Abstract
Objectives: To assess the effects of tocilizumab on pregnancy outcomes in Japanese patients with rheumatic disease.
Methods: Data from Chugai’s tocilizumab safety database (April 2005 to October 2014) were retrospectively analyzed to identify pregnancy outcomes in patients exposed to tocilizumab.
Results: Data were available for 61 pregnancies exposed to tocilizumab, and outcomes were reported for 50 of those pregnancies. In 36 births, no congenital anomalies were identified; however, six neonatal abnormalities were reported: five cases of low birth weight (<2500 g) and one case of neonatal asphyxia. Of 36 births, tocilizumab was resumed during lactation in two patients, with no subsequent adverse events reported in newborns. The spontaneous abortion rate was 18.0% (9 of 50 pregnancies), which is comparable to the rate in the general population. The five terminated pregnancies included one case of caudal regression syndrome.
Conclusions: The present retrospective study of 61 pregnancies exposed to tocilizumab at conception indicated no increased rates of spontaneous abortion or congenital abnormalities in patients with rheumatic disease. However, further study is necessary to confirm the benefit-risk profile of tocilizumab treatment during pregnancy.
Introduction
With the exception of ankylosing spondylitis and gout, rheumatic diseases are substantially more common in women than men [Citation1,Citation2]. For example, rheumatoid arthritis (RA) occurs in 2–3 times as many women as men. In the United States, the incidence of RA is 53.1 per 100,000 women vs 27.7 per 100,000 men and is rising faster among women than men (2.5% vs 0.5%, respectively, in 1995–2007) [Citation3]. Further, women are diagnosed with RA earlier than men, most commonly between 30 and 60 years of age, which includes peak childbearing age [Citation3,Citation4]. Although women with RA tend to experience improvement in symptoms during pregnancy [Citation5,Citation6], complications and poor pregnancy outcomes can result, and symptoms often flare postpartum [Citation7]. The relative risk of early (1.2 [95% CI, 1.1–1.3]) or late miscarriage (1.4 [95% CI, 1.1–1.7]) is slightly higher in women with RA than in women without RA [Citation8].
Conventional disease-modifying antirheumatic drugs (DMARDs; e.g. methotrexate) or targeted therapies (i.e. biologics such as etanercept, infliximab, tocilizumab, and tofacitinib) have been used as therapeutic agents. Safety concerns associated with treating active RA with these therapies during conception, pregnancy, and lactation include fetal toxicity, poor pregnancy outcomes, and potential risk to newborns [Citation9,Citation10]. However, successful outcomes can be achieved with appropriate choice of treatment and proactive management [Citation10–14]. Methotrexate, a fundamental therapeutic agent for RA management, is contraindicated in pregnancy; it is abortifacient and increases the risk of aminopterin syndrome, which is characterized by fetal central nervous system, skeletal, and cardiac abnormalities [Citation10,Citation15]. Leflunomide, which is frequently used in cases of methotrexate intolerance, has caused significant abnormalities in animal reproduction studies and is also contraindicated in pregnancy. Although biologics (e.g. tumor necrosis factor [TNF]-α inhibitors such as etanercept) did not pose a risk in animal reproduction studies, evidence in humans regarding transplacental passage and levels in breast milk are minimal and inconclusive [Citation16,Citation17].
Tocilizumab, an interleukin-6 (IL-6) receptor inhibitor with proven efficacy and safety as monotherapy and in combination with DMARDs [Citation18–22], posed no demonstrable risk to the fetus in animal reproduction studies [Citation23]. A study using the lipopolysaccharide-induced preterm delivery model in mice showed that tocilizumab may in fact help prevent preterm delivery [Citation24]. A study in monkeys did not indicate any dysmorphogenic potential but did yield more spontaneous abortions/embryofetal deaths at a high dose [Citation23]. The relevance of these data for humans is unknown because there are no adequate data on the use of tocilizumab in pregnant women. When tocilizumab was administered intravenously to cynomolgus monkeys during early gestation, no direct or indirect harmful effects on pregnancy or embryofetal development were observed. However, the rate of spontaneous abortion or embryofetal death increased at high doses (10 and 50 mg/kg/day, 1.25 and 6.25 times the human dose [8 mg/kg], respectively) [Citation23]. IL-6 has no demonstrated role in fetal growth or immunologic control of the maternal/fetal interface; therefore, the basis of this dose-dependent result is unknown.
