses commonly used for RA in other regions. No unexpected safety events were observed; however, this study was not powered to detect rare safety events.
In conclusion, the results demonstrate that treatment with golimumab 50 or 100 mg in combination with MTX provided sustained efficacy with no unexpected safety findings through 3 years of therapy in MTX-experienced Japanese patients with active RA.
Supplementary material available online
1109762_Supplemental_Table_1.pdf
Download PDF (79.2 KB)Acknowledgments
The authors thank Rebecca E. Clemente, PhD, and Mary Whitman, PhD, of Janssen Scientific Affairs, LLC, for their writing support.
Conflicts of interest
Y. T. has received research grants from AbbVie, Asahi-kasei, Astellas, Bristol-Myers Squibb K.K., Chugai, Eisai, Kyowa-Kirin, Mitsubishi-Tanabe, Takeda, and Taisho Toyama, and consulting fees from AbbVie, Asahi-kasei, Astellas, Bristol-Myers Squibb K.K., Chugai, Daiichi Sankyo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical K.K., Mitsubishi Tanabe, MSD, Pfizer Japan, Takeda, Teijin, and Santen. M. H. has received research grants from Abbvie Japan, Astellas, Bristol-Myers Squibb K.K., Chugai, Eisai, Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceuticals, Pfizer Japan, Sanofi-Aventis K.K., Santen Pharmaceutical, Takeda, Teijin Pharma, and UCB Japan, and consulting fees from AbbVie, Bristol-Myers Squibb, Chugai Pharmaceutical, Janssen Pharmaceutical K.K., and Teijin Pharma. T. T. has received research grants from AbbVie, Asahi-kasei Pharma Corp., Astellas Pharma, Bristol-Myers Squibb K.K., Chugai Pharmaceutical, Daiichi Sankyo Co. Ltd, Eisai, Mitsubishi Tanabe, Pfizer Japan, Santen Pharmaceutical, Symbio Pharmaceuticals, Taisho Toyama Pharmaceutical, Takeda Pharmaceutical Co., and Teijin Pharma; consulting fees from AbbVie, Asahi-kasei Pharma Corp., AstraZeneca, Bristol-Myers Squibb K.K., Daiichi Sankyo Co. Ltd, Eli Lilly Japan K.K., Mitsubishi Tanabe, Nippon Kayaku, and Novartis Pharma K.K., and speaking fees from AbbVie, Astellas Pharma, Bristol-Myers Squibb K.K., Celltrion, Chugai Pharmaceutical, Daiichi Sankyo Co. Ltd, Eisai, Janssen Pharmaceutical K.K., Mitsubishi Tanabe, Nippon Kayaku, Novartis Pharma K.K., and Takeda Pharmaceutical Co. H. Y. has received research grants from AbbVie, Asahi-kasei, Astellas Pharma, Astra Zeneca, Bristol-Myers Squibb K.K., Chugai, Daiichi Sankyo, Eisai, Janssen Pharmaceutical K.K., GlaxoSmithKline, Mitsubishi Tanabe Pharma Corporation, Nippon Kayaku, Pfizer Japan, Santen, Taisho Toyama, Takeda Pharmaceutical Co., and Teijin Pharma; consulting fees from AbbVie, Astellas Pharma, Astra Zeneca, Bristol-Myers Squibb K.K., Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Nippon Kayaku, Pfizer Japan, Takeda Pharmaceutical Co., and Teijin Pharma; and speaking fees from AbbVie, Astellas Pharma, Bristol-Myers Squibb K.K., Chugai, Eisai, Mitsubishi Tanabe, Pfizer Japan, Takeda Pharmaceutical Co., and Teijin Pharma. N. I. has received research grants from Astellas Pharmaceutical, AbbVie, Bristol-Myers Squibb K.K., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo, Eisai Pharmaceutical, Janssen Pharmaceutical K.K., Kaken, Mitsubishi Tanabe Pharma Corporation, Pfizer, and Takeda, and speaking fees from Astellas, AbbVie, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Hisamitsu, Janssen, Kaken, Mitsubishi Tanabe, Otsuka Pharmaceutical Company, Pharma Corporation, Pfizer Japan, Takeda, Taisho Toyama, and UCB. K. Y. has received research support from AbbVie, Astellas, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Janssen Pharmaceutical K.K., Mitsubishi Tanabe, Pfizer Japan, Sanofi, Santen, Takeda, and Teijin. N. M. has received research support from AbbVie, Astellas, Chugai, Eisai, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Teijin, and Takeda. T. K. has received speaking fees from AbbVie, Astellas, Bristol-Myers Squibb, Chugai, Eisai, Mitsubishi Tanabe Pharma Corporation, MSD KK, Pfizer, Takeda, and UCB and research support from Janssen Pharmaceutical K.K. D. B. is an employee of Janssen Research & Development, LLC. Y. I. is an employee of Janssen Pharmaceutical K.K. T. Y. is an employee of Mitsubishi Tanabe Pharma Corporation. This trial was funded by Janssen Research & Development, LLC., Janssen Pharmaceutical K.K., and Mitsubishi Tanabe Pharma Corporation.
