Abstract
Objective: To estimate rates of suicidal behaviors and treated depression in patients with psoriatic arthritis (PsA) in comparison to non-PsA patients.
Methods: Using the Clinical Practice Research Datalink, we conducted a cohort study of patients with PsA compared to non-PsA patients. Patients with codes for suicidal behaviors (ideation, attempts, and suicide) and treated depression (diagnosis plus anti-depressant prescription) recorded during follow-up were identified as cases. We estimated incidence rates (IRs) and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) for each outcome and stratified results in the PsA cohort by receipt of systemic PsA drugs.
Results: The rates of suicide ideation, attempt, and suicide were similar for PsA and non-PsA patients [IRR = 0.99 (95%CI: 0.67–1.47), IRR = 1.07 (95%CI: 0.86–1.34), and 0.34 (95%CI: 0.05–2.48), respectively] and rates of suicidal behaviors were slightly higher among PsA patients who received PsA drugs compared to those who did not. PsA patients had slightly higher rate of treated depression compared to non-PsA patients [IRR = 1.38 (95%CI: 1.27–1.49)] and were significantly higher in PsA patients who received drugs [IRR = 1.59 (95%CI: 1.35–1.86)].
Conclusions: Rate of depression was higher in patients with PsA compared to non-PsA patients. The rate of suicidal behaviors was similar between the two cohorts.
Introduction
Rheumatic diseases have been associated with an increased prevalence of depression; however few published studies have examined the risk of depression in patients with psoriatic arthritis (PsA). A few small studies have reported the prevalence of depression in PsA patients to be 11.6–22.2%. A study of 306 consecutive PsA patients attending a dermatologic clinic reported higher prevalence of anxiety (36.6% versus 24.4%) and depression (22.2% versus 9.6%) as measured using the Hospital Anxiety and Depression Scale (HADS) in comparison to patients without PsA [Citation1]. In the multivariate model analysis, unemployment, female gender, high joint count, pain, and fatigue were associated with anxiety and depression suggesting that these factors maybe proxies for disease severity and degree of function in these patients [Citation1]. A study of 394 PsA patients in Canada reported that depression was likely associated with pain [Citation2]. Another study of 98 eligible PsA patients from the Rheumatology Department in the University Hospital of Ioannina, Greece reported that 21.7% of PsA patients had moderate or severe depressive symptoms as measured using the Patient Health Questionnaire (PHQ-9) [Citation3]. One study conducted in a Spanish PsA population reported a prevalence of 17.6% [Citation4] while another study conducted on a network of 18 clinical sites across 10 countries reported a prevalence of 11.6% [Citation5]. Both studies used the HADS instrument with the same cutoff for pathological threshold (HADS ≥11). In patients with rheumatoid arthritis, inflammatory cytokines may affect serotonin transport, contributing to the development of depression [Citation6]. A similar biological mechanism may contribute to the development of depression in patients with PsA.
These small studies suggest that there is an increased prevalence of depression in patients with PsA, however no studies to date have estimated the incidence of depression in PsA patients, and no studies to date have evaluated the rate of suicidal behaviors in this patient population. The objective of this study is to estimate IRs of treated depression and suicidal behaviors (ideation, attempts, and suicide) in patients with PsA compared with patients without PsA or psoriasis in the United Kingdom Clinical Practice Research Datalink.
Methods
Data source
The UK CPRD is a large, longitudinal, population-based electronic medical record database that contains data of approximately 10 million people. The UK National Health Service (NHS) provides universal coverage; therefore the database contains a representative sample of the UK general population [Citation7]. Participating general practitioners (GPs) contribute data in an anonymous format including medical diagnoses, physical findings, symptoms, details of hospital stays and specialist visits, and deaths. Several validation studies have been published on the accuracy of information recorded in the CPRD [Citation8–10], which indicate that the data are of high accuracy with regards to recorded clinical diagnoses with more than 90% of information from the manual medical records present in the GP’s office recorded on the computer.
Study design and cohort identification
This cohort study was conducted using the CPRD data from January 1, 1988 through December 31, 2012. The PsA cohort included all patients in the CPRD with a diagnosis of PsA who had at least one year of medical information in their record before the first PsA diagnosis code. One year of history was required to ensure that we identified patients with new diagnoses of PsA that were not diagnosed before the start of the patient record. The cohort entry date was the date of the first recorded PsA diagnosis. For each PsA patient we identified up to 10 random patients with no recorded diagnoses of psoriasis or PsA (henceforth referred to as the non-PsA cohort) matched on age, sex, and general practice attended. All non-PsA patients were required to be present in the database on the cohort entry date of their matched PsA patient and the cohort entry date of each patient with PsA was assigned to the matched non-PsA patients.