Women with childbearing potential were required to use a reliable contraception method in tocilizumab clinical trials. Therefore, information on pregnancy outcomes in women exposed to tocilizumab during pregnancy is limited. The objective of the present study was to retrospectively analyze pregnancy outcomes in patients receiving tocilizumab.
Methods
Data collection
After the approval of tocilizumab in Japan in April 2005, pregnancies in women receiving tocilizumab treatment were reported and entered into Chugai’s safety database. The database comprises data from various sources, including postmarketing surveillance reports, spontaneous reports, and reports from literature. Data were retrieved from cases reported from April 11, 2005, to October 10, 2014. Data were analyzed for women with pregnancies confirmed after they started treatment with tocilizumab for an indication approved in Japan (RA, systemic juvenile idiopathic arthritis, polyarticular juvenile idiopathic arthritis, or multicentric Castleman disease). As much pregnancy-associated information as possible was collected, including details of treatment with tocilizumab; pregnancy outcome; abnormalities in patients, fetuses, or newborns; and lactation status.
Pregnancies were identified, and adverse events were extracted using predetermined Medical Dictionary for Regulatory Activities (version 17.1) system organ classes and preferred terms. Timing of tocilizumab exposure during or before pregnancy was estimated using gestational age, last menstrual period (LMP), and delivery date. If the gestational age was not reported, we calculated it based on the delivery date, and the gestational age was assumed as 40 weeks. In patients who discontinued tocilizumab treatment before LMP, tocilizumab exposure was determined based on the calculated date regardless of the blood concentration.
Results
Patient demographics and characteristics
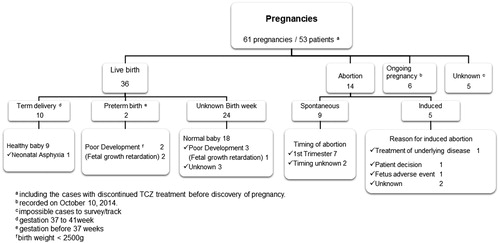
Data were extracted for 61 pregnancies. Mean age in the 42 pregnacies with reported age was 30.5 (range, 19–41) years. Most patients (86.9%; 53 of 61 pregnancies) recieved tocilizumab for the treatment of RA (). Because most data were collected before the subcutaneous formulation of tocilizumab was available, most patients (91.8%; 56 of 61 pregnancies) were treated intravenously. Information on tocilizimab dose was not rigorously collected, but it was assumed that tocilizumab was administered according to the intravenous (8 mg/kg/four weeks) and subcutaneous (162 mg/two weeks) dosages approved in Japan.
Table 1. Patient demographics and characteristics,.
Tocilizumab was discontinued before LMP in 10 pregnancies. Women were exposed to tocilizumab by the first trimester in 30 pregnancies, and tocilizumab was resumed during two pregnancies (the timing of resumption was unknown). No patients discontinued treatment in the second or third trimester, and timing of exposure in the remaining 19 pregnancies was unknown. Tocilizumab was continued throughout pregnancy in two patients ().
Live births
Of the 50 pregnancies with confirmed pregnancy outcome, there were 36 deliveries. Overall, tocilizumab was discontinued before LMP in six pregnancies and in the first trimester in 23 pregnancies. Of these 23 pregnancies during which tocilizumab was discontinued in the first trimester, the drug was resumed during two pregnancies. One patient continued tocilizumab throughout the pregnancy. The exposure to tocilizumab during pregnancy was unknown in six patients.
Ten pregnancies were term deliveries, and preterm births were reported for two pregnancies. For the remaining 24 pregnancies, the gestational ages at birth were unknown (). No congenital anomalies were identified in the 36 newborns. However, neonatal abnormalities were reported in six of the 36 births (16.7%): one case of neonatal asphyxia (reported as postnatal death) and five cases of low birth weight (<2500 g), three of which were considered fetal growth restriction on the basis of the reported term for adverse events or gestation week ().
Table 2. Summary of abnormal neonatal outcomes.
Tocilizumab was resumed during lactation in two patients who had normal births, with no reports of adverse events in the newborns.
Abortions
Of the 50 pregnancies with available outcomes, there were nine spontaneous abortions and five induced abortions ().
