When it comes to thinking about musculoskeletal imaging, traditionally beset with techniques as conventional radiography (CR) and computerized tomography (CT), the scenery has dramatically changed. Particularly in rheumatology, an explosion in the use of ultrasonography (US) and magnetic resonance imaging (MRI) has been observed in recent years. The common interest of both clinical and academic rheumatologists in US seems to be driven by various perspectives. From the setting of the daily practice of the clinical rheumatologist, the technology is easy to employ at the point-of-care, versatile, patient friendly, radiation free, and inexpensive. For the academic rheumatologist, US may serve as an interesting outcome measure centered at the heart of the rheumatic process, which is all about inflammation. Both MRI and US are able to visualize the inflammatory process at the level of joints and tendons synovia, whereas, in contrast, radiographic progression seems to have fallen out of line with modern outcomes as erosive disease becomes more and more a phenomenon of the past. At least in theory, US may be able to accurately classify or diagnose, to prognosticate, or to sensitively measure inflammatory response of the synovial membrane over time.
In keeping with these views, US has attracted attention as a potential tool for clinical decision-making in patients with rheumatoid arthritis (RA), who are treated with biologicals [Citation1,Citation2]. When clinical judgment fails to provide the complete answer, including the US assessment of synovitis as a part of the clinical decision-making algorithm for tapering biological therapy makes sense. Using US criteria to define remission in RA patients on biologicals, Marks et al. [Citation3] have shown that it is possible to identify patients who may be suitable for TNF dose reduction. However, the question is, what premium has US to offer compared to a thorough clinical examination? Can US make the distinction between biologic drug users who are likely to flare and those who will not following dose reduction? Synovitis detected by power Doppler was the strongest predictor of failed biologic therapy tapering in RA patients in sustained clinical remission [Citation4]. The jury is still out.
Next comes the issue of diagnosing RA at an early or too early stage. The new ACR/EULAR criteria for RA (2010) are based on the identification of clinical synovitis and radiographic bone damage. Yet there is a substantial number at risk who do not fulfill these criteria, e.g. those patients who have arthralgias and positive rheumatic factor or anti-CCP antibodies. Including US for the assessment of synovitis makes sense, especially after it was shown that early institution of MTX can lead to better clinical outcomes in RA, in terms of survival, function, and quality of life [Citation5].
Unfortunately, all that glitters is not gold. Specific pitfalls of ultrasound are its subjectivity in interpretation of images, the lack of standardization of the acquisition of images, and the considerable amount of training the technique requires. Since 2004, an US special interest group under the umbrella of Outcome Measures in Rheumatology (OMERACT), an international informal platform striving to improve outcome measures in rheumatology, is working on the applicability of this technique for measuring outcome measures in clinical trials. For selection of an outcome measure, e.g. synovitis, the measure has to pass a 3-way filter consisting of the fundamental principles of truth (validity), discrimination (responsiveness), and feasibility [Citation6].
The OMERACT US definition of the composite entity synovitis states that there are several components to it, i.e. effusion, synovial hypertrophy, and power Doppler positivity [Citation7]. The definition comes, however, with all sorts of snags to be sorted out. For instance, any amount of fluid or hypoechoic thickening in a healthy subject is regarded pathologic. Among ultrasonographers, it is well known that the first MTP joint in non-arthritic persons frequently shows a proximal dorsal effusion on US. Still another report mentioned false-positivity of synovitis in the MCP joints of healthy persons [Citation8]. They scanned for the so called distal anechoic area in the second MCP joint of 24 non-arthritic persons, resulting in a prevalence of 54%. As they performed 3D US and dissected two postmortem specimens, the distal anechoic area appeared not to be an US artifact but a distal extension of the joint, i.e. a recess [Citation8]. Miscellaneous factors often forgotten are incorrect position of the probe and incomplete examination, i.e. one has to scan all around the structure of interest [Citation9].
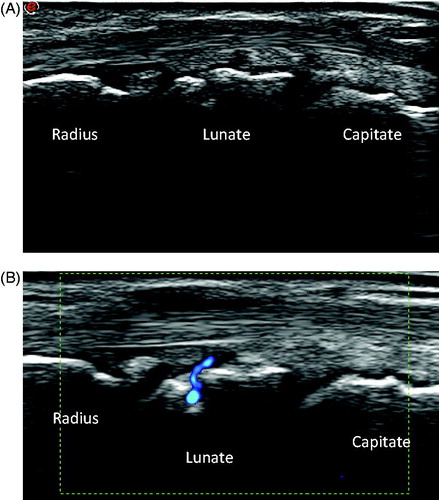
In Doppler US, a variety of causes of false positivity are well known. The most notorious are intra-articular vessels, normal feeding vessels () and reverberation of superficial vessels on the bone, i.e. so called mirroring. In addition, 11% of non-arthritic joints may show Doppler signals [Citation10]. Modern US machines are equipped with highly sensitive Doppler modalities detecting blood flow at very low levels. Flow per se does not necessarily imply inflamed synovium [Citation11], as has been demonstrated by the presence of Doppler signals in healthy joints. With increasing sensitivity of modern machines, we have to become aware of increased vascularization in otherwise normal synovium.
