Abstract
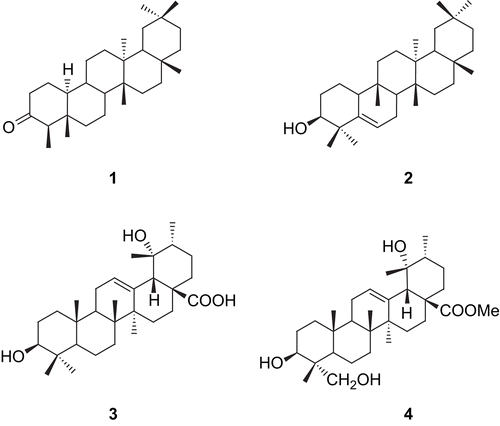
In the course of screening antifibrotic activity of natural products employing HSC-T6, a rat hepatic stellate cell line, as an in vitro assay system, the methanolic extract of aerial parts of Euscaphis japonica (Tunb.) Kantiz (Staphyleaceae) showed significant inhibitory activity on HSC proliferation. Activity-guided fractionation led to the isolation of four triterpenoids, friedeline (1), glut-5-en-ol (2), pomolic acid (3), and methylrotundate (4). Among the triterpenoids isolated, pomolic acid (3) significantly inhibited the proliferation of HSCs at concentrations 10 and 100 μM.
Introduction
Hepatic fibrosis occurs as the consequence of a sustained wound-healing response of liver to toxic, infectious, or metabolic agents, and is characterized by excessive accumulation of extracellular matrix (ECM) leading to ultimate liver dysfunction and irreversible cirrhosisCitation1. Hepatic stellate cells (HSCs) play important functions in normal liver, such as retinoid storage, remodeling of ECM, and production of growth factors and cytokines. However, in response to liver damage, HSCs undergo a process of activation, developing a myofibroblast-like phenotype associated with increased proliferation, and/or excessive production and deposition of ECM components, which is the major pathological feature of hepatic cirrhosisCitation2,Citation3. Therefore, HSCs are considered to play a key role in the pathogenesis of liver fibrosis, and suppression of HSC activation has been proposed as a therapeutic target against hepatic fibrosisCitation4,Citation5.
In the course of screening antifibrotic activity of natural products employing HSC-T6, a rat hepatic stellate cell line, as an in vitro assay system, the methanolic extract of aerial parts of Euscaphis japonica (Tunb.) Kantiz (Staphyleaceae) significantly inhibited the proliferation of HSCs. E. japonica has been used in the treatment of inflammation in folk medicineCitation6. We previously reported anti-inflammatory compounds from E. japonicaCitation7. In a continuation of our work, we have attempted to isolate the antifibrotic constituents from E. japonica. In the present study, we isolated four triterpenoids from E. japonica: friedeline (1), glut-5-en-ol (2), pomolic acid (3), and methylrotundate (4). Among the triterpenoids isolated, pomolic acid (3) significantly inhibited the proliferation of HSCs at concentrations 10 and 100 μM.
Methods and materials
General experimental procedures
1H and 13C nuclear magnetic resonance (NMR) measurements were carried out using a Bruker AMX 400 spectrometer operating at 300 and 100 MHz, respectively. Solvent signals were used as internal standards. 1H−1H COSY (correlation spectroscopy), HMQC (heteronuclear multiple quantum correlation), and HMBC (heteronuclear multiple bond correlation) NMR experiments were performed on the same spectrometer. Electron ionization (EI) mass spectra were obtained on a Jeol JMS 700 spectrometer with a 70 eV ionizing potential. Thin layer chromatography (TLC) and column chromatography were carried out on precoated silica gel F254 plates (art. 5715; Merck) and silica gel 60 (230–400 mesh; Merck).
Plant materials
The aerial parts of E. japonica were collected from Mt. Baekwoon (Kwangyang, Korea) in August 2004 and identified by Dr. Jong Hee Park, a professor of the College of Pharmacy, Pusan National University (Busan, Korea). A voucher specimen (SNU-2004-08) has been deposited in the Herbarium of the Medicinal Plant Garden, College of Pharmacy, Seoul National University.