The nine patients who experienced spontaneous abortions were slightly older (age was reported for eight patients; mean age, 32.4 years) than those in the full population (age was reported for 42 patients; mean age, 30.5 years). Tocilizumab was discontinued before LMP in two pregnancies, and patients were exposed to tocilizumab in the first trimester in four pregnancies. Exposure to tocilizumab during pregnancies was unknown for three pregnancies. Methotrexate was administered with tocilizumab in five pregnancies that resulted in spontaneous abortions. Methotrexate was continued in two pregnancies when pregnancy was confirmed. The seven pregnancies for which the timing of spontaneous abortion was known all occurred in the first trimester ().
Table 3. Summary of spontaneous abortions.
Of the five terminated pregnancies, tocilizumab was discontinued before LMP in one pregnancy and continued in the first trimester in one pregnancy. One patient continued tocilizumab during the pregnancy. Exposure to tocilizumab during pregnancy was unknown for the remaining two pregnancies. One abortion was induced because of fetal abnormalities (caudal regression syndrome) in a woman who received methotrexate and leflunomide with tocilizumab before confirmation of pregnancy.
Discussion
Although biologic therapies have presented effective treatment options for patients with RA and other rheumatic diseases, there are still concerns about their use during conception, pregnancy, and breastfeeding [Citation25]. There are limited data regarding pregnancy outcomes after exposure to biologics other than TNF-α inhibitors, including rituximab, abatacept, anakinra, and tocilizumab, and their use in pregnancy cannot currently be recommended [Citation26].
In this study, we extracted data to retrospectively evaluate pregnancy outcomes of patients who were exposed to tocilizumab during conception, pregnancy, or lactation. In general, rates of spontaneous abortion, congenital abnormalities, and other pregnancy outcomes were not different than those seen in the general population [Citation27–29]. Rates are also similar to that reported from an analysis of tocilizumab clinical trials, which reported 39.4% (13 of 33) therapeutic abortions, 21.2% (7 of 33) spontaneous abortions, and 33.3% (11 of 33) term deliveries [Citation30].
No congenital anomalies were identified in the 36 live births in this study. However, six neonatal abnormalities were reported among the 36 live births: five cases of low birth weight and one case of neonatal asphyxia. Active RA can negatively affect the birth weight of newborns [Citation31] and may have long-term effects on their future health status [Citation32]. Further, intrauterine growth restriction is associated with elevated IL-6 and IL-18 levels, suggesting that inflammation plays a role in this fetal abnormality [Citation33,Citation34]. Of the five cases of low birth weight in this study, two of the reported gestational ages at birth were not within 1.5 SD of the standard curve for estimated fetal weight vs gestational age in Japan [Citation35]. However, these two patients discontinued tocilizumab in the first trimester and the patients experienced high disease activity. The gestational age at birth in the other three cases of low birth weight was unknown; therefore, the assessment of the reason for low birth weight has limited value. In the case of neonatal asphyxia, information, including the grade of neonatal asphyxia, was not available. However, the complication of systemic lupus erythematosus might have played a role in the abnormality.
Among the 50 pregnancies with known outcomes, the rate of spontaneous abortion was 18.0% (9 of 50), which is in line with the reported rate of 8% to 20% in the general population [Citation27–29]. Half of the patients who experienced spontaneous abortions in this study had been co-treated with methotrexate, which is reported to cause an increased risk of spontaneous abortion [Citation36]. Advanced maternal age is a known risk factor for female infertility, pregnancy loss, fetal anomalies, stillbirth, and obstetric complications [Citation37,Citation38] and may have – along with exposure to methotrexate – contributed to the risk of spontaneous abortion. The mean age of patients who experienced spontaneous abortion was 32.4 years.
One case of caudal regression syndrome was confirmed in this study. For this case, a causal relationship between tocilizumab and caudal regression syndrome could not be appropriately assessed because this patient was also exposed to leflunomide and methotrexate before confirmation of the pregnancy. In a report from the British Society for Rheumatology Biologics Register, the spontaneous abortion rate among 130 pregnant women was 24% in women receiving TNF-α inhibitors and 33% in women receiving TNF-α inhibitors plus methotrexate or leflunomide [Citation39].
In this study, tocilizumab was resumed during lactation in two patients, and no adverse events were reported in the newborns. However, further studies are needed to confirm the safety of tocilizumab exposure during lactation.
Limitations of this study include its small sample size, lack of data on disease severity of RA and of efficacy assessment in pregnant patients with RA, as well as missing information on tocilizumab dose and duration of exposure. Further, information on pregnancies tends to be reported more often when adverse events are involved in the real-world clinical setting after approval, potentially resulting in reporting bias. Case report forms were not used because the data for this analysis were obtained from a variety of reporting sources, but researchers tried to obtain as many pregnancy outcomes and as much relevant information as possible. The number of reported pregnancies is probably an underestimate of the actual number. This report was also limited by the absence of a control group; therefore, further analyses will be necessary to confirm the benefit-risk profile of tocilizumab treatment during pregnancy.