References
- Yamanaka H, Sugiyama N, Inoue E, Taniguchi A, Momohara S. Estimates of the prevalence of and current treatment practices for rheumatoid arthritis in Japan using reimbursement data from health insurance societies and the IORRA cohort (I). Mod Rheumatol. 2014;24:33–40.
- Welsing PM, van Gestel AM, Swinkels HL, Kiemeney LA, van Riel PL. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001;44:2009–17.
- Magnano MD, Genovese MC. Management of co-morbidities and general medical conditions in patients with rheumatoid arthritis. Curr Rheumatol Rep. 2005;7:407–15.
- Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907–27.
- Baumgartner SW, Fleischmann RM, Moreland LW, Schiff MH, Markenson J, Whitmore JB. Etanercept (Enbrel) in patients with rheumatoid arthritis with recent onset versus established disease: improvement in disability. J Rheumatol. 2004;31:1532–7.
- Lillegraven S, van der Heijde D, Uhlig T, Kvien TK, Haavardsholm EA. What is the clinical relevance of erosions and joint space narrowing in RA? Nat Rev Rheumatol. 2012;8:117–20.
- Quinn MA, Emery P. Window of opportunity in early rheumatoid arthritis: possibility of altering the disease process with early intervention. Clin Exp Rheumatol. 2003;21:S154–7.
- Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, et al. Golimumab, a human anti-tumor necrosis factor a monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272–83.
- Keystone E, Genovese MC, Klareskog L, Hsia EC, Hall S, Miranda PC, et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann Rheum Dis. 2010;69:1129–35.
- Smolen JS, Kay J, Doyle MK, Landewe R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor a inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210–21.
- Tanaka Y, Harigai M, Takeuchi T, Yamanaka H, Ishiguro N, Yamamoto K, et al. Golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis: results of the GO-FORTH study. Ann Rheum Dis. 2012;71:817–24.
- van Riel PL, van Gestel AM, Scott DL. EULAR handbook of clinical assessments in rheumatoid arthritis. Alphen Aan Den Rijn, The Netherlands: Van Zuiden Communications, BV; 2000.
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45.
- Lubeck DP. Patient-reported outcomes and their role in the assessment of rheumatoid arthritis. Pharmacoeconomics. 2004;22:27–38.
- van der Heijde DM, van Leeuwen MA, van Riel PL, Koster AM, van‘t Hof MA, van Rijswijk MH, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35:26–34.
- Bruynesteyn K, van der Heijde D, Boers M, Saudan A, Peloso P, Paulus H, et al. Detecting radiological changes in rheumatoid arthritis that are considered important by clinical experts: influence of reading with or without known sequence. J Rheumatol. 2002;29:2306–12.
- Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63:573–86.
- Aletaha D, Nell VPK, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:R796–806.
- Kay J, Matteson EL, Dasgupta B, Nash P, Durez P, Hall S, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58:964–75.
- Jansen LM, van der Horst-Bruinsma IE, van Schaardenburg D, Bezemer PD, Dijkmans BA. Predictors of radiographic joint damage in patients with early rheumatoid arthritis. Ann Rheum Dis. 2001;60:924–7.
- Emery P, Fleischmann RM, Doyle MK, Strusberg I, Durez P, Nash P, et al. Golimumab, a human anti-tumor necrosis factor monoclonal antibody, injected subcutaneously every 4 weeks in patients with active rheumatoid arthritis who had never taken methotrexate: 1-year and 2-year clinical, radiologic, and physical function findings of a phase III, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res (Hoboken). 2013;65:1732–42.
- Kay J, Fleischmann R, Keystone E, Hsia EC, Hsu B, Mack M, et al. Golimumab 3-year safety update: an analysis of pooled data from the long-term extensions of randomised, double-blind, placebo-controlled trials conducted in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. Ann Rheum Dis. 2015;74:538–46.
- Keystone EC, Genovese MC, Hall S, Miranda PC, Bae SC, Palmer W, et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: results through 2 years of the GO-FORWARD study extension. J Rheumatol. 2013;40:1097–103.