Extraction and isolation
The aerial parts of E. japonica (8.1 kg) were extracted three times with 80% MeOH in an ultrasonic apparatus. Upon removal of the solvent in vacuo, the methanolic extract yielded 1.7 kg. The methanolic extract was suspended in H2O and partitioned successively with n-hexane, EtOAc, and n-BuOH. The n-hexane and EtOAc fractions, which significantly inhibited HSC proliferation, were subjected to column chromatography (CC) to isolate active compounds. The n-hexane fraction (77 g) was subjected to CC over silica gel eluted with n-hexane−EtOAc−MeOH mixture to afford 14 fractions (H1−H14). Compound 1 (137 mg) was obtained from H2 by recrystallization using MeOH. The residual H2 was subjected to silica gel CC with n-hexane−EtOAc−MeOH mixture to yield eight fractions (H2-1−H2-8). Compound 2 (1270 mg) was isolated from H2-7 by recrystallization using MeOH. The EtOAc fraction (349 g) was subjected to CC over silica gel eluted with n-hexane−EtOAc−MeOH mixture to afford 17 fractions (E1−E17). E7 was subjected to CC over silica gel eluted with n-hexane−EtOAc−MeOH mixture to afford seven fractions (E7-1−E7-7). Compound 3 (8470 mg) was obtained from H7-5 by recrystallization using n-hexane. E10 was subjected to CC over silica gel eluted with n-hexane−EtOAc−MeOH mixture to afford eight fractions (E10-1−E10-8). Compound 4 (844 mg) was obtained from E10-7 by recrystallization using n-hexane.
Friedeline (1). White amorphous powder. Positive FAB MS m/z: 426 [M]+. 1H-NMR (300 MHz, CDCl3): γ 2.20–2.40 (3H, m, H-2, 4), 1.11 (3H, s, H-28), 0.98 (3H, s, H-27), 0.94 (3H, s, H-30), 0.88 (3H, s, H-29), 0.82 (3H, d, J = 6.4 Hz, H-23), 0.80 (3H, s, H-25), 0.66 (3H, s, H-24) ppm. 13C-NMR (75 MHz, CDCl3): γ 213.3 (C-3), 59.4 (C-10), 58.2 (C-4), 53.1 (C-8), 42.8 (C-18), 42.1 (C-5), 41.5 (C-2), 41.3 (C-6), 39.7 (C-13), 35.3 (C-19), 35.0 (C-29), 32.7 (C-21), 32.4 (C-15), 32.1 (C-28), 31.8 (C-30), 30.5 (C-12), 30.0 (C-17), 28.2 (C-20), 22.3 (C-1), 20.2 (C-26), 18.7 (C-27), 18.2 (C-7), 18.0 (C-25), 14.6 (C-24), 6.8 (C-23) ppm.
Glut-5-en-ol (2). White amorphous powder. IR νmax (KBr): 3425 (OH), 1635 (C=C-H), 1380, 1360 (geminal dimethyl) cm−1. EIMS m/z (rel. int.): 426 [M]+ (14), 411 (10), 408 (10), 274 (100), 205 (65). 1H-NMR (300 MHz, CDCl3): γ 5.61 (1H, d, J = 6.3 Hz, H-6), 3.44 (1H, t, J = 2.8 Hz, H-3), 1.14 (3H, s, H-30), 1.12 (3H, s, H-24), 1.07 (3H, s, H-27), 1.02 (3H, s, H-23), 0.98 (3H, s, H-28), 0.97 (3H, s, H-26), 0.93 (3H, s, H-29), 0.83 (3H, s, H-30) ppm. 13C-NMR (100 MHz, CDCl3): γ 141.6 (C-5), 122.0 (C-6), 76.3 (C-3), 49.7 (C-10), 47.4 (C-18), 43.0 (C-8), 40.7 (C-14), 39.3 (C-4), 38.9 (C-22), 37.8 (C-13), 36.0 (C-16), 35.0 (C-19), 34.8 (C-9), 34.6 (C-29), 34.5 (C-11), 33.1 (C-21), 32.4 (C-28), 32.0 (C-15), 27.7 (C-7), 25.5 (C-24), 23.6 (C-1), 19.6 (C-27), 18.4 (C-27), 18.2 (C-2), 16.2 (C-25) ppm.