Tocilizumab’s prescribing information states that it is recommended for women who are or may be pregnant only when the benefits of treatment outweigh the risks [Citation23]. Although this study did not uncover increased rates of untoward effects associated with exposure to tocilizumab before, during, or after pregnancy, tocilizumab should only be used in such patients when the benefits outweigh the risks, and treatment should be carefully considered in women of childbearing age who want to become pregnant. The treatment plan should be reviewed for such patients, taking into consideration the timing of the desired pregnancy.
In conclusion, this retrospective analysis of 61 pregnancies exposed to tocilizumab before or during pregnancy indicated no increased rates of spontaneous abortion or congenital abnormalities. However, further information is necessary to confirm the benefit-risk profile of tocilizumab treatment during pregnancy.
Acknowledgments
Medical writing services were provided by Cactus Communications and funded by Chugai Pharmaceutical Co, Ltd. The authors retained full control of the manuscript content.
Conflict of interest
Atsuko Murashima has received speaker honoraria from Astellas, Tanabe-Mitsubishi, Takeda, Eisai, Chugai, Kyorin-Pharma, and MSD-K. Atsuko Murashima has received research grants from Japan Blood Products Organization and Tanabe-Mitsubishi. Ayako Nakasone and Nobuhiko Ishizuka are employees of Chugai. There are no other competing interests for the rest of authors.
This study was funded by Chugai Pharmaceutical Co, Ltd.
References
- Sangha O. Epidemiology of rheumatic diseases. Rheumatology (Oxford). 2000;39(Suppl 2):3–12.
- Lockshin MD. Invited review: sex ratio and rheumatic disease. J Appl Physiol (1985). 2001;91:2366–73.
- Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62:1576–82.
- Golding A, Haque UJ, Giles JT. Rheumatoid arthritis and reproduction. Rheum Dis Clin North Am. 2007;33:319–43, vi–vii.
- Ostensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol. 2007;29:185–91.
- de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008;59:1241–8.
- Wallenius M, Salvesen KA, Daltveit AK, Skomsvoll JF. Rheumatoid arthritis and outcomes in first and subsequent births based on data from a national birth registry. Acta Obstet Gynecol Scand. 2014;93:302–7.
- Wallenius M, Salvesen KA, Daltveit AK, Skomsvoll JF. Miscarriage and stillbirth in women with rheumatoid arthritis. J Rheumatol. 2015;42:1570–2.
- Chakravarty EF, Sanchez-Yamamoto D, Bush TM. The use of disease modifying antirheumatic drugs in women with rheumatoid arthritis of childbearing age: a survey of practice patterns and pregnancy outcomes. J Rheumatol. 2003;30:241–6.
- Krause ML, Amin S, Makol A. Use of DMARDs and biologics during pregnancy and lactation in rheumatoid arthritis: what the rheumatologist needs to know. Ther Adv Musculoskelet Dis. 2014;6:169–84.
- Umeda N, Ito S, Hayashi T, Goto D, Matsumoto I, Sumida T. A patient with rheumatoid arthritis who had a normal delivery under etanercept treatment. Intern Med. 2010;49:187–9.
- Natsumi I, Matsukawa Y, Miyagawa K, Kodaira H, Tanaka T, Horikoshi A, et al. Successful childbearing in two women with rheumatoid arthritis and a history of miscarriage after etanercept treatment. Rheumatol Int. 2013;33:2433–5.
- Murashima A, Watanabe N, Ozawa N, Saito H, Yamaguchi K. Etanercept during pregnancy and lactation in a patient with rheumatoid arthritis: drug levels in maternal serum, cord blood, breast milk and the infant’s serum. Ann Rheum Dis. 2009;68:1793–4.
- Chambers C, Koren G, Tutuncu ZN, Johnson D, Jones KL. Are new agents used to treat rheumatoid arthritis safe to take during pregnancy? Organization of Teratology Information Specialists (OTIS) study. Can Fam Physician. 2007;53:409–12.
- Hausknecht RU. Methotrexate and misoprostol to terminate early pregnancy. N Engl J Med. 1995;333:537–40.