Separate sub-cohorts were identified for each outcome of interest (i.e. suicidal behaviors and treated depression). We did not exclude any patients from the suicidal behaviors sub-cohort because patients could recover from suicidal behaviors with treatment or consultation. Any patient who had a diagnosis of depression or a prescription for an antidepressant drug recorded before the cohort entry date was excluded from the Treated Depression sub-cohort. In each sub-cohort, patient follow-up began at the cohort entry date and continued until the end of the study period, end of registration with the practice, death, or until they developed the outcome of interest. The accumulated time was expressed as person-years (PY) either exposed to PsA or not exposed.
Case identification
Diagnoses of interest were identified using automated searches of the electronic records of all patients in each sub-cohort. For suicidal behaviors, a patient was identified as a case if they had a diagnosis of suicidal ideation, suicide attempt, and/or suicide recorded after the cohort entry date. We reviewed the electronic records of a sample of identified cases with suicidal ideation or attempt, and all cases with suicide to assess the accuracy of the relevant diagnoses, and, in the case of suicide, to confirm that the patient had died. If a patient had a code for suicide, but had not died, the event was classified as a suicide attempt. Suicidal ideation, attempts, and suicide were considered separately; thus a patient may have been included in more than one analysis. A patient was required to have at least one prescription for an antidepressant drug in addition to a diagnosis code for depression within 60 days of each other to qualify as a case of treated depression. The electronic records for a sample of identified depression cases were reviewed to assess the accuracy the relevant diagnoses.
Stratification by exposure to PsA systemic therapy
Among those with PsA, we further evaluated the effects of having treated versus untreated PsA. For each patient in the PsA cohort we accumulated person-time on any PsA systemic therapy and for non-exposed time. Systemic therapies included disease-modifying antirheumatic drugs (DMARDs)/biologics (e.g. methotrexate, sulfasalazine, and adalimumab), immunosuppressants (e.g. azathioprine and leflunomide), and corticosteroids (codes are available upon request). Person-time of exposure to these drugs was accrued between the dates of the prescription issued through 3 months after the end of the supply (based on the amount prescribed divided by the number of times per day). Otherwise the follow-up time was treated as not exposed to the systemic therapies. To assess the appropriateness of this exposure time window definition we conducted sensitivity analyses where the period after the end of supply was varied from 1 month to 6 months.
Statistical analysis
Within each sub-cohort, we estimated incidence rates (IRs) per 1000 PY and 95% confidence intervals (CIs) using Byar’s method [Citation11], and incidence rate ratios (IRRs) with 95% CIs for PsA compared to non-PsA cohorts, and stratified by sex and age (<30, 30–49, 50–69, and ≥70 years). In the suicidal behaviors sub-cohort, we stratified the estimates based on whether the patient had a recorded history of suicidal behavior before the cohort entry date to assess effect modification. We estimated the risk (cumulative hazard function) of each study outcome using the Kaplan–Meier method and tested the differences using the log-rank test. In the PsA cohort, the results were stratified by receipt or non-receipt of prescriptions for systemic PsA therapy and type of drug prescribed. Statistical analyses were carried out using SAS Release 9.2 (SAS Institute Inc., Cary, NC). The protocol for this study was reviewed and approved by the Independent Scientific Advisory Committee of the CPRD.
Results
Suicidal behaviors
We identified 8677 PsA patients and 86,413 non-PsA patients in the suicidal behaviors sub-cohort. Their characteristics are presented in . provides the rates according to the type of suicidal behavior (suicidal ideation, suicide attempt, and suicide) and exposure cohort (PsA versus non-PsA). Overall rates for these outcomes were low. There were 27 cases of suicidal ideation, 85 cases of suicide attempt, and only one case of suicide in the PsA cohort. Rates were highest for suicide attempt [(1.3/1000 PY (95% CI: 1.0–1.6) and 1.2/1000 PY (95% CI: 1.1–1.3), for PsA and non-PsA], followed by suicidal ideation [0.4/1000 PY (95% CI: 0.3–0.6) and 0.4/1000 PY (95% CI: 0.4–0.5)]; and there was little difference between the PsA and non-PsA cohorts [IRR = 1.07 (95% CI: 0.86–1.34) for suicide attempt and IRR = 0.99 (95% CI: 0.67–1.47) for suicide ideation]. For both the PsA and non-PsA cohorts, rates of suicidal ideation and suicide attempt were higher among patients with a history of suicidal behaviors in comparison to patients with no history of suicidal behaviors before the cohort entry date. There was only one PsA exposed and 25 non-PsA cases of suicide, with no difference in the IRs between cohorts [<0.001/1000 PY (95% CI: 0.0–0.l) for both cohorts; IRR = 0.34 (95% CI: 0.05–2.48)]. The IR of suicide in the non-PsA cohort was significantly higher in patients with a history of suicidal behaviors compared to those without. The analysis of cumulative hazards demonstrated that there were no statistically significant differences between the PsA and non-PsA cohorts in any of the suicidal behavior outcomes (p > 0.2 for all outcomes) ().