Pomolic acid (3). White amorphous powder. IR νmax (KBr): 3400 (OH), 1690 (C=O), 1045, 1025 cm−1. EIMS m/z (rel. int.): 472 [M]+ (13), 427 (22), 302 (100), 286 (20), 273 (10), 121 (10). 1H-NMR (300 MHz, pyridine-d5): γ 5.61 (1H, t, J = 3.6 Hz, H-12), 3.60 (1H, s, H-3), 3.06 (1H, s, H-18), 1.73 (3H, s, H-29), 1.45 (3H, s, H-27), 1.23 (3H, s, H-25), 1.12 (3H, s, H-23), 1.11 (3H, d, J = 7.2 Hz, H-30), 1.02 (3H, s, H-24), 0.91 (3H, s, H-26) ppm. 13C-NMR (75 MHz, pyridine-d5): γ 180.6 (C-28), 139.9 (C-13), 128.0 (C-12), 78.1 (C-3), 72.6 (C-19), 55.8 (C-5), 54.6 (C-18), 48.2 (C-17), 47.7 (C-9), 42.3 (C-20), 42.0 (C-14), 40.3 (C-8), 39.3 (C-4), 38.9 (C-1), 38.4 (C-22), 37.3 (C-10), 33.5 (C-7), 29.2 (C-15), 28.7 (C-23), 28.0 (C-2), 27.1 (C-21), 26.9 (C-29), 26.3 (C-16), 24.6 (C-27), 24.0 (C-11), 18.9 (C-6), 17.1 (C-26), 16.7 (C-24), 16.5 (C-30), 15.5 (C-25) ppm.
Methylrotundate (4). White amorphous powder. IR νmax (KBr): 3429 (OH), 2935 (OH), 1692 (C=O), 1236 (COO-), 1160, 1043 (CO), 534 cm−1. Positive FAB MS m/z: 525 [M + Na]+, 503 [M + H]+, 471 [M-OCH3]+, 453, 395. 1H-NMR (300 MHz, pyridine-d5): γ 3.87 (1H, d, J = 10.1, H-23a), 3.16 (1H, d, J = 10.1, H-23b), 3.97 (3H, s, OCH3), 1.38 (3H, s, H-27), 1.09 (3H, s, H-29), 0.83 (3H, s, H-24), 0.80 (3H, d, J = 7.2 Hz, H-30), 0.75 (3H, s, H-25), 0.68 (3H, s, H-26) ppm. 13C-NMR (75 MHz, pyridine-d5): γ 180.6 (C-28), 140.0 (C-13), 128.0 (C-12), 73.5 (C-3), 72.7 (C-19), 68.0 (C-23), 54.6 (C-18), 52.3 (OCH3), 48.6 (C-5), 48.6 (C-17), 48.2 (C-9), 42.8 (C-4), 42.3 (C-20), 42.1 (C-14), 40.3 (C-8), 38.8 (C-1), 38.4 (C-22), 37.1 (C-10), 33.2 (C-7), 30.8 (C-15), 27.6 (C-2), 27.0 (C-29), 26.9 (C-21), 26.3 (C-16), 24.6 (C-27), 24.0 (C-11), 18.7 (C-6), 17.2 (C-25), 16.7 (C-26), 15.9 (C-30), 13.0 (C-24) ppm.
Culture of HSC-T6 hepatic stellate cells
An immortalized rat hepatic stellate cell line, “HSC-T6” instead of HSC-T68, was kindly provided by Professor S. L. Friedman (Columbia University, New York). HSC-T6 cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 95% air–5% CO2Citation9.
Evaluation of antifibrotic activity
Compounds to be tested were dissolved in dimethylsulfoxide (DMSO). Our preliminary study showed that DMSO at a final concentration of 0.1% in media did not affect the cell viability. HSC-T6 cells were treated with vehicle or compounds to be tested for 48 h. β-Glycyrrhetinic acid (Sigma-Aldrich Co.) was used as a positive control. Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) assay. HSC-T6 cells were incubated with 0.5 mg/mL of MTT in the last 2 h of the culture period tested. Reduction of MTT to formazan was assessed using an enzyme-linked immunosorbent assay (ELISA) plate reader.
Statistical analysis
The evaluation of statistical significance was determined by Student’s t-test with a value of p < 0.05 or less considered to be statistically significant.