Figure 1. Dorsal longitudinal ultrasound scan of the wrist of a healthy person. Clearly seen is a positive power Doppler signal superficial to and entering the lunate bone. The Doppler signal is due to a feeding vessel; the signal can easily be mistaken for synovitis or erosion.
In this issue of the journal (http://dx.doi.org/10.3109/14397595.2015.1091123), Ikeda et al. report a comprehensive evaluation of potential causes of false positive US scans. Their important findings shed light on the complexity of US assessment of synovial inflammation. Previously, they showed that US assessment of mild synovial inflammation is less reproducible than severe synovial inflammation, notwithstanding the track record of rheumatologists, suggesting a limited specificity of low/mild synovitis scans. First, all factors were categorized by a systematic literature review. Subsequently, they performed a Delphi consensus process to identify the most appropriate candidates and a second one for consensus finding of representative scans. After identification, they provided an atlas with representative images of false-positive scans. Of note, the authors only used one type of US machine, so precluding a major cause of US error. Not surprisingly, the consensus among the Japanese authors was that the most confounding factors for B-mode were non-specific thickening of synovial membrane and fluid collection. In addition, anatomic structures like muscle, ligament, pulleys, and retinaculum could be mistakenly interpreted as synovitis. The latter may come as a surprise, since recognition of anatomic pitfalls could be rigorously trained. False positive Doppler signals, due to mirror imaging was felt the most confounding, an artifact that can easily be prevented by training.
In conclusion, it is difficult to imagine management of inflammatory arthritis in our twenty-first century without US imaging. Therefore, the findings of Ikeda et al. have to lead to recommendations and reform of the existing scoring systems. Although it is unlikely that new scoring systems will completely eliminate the presence of false positive US findings, changes in cut-off values, e.g. abolishment of the current grades B-mode 1 and power Doppler grade 1, will significantly increase the accuracy. The small decrease in sensitivity will largely be offset by an increased specificity of diagnosing synovitis. That’s not a heavy drawback, as modern US has simply become too sensitive.
Conflict of interest
This work is supported by the project (Ministry of Health, Czech Republic) for conceptual development of research organization 023728 (Institute of Rheumatology) and by project no. NT12437.
References
- Bruyn GA. The Swiss musculoskeletal ultrasound recommendations and the SONAR score: do they meet current standards? Swiss Med Wkly. 2013;143:w13893.
- Hanova P, Zavada J, Hurnakova J, Klein M, Forejtova S, Sleglova O, et al. Potential of US 7 score in evaluating disease activity in patients with rheumatoid arthritis in the state of remission. Ann Rheum Dis. 2014;73(S2):abstract 0205.
- Marks JL, Holroyd CR, Dimitrov BD, Armstrong RD, Calogeras A, Cooper C, et al. Does combined clinical and ultrasound assessment allow selection of individuals with rheumatoid arthritis for sustained reduction of anti-tumor necrosis factor therapy? Arthritis Care Res (Hoboken). 2015;67:746–53.
- Foltz V, Gandjbakhch F, Etchepare F, Rosenberg C, Tanguy M.L, Rozenberg S, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum. 2012;64:67–76.
- Nakagomi D, Ikeda K, Okubo A, Iwamoto T, Sanayama Y, Takahashi K, et al. Ultrasound can improve the accuracy of the 2010 American College of Rheumatology/European League against rheumatism classification criteria for rheumatoid arthritis to predict the requirement for methotrexate treatment. Arthritis Rheum. 2013;65:890–8.
- Boers M, Brooks P, Strand C.V, Tugwell P. The OMERACT filter for outcome measures in rheumatology. J Rheumatol. 1998;25:198–9.
- Wakefield R, Balint PV, Skzudlarek M, Filippucci E, Backhaus M, D’Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–7.
- Ten Cate DF, Luime JJ, Hazes JM, Kleinrensink GJ, Jacobs JW. Is the frequent sonographic anechoic area distally in metacarpophalangeal joints a sign of arthritis? Ultrasound Med Biol. 2014;40:2537–41.
- Bruyn GA, Möller I, Garrido J, Bong D, d’Agostino M.A, Iagnocco A, et al. Reliability testing of tendon disease using two different scanning methods in patients with rheumatoid arthritis. Rheumatology (Oxford). 2012;51:1655–61.
- Terslev L, Torp-Pedersen S, Qvistgaard E, von der Recke P, Bliddal H. Doppler ultrasound findings in healthy wrists and finger joints. Ann Rheum Dis. 2002;46:647–53.
- Terslev L, von der Recke P, Torp-Pedersen S, Koenig M, Bliddal H. Diagnostic sensitivity and specificity of Doppler ultrasound in rheumatoid arthritis. J Rheumatol. 2008;35:49–53.