Figure 1. Cumulative incidence of suicidal ideation (A), suicide attempts (B), and suicide (C) in the psoriatic arthritis and comparison cohorts.
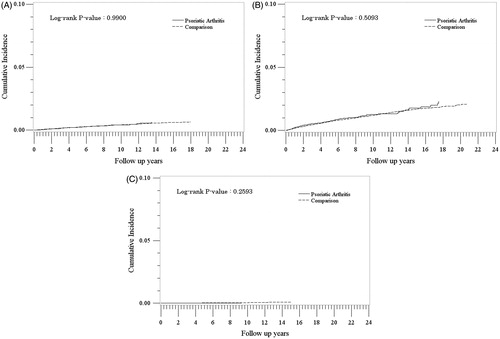
Table 1. Distribution of characteristics of patients with and without psoriatic arthritis (PsA) – suicidal behaviors sub-cohort.
Table 2. Rates of suicidal behavior in patients with and without psoriatic arthritis (PsA).
In the PsA cohort, patients who received prescriptions for PsA drugs had slightly higher rates of suicidal behaviors compared to those who did not, although not statistically significant (). IRs were highest among those treated with immunosuppressants, followed by DMARDs/biologics. There were no cases exposed to corticosteroids. The results did not differ when we redefined the exposure windows in the sensitivity analyses.
Table 3. Rates of suicidal behavior in patients with psoriatic arthritis (PsA), stratified by receipt of PsA drug prescriptions.
Treated depression
There were 7643 PsA patients and 69,526 non-PsA patients in the treated depression analysis following exclusion of patients with treated depression before cohort entry. Their characteristics are presented in . Patients in the PsA cohort had slightly higher rates of treated depression compared to non-PsA patients [13.3/1000 PY (95% CI: 12.3–14.3) and 9.7/1000 PY (95% CI: 9.4–9.9), respectively; IRR = 1.38 (95% CI: 1.27–1.49)] (). Rates were higher for females and highest in the younger age groups. The cumulative incidence of treated depression was consistently higher in the PsA cohort compared to the non-PsA cohort at each time point after cohort entry (p < 0.0001) ().
Figure 2. Cumulative incidence of treated depression in the psoriatic arthritis and comparison cohorts.
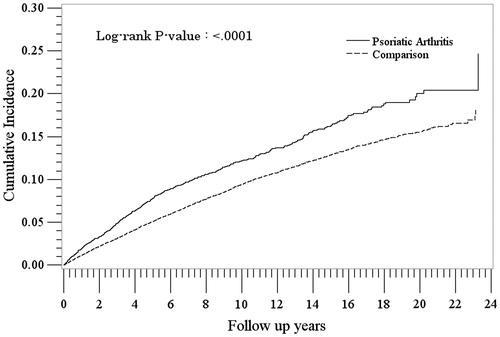
Table 4. Distribution of characteristics of patients with and without psoriatic arthritis (PsA) – treated depression sub-cohort.
Table 5. Rates of incident treated depression in patients with and without psoriatic arthritis (PsA).
In the PsA cohort, rates of incident treated depression were higher in patients who received prescriptions for PsA drugs compared to those who did not [IRs = 18.8/1000 PY (95% CI: 16.4–21.6) and 11.9/1000 PY (95% CI: 10.9–12.9), respectively: IRR = 1.59 (95% CI: 1.35–1.86)] (). Among those who received prescriptions for PsA drugs, IRs were similar in current users of immunosupressants (23.2/1000 PY, 95% CI: 13.9–36.2), and DMARDs/biologics (18.5/1000 PY (95% CI: 15.9–21.3), but were higher among current users of corticosteroids (53.3/1000 PY, 95% CI: 25.5–90.0). The results did not differ when we redefined the exposure window in the sensitivity analyses.