Results and discussion
Hepatic stellate cells (HSCs) are considered to play a key role in the pathogenesis of liver fibrosis. During liver fibrogenesis, HSCs are activated and acquire a myofibroblast-like phenotype which is accompanied by increased proliferation and ECM synthesisCitation2,Citation3. HSC activation in vivo can be induced by many factors, such as cytokines and soluble factors derived from damaged hepatocytes and Kupffer cells. HSC activation can also be induced in various conditions in vitro. Culturing HSCs on uncoated plastic plates is known to cause spontaneous activation, mimicking the process seen in vivo. The HSC-T6 cell line comprises immortalized rat hepatic stellate cells, and has been known to retain all features of activated stellate cells, including expression of desmin, α-smooth muscle actin, and glial fibrillary acidic protein, and it can esterify retinol into retinyl estersCitation8. Thus, we evaluated antifibrotic activity employing HSC-T6 cells by assessment of cell viability using the MTT assay.
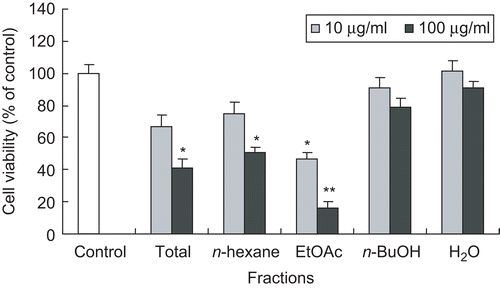
The methanolic extract of the aerial parts of E. japonica was further fractionated into n-hexane, EtOAc, and n-BuOH fractions. Among them, the n-hexane and EtOAc fractions significantly reduced cell viability at the concentration of 100 μg/mL (). Therefore, activity-guided fractionation of n-hexane and EtOAc fractions was carried out for the isolation of active constituents. Further fractionation and separation by several chromatographic methods yielded two triterpenoids (1–2) from the n-hexane fraction and two triterpenoids (3–4) from the EtOAc fraction. The structures of the triterpenoids were identified as friedeline (1), glut-5-en-ol (2), pomolic acid (3), and methylrotundate (4) (), by direct comparison of their physicochemical and spectroscopic data with those previously reportedCitation10–14. Among the four triterpenoids isolated, friedeline (1), glut-5-en-ol (2), and methylrotundate (4) are first reported from this plant.
Figure 1. Effect of total methanolic extract and the fractions of E. japonica on cell viability of HSC-T6 cells. HSC-T6 cells were incubated with test samples at the concentration of 10 or 100 μg/mL for 48 h. Cell viability was measured by the MTT assay. The percent of cell viability (%) was calculated as 100 × (absorbance of compound-treated/absorbance of control). Results are expressed as the mean ± SD of three independent experiments, each performed using triplicate wells. *p < 0.05, **p < 0.001 compared with control.
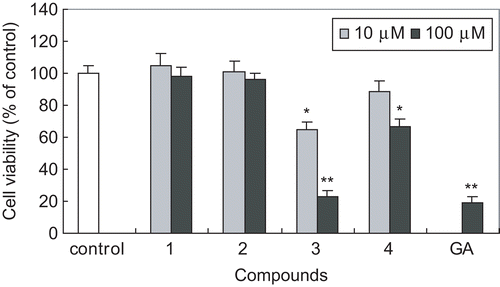
We further investigated the antiproliferative activity of these triterpenoids in HSC-T6 cells by assessing cell viability using the MTT assay. Among the compounds tested, compound 3 showed the most potent inhibitory activity on HSC cell viability at concentrations of 10 and 100 μM for 48 h incubation (). At the concentration of 100 μM, compound 3 inhibited cell viability up to 21%, which is comparable to that of β-glycyrrhetinic acid (GA), a positive control. Compound 4 showed weak activity; however, compounds 1 and 2 showed little activity ().
Figure 3. Effect of triterpenoids isolated from E. japonica on cell viability of HSC-T6 cells. HSC-T6 cells were incubated with compounds at the concentration of 10 or 100 μM for 48 h. GA (β-glycyrrhetinic acid) was used as a positive control. Cell viability was measured by the MTT assay. The percent of cell viability (%) was calculated as 100 × (absorbance of compound-treated/absorbance of control). Results are expressed as the mean ± SD of three independent experiments, each performed using triplicate wells. *p < 0.05, **p < 0.001 compared with control.