- Skomsvoll JF, Wallenius M, Koksvik HS, Rødevand E, Salvesen KA, Spigset O, et al. Drug insight: anti-tumor necrosis factor therapy for inflammatory arthropathies during reproduction, pregnancy and lactation. Nat Clin Pract Rheumatol. 2007;3:156–64.
- Roux CH, Brocq O, Breuil V, Albert C, Euller-Ziegler L. Pregnancy in rheumatology patients exposed to anti-tumour necrosis factor (TNF)-alpha therapy. Rheumatology (Oxford). 2007;46:695–8.
- Hashimoto J, Garnero P, van der Heijde D, Miyasaka N, Yamamoto K, Kawai S, et al. Humanized anti-interleukin-6-receptor antibody (tocilizumab) monotherapy is more effective in slowing radiographic progression in patients with rheumatoid arthritis at high baseline risk for structural damage evaluated with levels of biomarkers, radiography, and BMI: data from the SAMURAI study. Mod Rheumatol. 2011;21:10–15.
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the Tocilizumab in Combination With Traditional Disease-Modifying Antirheumatic Drug Therapy study. Arthritis Rheum. 2008;58:2968–80.
- Strand V, Burmester GR, Ogale S, Devenport J, John A, Emery P. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE study. Rheumatology (Oxford). 2012;51:1860–9.
- Burmester GR, Rubbert-Roth A, Cantagrel A, Hall S, Leszczynski P, Feldman D, et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis. 2014;73:69–74.
- Ogata A, Tanimura K, Sugimoto T, Inoue H, Urata Y, Matsubara T, et al. Phase III study of the efficacy and safety of subcutaneous versus intravenous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66:344–54.
- Prescribing Information: Actemra (tocilizumab) 2013. Available from: http://www.gene.com/download/pdf/actemra_prescribing.pdf Accessed September 20, 2015.
- Wakabayashi A, Sawada K, Nakayama M, Toda A, Kimoto A, Mabuchi S, et al. Targeting interleukin-6 receptor inhibits preterm delivery induced by inflammation. Mol Hum Reprod. 2013;19:718–26.
- Hyrich KL, Verstappen SM. Biologic therapies and pregnancy: the story so far. Rheumatology (Oxford). 2014;53:1377–85.
- Calligaro A, Hoxha A, Ruffatti A, Punzi L. Are biological drugs safe in pregnancy? Reumatismo. 2015;66:304–17.
- Yamamoto T. Abnormal pregnancy [in Japanese]. Acta Obster Gymnaeccol Jpn. 2007;59:N663–71.
- MedlinePlus® 2014; Miscarriage. Available from: http://www.nlm.nih.gov/medlineplus/ency/article/001488.htm Accessed November 16, 2015.
- Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–94.
- Rubbert-Roth A, Goupille P, Moosavi S. First experiences with pregnancies n RA patients (pts) receiving tocilizumab (TCZ) therapy. Arthritis Rheum. 2010;62(Suppl 10):384.
- de Man YA, Hazes JM, van der Heide H, Willemsen SP, de Groot CJ, Steegers EA, et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. 2009;60:3196–206.
- Østensen M, Andreoli L, Brucato A, Cetin I, Chambers C, Clowse ME, et al. State of the art: reproduction and pregnancy in rheumatic diseases. Autoimmun Rev. 2015;14:376–86.
- Krajewski P, Sieroszewski P, Karowicz-Bilinska A, Kmiecik M, Chudzik A, Strzalko-Gloskowska B, et al. Assessment of interleukin-6, interleukin-8 and interleukin-18 count in the serum of IUGR newborns. J Matern Fetal Neonatal Med. 2014;27:1142–5.
- de Steenwinkel FD, Hokken-Koelega AC, de Man YA, de Rijke YB, de Ridder MA, Hazes JM, et al. Circulating maternal cytokines influence fetal growth in pregnant women with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1995–2001.
- Itabashi K, Miura F, Uehara R, Nakamura Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr Int. 2014;56:702–8.
- Weber-Schoendorfer C, Chambers C, Wacker E, Beghin D, Bernard N, Network of French Pharmacovigilance C, et al. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: a prospective multicenter cohort study. Arthritis Rheumatol. 2014;66:1101–10.
- Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–11.
- Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril. 2015;103:1136–43.
- Verstappen SM, King Y, Watson KD, Symmons DP, Hyrich KL, BSRBR Control Centre Consortium, BSR Biologics Register. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823–6.