Table 6. Rates of treated depression in patients with psoriatic arthritis (PsA), stratified by receipt of PsA drug prescriptions.
Discussion
There were no significant differences in the rate of suicidal behaviors (ideation, attempt, or suicide), between PsA patients and non-PsA patients in this study. Furthermore, among patients with PsA, rates did not differ among those who received prescriptions for systemic PsA therapy compared to those who did not. IRs of treated depression were, however, higher in patients with PsA compared to non-PsA patients. The rate of depression was higher among PsA patients who received prescriptions for systemic therapy compared to PsA patients who did not, particularly for those who were current users of corticosteroids. However, the number of patients exposed to corticosteroids and immunosuppressants was small. It is possible that the higher risk of treated depression in PsA patients who receive treatments compared to non-treated PsA patients may be explained by the severity of the PsA disease: Patients who receive treatments are likely to have more severe disease than those who do not.
No other published studies provide IRs of depression and suicidal behaviors in patients with PsA; however, similar studies have been published for patients with psoriasis. The IRs from our analysis, where the rate of suicidal behaviors ranged from 0.4 to 1.3 per 1000 PY, are similar to an earlier study of psoriasis patients in the CPRD which reported an IR of 0.9 per 1000 PY for suicidality (a combination of ideation, attempt, and completed suicide) [Citation10]. This study found a significant difference in the rate of suicidality between psoriasis patients compared to patients without psoriasis (HR = 1.44, 95% CI: 1.32–1.57); however, we did not observe any significant differences in rates of suicidal ideation, attempt, or suicide in our analysis. This may be due to the underlying study population; we evaluated PsA patients while the earlier study was on psoriasis patients.
There are no reported IRs of depression in a PsA population to compare with our estimates. The Kurd et al. study [Citation12] reported an incidence of depression for psoriasis patients ranging from 25.7 to 31.8 per 1000 PY, which is higher than our estimate of 13.3 per 1000 PY. This difference could be due to the definition of depression used to identify cases. In our analysis, we defined depression as treated depression which requires both a depression diagnosis and a prescription for an antidepressant medication, whereas the Kurd study appears to only require a diagnosis of depression.
Our population-based study had a number of strengths. We used a very large and well-established, validated, longitudinal primary care database, the CPRD, which is known for its high accuracy and completeness. The mean length of follow-up was over 7 years in both the PsA and non-PsA cohorts for each outcome of interest. By excluding patients who had less than 1 year in their history before the index date we reduced the risk of including prevalent, rather than incident, PsA patients.
There were a few potential limitations to consider. It is possible that we included some people who did not actually have PsA. Results of validation studies have indicated that the diagnosis of psoriasis and PsA in the CPRD has high positive predictive value [Citation13,Citation14]. In addition, we reviewed the electronic medical records of the PsA patients and assessed the presence of and number of PsA diagnoses, codes for PsA symptoms, and treatments for PsA to provide confidence in the PsA diagnosis. We found that around 98% of potential PsA patients identified had at least one of these supporting codes, so we are confident that the vast majority of people in the PsA cohort did have the disease. To be eligible to be included in the PsA cohort, a patient had to have a recorded diagnosis of PsA, thus if a general practitioner did not record the diagnosis, the patient was not included in the study. Therefore, the results of this study are generalized to patients with diagnosed PsA that comes to the attention of the general practitioner. Also, the CPRD does not contain information on PsA severity or activity. PsA patients who receive treatments are likely to have more severe or active disease than patients with PsA who do not, thus the elevated risks observed among treated PsA patients compared to PsA patients who did not receive treatment may be explained by disease severity or activity. It is also possible that some PsA patients stopped taking systemic PsA therapies sooner than was assumed in our estimation of person-time. To assess this, we conducted sensitivity analyses where we extended and shortened the exposure time window and there were no material differences in the rates of suicidal behaviors and depression in these analyses. In the UK, biologics are often prescribed by consultants (specialists) and thus are may not be captured in the GP record, thus it is possible that we misclassified some exposed person-time as non-exposed. The incomplete capture of exposure to biologics also limits our ability to separate independent effects of biologics from DMARDs. There were only 10 PsA patients who were exposed to corticosteroids, thus we were unable to evaluate the effect of corticosteroid dose on the rate of depression. Fibromyalgia has been reported to be associated with both PsA and risk of suicidal ideation/suicide [Citation15–17]. Very few patients in our study population had a diagnosis of fibromyalgia at cohort entry (1.6% of the PsA cohort and 0.7% of the non-PsA cohort): therefore, we did not include fibromyalgia in our analysis. The study results cannot be explained by the small number of patients with fibromyalgia. We only considered patients who were diagnosed with depression that required treatment with an antidepressant; thus, this study is generalizable to those who have treated depression. We may have missed some instances of suicidal behavior (ideation, attempt, or suicide) since not all events are reported to the GP. However, there should not have been any differences in reporting between PsA and non-PsA patients.