Recently, there has been growing interest in the search for antifibrotic compounds from natural products. As a result, a diverse skeleton of natural products including flavonoids, alkaloids, and terpenoids have been suggested to have antifibrotic activityCitation15–19. We previously reported the antifibrotic activity of triterpenoids from Eclipta prostrataCitation9. In addition, many other triterpenoids have been reported to have antifibrotic activity. Glycyrrhetinic acid and carbenoxolone, a derivative of glycyrrhetic acid, inhibited HSC proliferation and ECM productionCitation15,Citation20. Asiatic acid and its derivatives were reported to exert an antifibrotic effect in HSC-T6 cells by reduction of collagen synthesis and cell proliferationCitation21. These results together with those from our present study suggest the therapeutic potential of triterpenoids in liver fibrosis.
Acknowledgments
We thank Professor S. L. Friedman (Columbia University, New York) for kindly gifting the HSC-T6 cells.
Declaration of interest: This work was supported by a Seoul R&BD Program (10541).
References
- Friedman SL. Liver fibrosis – from bench to beside. J Hepatol 2003;38:S38–53.
- Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J Gastroenterol Hepatol 1999;14:618–33.
- Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta 2006;364:33–60.
- Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol 2000;35:665–72.
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209–18.
- Bae KH. Dictionary of Korean Folk Medicine. Seoul: Kyu Hak Sa, 2000:317.
- Lee MK, Jeon HY, Lee KY, Kim SH, Ma CJ, Sung SH, et al. Inhibitory constituents of Euscaphis japonica on lipopolysacchride-induced nitric oxide production in BV2 microglia. Planta Med 2007;73:782–6.
- Vogel SR, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, et al. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res 2000;41:882–93.
- Lee MK, Ha NR, Yang H, Sung SH, Kim GH, Kim YC. Anti-proliferative activity of triterpenoids from Eclipta prostrata on hepatic stellate cells. Phytomedicine 2008;15:775–80.
- Akihisa T, Yamamoto K, Tamura T, Kimura Y, Iida T, Nambara T, et al. Triterpenoid ketone from Lingnania chungii Mcclure: arborinone, friedelin, and glutinone. Chem Pharm Bull 1992;40:789–91.
- El-Seedi HR. Antimicrobial triterpenes from Poulsenia armata Miq. Standl. Nat Prod Res 2005;19:197–202.
- Mahaho SB, Kundu AP. 13C NMR spectra of pentacyclic triterpenoids – a complication and some salient features. Phytochemistry 1994;37:1517–75.
- Cheng DL, Cao XP. Pomolic acid derivatives from the root of Sanguisorba officinalis. Phytochemistry 1992;31:1317–20.
- Nakatani M, Miyazaki Y, Iwashita T, Naoki H, Hase T. Triterpenes from Ilex rotunda fruit. Phytochemistry 1989;23:1479–82.
- Uyama N, Shimahara Y, Okuyama H, Kawada N, Kamo S, Ikeda K, et al. Carbenoxolone inhibits DNA systhesis and collagen gene expression in rat hepatic stellate cells in culture. J Hepatol 2003;39:745–99.
- Chen A, Zhang L. The antioxidant (-)-epigallocatechin-3-gallate inhibits rat hepatic stellate cell proliferation in vitro by blocking the tyrosine phosphorylation and reducing the gene expression of platelet-derived growth factor-beta receptor. J Biol Chem 2003;278:23381–9.
- Sakata R, Ueno T, Nakamura T, Sakamoto M, Torimura T, Sata M. Green tea polyphenol epigallocatechin-3-gallate inhibits platelet-derived growth factor-induced proliferation of human hepatic stellate cell line LI90. J Hepatol 2004;40:52–9.
- Chen YW, Li DG, Wu JX, Chen YW, Lu HM. Tetrandrine inhibits activation of rat hepatic stellate cells stimulated by transforming growth factor-β in vitro via up-regulation of Smad 7. J Ethnopharmacol 2005;100:299–305.
- Lin YL, Lee TF, Huang YJ, Huang YT. Antiproliferative effect of salvianolic acid A on rat hepatic stellate cells. J Pharm Pharmacol 2006;58:933–9.
- Wang JY, Zhang OS, Guo JS, Hu MY. Effects of glycyrrhetinic acid on collagen metabolism of hepatic stellate cells at different stages of liver fibrosis in rats. World J Gastroenterol 2001;7:115–19.
- Dong MS, Jung SH, Kim HJ, Kim JR, Zhao LX, Lee ES, et al. Structure-related cytotoxicity and anti-hepatofibric effect of asiatic acid derivatives in rat hepatic stellate cell-line. Arch Pharm Res 2004;27:512–17.