In summary, we found that patients with PsA had a higher risk of developing depression compared to an age and sex matched cohort of people without PsA, which adds support to current published literature suggesting there is an increase in depression in PsA patients. The rate of suicidal behaviors was similar in the two cohorts.
Conflict of interest
This study was sponsored by Celgene Corporation. Hagberg, Li, and Jick received funding from Celgene Corporation to conduct the study. Peng, Shah, and Paris are all employees of and own stock/have stock options in Celgene Corporation.
References
- McDonough E, Ayearst R, Eder L, Chandran V, Rosen CF, Thavaneswaran A, Gladman DD. Depression and anxiety in psoriatic disease: prevalence and associated factors. J Rheumatol. 2014;41(5):887–96.
- Husted JA, Tom BD, Farewell VT, Gladman DD. Longitudinal study of the bidirectional association between pain and depressive symptoms in patients with psoriatic arthritis. Arthritis Care Res (Hoboken). 2012;64(5):758–65.
- Kotsis K, Voulgari PV, Tsifetaki N, Machado MO, Carvalho AF, Creed F, et al. Anxiety and depressive symptoms and illness perceptions in psoriatic arthritis and associations with physical health-related quality of life. Arthritis Care Res (Hoboken). 2012;64(10):1593–601.
- Cauli A, Gladman DD, Mathieu A, Olivieri I, Porru G, Tak PP, et al. Patient global assessment in psoriatic arthritis: a multicenter GRAPPA and OMERACT study. J Rheumatol. 2011;38(5):898–903.
- Freire M, Rodríguez J, Möller I, Valcárcel A, Tornero C, Díaz G, et al. Prevalence of symptoms of anxiety and depression in patients with psoriatic arthritis attending rheumatology clinics. Rheumatol Clin. 2011;7(1):20–6.
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Eng J Med. 2011;365:2205–19.
- Lawson DH, Sherman V, Hollowell J, for the Scientific and Ethical Advisory Group. The general practice research database. Q J Med. 1998;91:445–52
- Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerized data resource in the United Kingdom. BMJ. 1991;302:766–8.
- Jick H, Terris BZ, Derby LE, Jick SS. Further validation of information recorded on a general practitioner based computerized data resource in the United Kingdom. Pharmacoepidemiol Drug Saf. 1992;1:347–9.
- Jick SS, Kaye JA, Vasilakis-Scaramozza C, Garacia Rodriguez LA, Ruigómez A, Meier CR, et al. Validity of the general practice research database. Pharmacotherapy. 2003;23:686–9.
- Rothman KJ, Boice Jr JD. Epidemiologic analysis with a programmable calculator (NIH Publication 79-1649). Washington (DC): US Government Printing Office; 1979.
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–5.
- Huerta C, Rivero E, Rodríguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143:1559–65.
- Gelfand JM, Wang X, Qing L, Neimann AL, Weinstein R, Margolis DJ, et al. Epidemiology and treatment patterns of psoriasis in the General Practice Research Database (GPRD). Pharmacoepidemiol Drug Saf. 2005;14(Suppl):S23.
- Magrey MN, Antonelli M, James N, Khan MA. High frequency of fibromyalgia in patients with psoriatic arthritis: a pilot study. Arthritis. 2013;2013:762921.
- Jimenez-Rodríguez I, Garcia-Leiva JM, Jimenez-Rodriguez BM, Condés-Moreno E, Rico-Villademoros F, Calandre EP. Suicidal ideation and the risk of suicide in patients with fibromyalgia: a comparison with non-pain controls and patients suffering from low-back pain. Neuropsychiatr Dis Treat. 2014;10:625–30. eCollection 2014.
- Crofford LJ. Psychological aspects of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2015;29(1):147–55.
