Abstract
Enzyme-assisted water extracts (EWEDL) and ethanol extracts of Du-zhong leaves (EEDL) were evaluated for their antioxidant activities using the DPPH radical-scavenging assay, Fe2+-chelating assay, and inhibition ability of the linoleic acid peroxidation assay. In general, the antioxidant activity of Du-zhong leaf extracts increased with increasing concentration. Based on the two extracting methods with different antioxidative reactions, it was shown that the enzyme-assisted water extracting method was more effective for antioxidant extraction from Du-zhong leaves. By HPLC-MS analysis, the main phenolic compounds (geniposidic acid, epicatechin, and chlorogenic acid) identified in EWEDL and EEDL were similar. EWEDL and EEDL had total phenolic contents of 13.84 ± 0.11 and 14.72 ± 0.14 mg chlorogenic acid equivalents (CAE) in each gram of extract, respectively. However, there was no positive correlation between total phenolic content and antioxidant activities of EWEDL and EEDL measured by the three different assays.
Introduction
Du-zhong (Eucommia ulmoides Oliv.) is an important tonic herb widely used in China, Japan, and Korea. According to ancient records, Du-zhong possesses many pharmacological effects, including reinforcement of the muscles and lungs, prevention of abortion, lowering of blood pressure, and antioxidant activityCitation1,2. Previously, only the bark of the Du-zhong was thought to contain the medicinally effective components, but lately, interest has focused on the leaves, as the bark of Eucommia ulmoides Oliv. tree can only be peeled off after 20 years, and after that the tree will probably dieCitation3. Moreover, it has been reported that Du-zhong leaves contain similar medical components and nutrient components to barkCitation4. In Japan, Du-zhong tea, an aqueous extract of Du-zhong leaves and a popular beverage, is used in the treatment of hypertension, and is thought to be a functional healthy foodCitation2,5. Thus, there has been increasing interest in research study of Du-zhong leaves.
Previous literature reveals that several studies have been performed on the extraction of bioactive components from Du-zhong leaves, including aqueous extractionCitation6,7, organic solvent extraction (ethanol, methanol, and acetone)Citation3,8, and physical extraction (ultrasonic technique, microwave technique, and supercritical fluid extraction)Citation9,10. Application of these procedures presents some disadvantages, such as the loss of bioactive components due to ionization, hydrolysis, and oxidation during extraction, and the consumption of a large amount of solvent and energyCitation10. Enzyme extraction, as a fairly new procedure, has been studied by some researchers. Treatment with several enzymes (mainly cellulase, pectinase, and hemicellulase) can disintegrate and hydrolyze cell-wall materials (mainly cellulose and pectin) for better separation and solvent extraction of the intracellular componentsCitation11,12. Enzyme-assisted solvent extraction has been widely used for bioactive component extraction in plant materials such as sweet potato, orange peel, carrot, and soybeanCitation11,13,14. In particular, enzyme-assisted water extraction enhances the extraction yield, and improves product quality compared with the original aqueous process without enzymes in oil extraction. Moreover, it eliminates solvent consumption and the energy requirementCitation15,16. However, few reports are related to the application of enzyme extraction in Chinese medicinal herbs.
Natural antioxidants from plant extracts have attracted increasing interest due to consumer concern about the safety of synthetic antioxidants in foodCitation17. The extracts from Du-zhong leaves, being a potential resource of natural antioxidant, are capable of scavenging reactive oxygen species, inhibiting Fenton reaction-induced oxidative damage in biomoleculesCitation2,18. Recently, it was indicated that the inhibitory activity of water extracts of Du-zhong (leaves, roasted cortex, and raw cortex) on the peroxidation of linoleic acid measured by the thiocyanate method followed the order leaves > roasted cortex > raw cortex at 60 h of incubation, and all water extracts of Du-zhong were found to possess inhibitory effects on the oxidative modification of low-density lipoprotein (LDL) induced by Cu2+Citation19,20. However, investigations into the effects of different extraction methods on the antioxidant activity of Du-zhong leaves are still relatively rare.
The main objectives of this work were to prepare extracts from Du-zhong leaves using enzyme-assisted water extraction and ethanol extraction, and evaluate the antioxidant activity with different methods including the radical-scavenging effect, Fe2+-chelating ability, and inhibition ability of linoleic acid peroxidation. The results for antioxidant activity have been compared with those of a synthetic antioxidant (tertiary butylhydroxyquinone (TBHQ)). Furthermore, the total phenolic content (TPC) has also been evaluated.
Materials and methods
Materials and reagents
Dried Du-zhong leaves were purchased from a medicinal herbs base (Hanzhong, China), homogenized to a fine powder, and stored at 5°C until use.
Cellulase (15,000 U/g), linoleic acid, trichloroacetic acid (TCA), and thiobarbituric acid (TBA) were purchased from Shanghai Chemical Co. (Shanghai, China). Epicatechin, chlorogenic acid, α,α-diphenyl-β-picrylhydrazyl (DPPH), and {4,49-[3-(2-pyridinyl)-1,2,4-triazine-5,6-diyl]bisbenzenesulfonic acid} (Ferrozine) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Tertiary butylhydroxyquinone (TBHQ) was purchased from Liyuan Industries, Ltd. (Beijing, China). All other chemicals and solvents were of HPLC (high performance liquid chromatography) grade or analytical reagent grade.
Preparation of enzyme-assisted water extracts of Du-zhong leaves (EWEDL)
One hundred grams of Du-zhong leaf powder were finely mixed with 1000 mL distilled water (pH 4.5, adjusted with citric acid) in a large beaker. One gram of cellulase was added and the mixture was stirred on a magnetic stirrer for 5 min. This mixture was placed in a water bath (Model HH-S; Jiangsu Zhenjiang Instrument Co. Ltd., Jiangsu, China) at 50°C. After 2.5 h of enzyme treatment, the mixture was vacuum-filtered through Whatman No. 2 filter paper using a Buchner funnel. The residue was washed with distilled water twice. Combined filtrates were evaporated under vacuum below 40°C using a rotary evaporator (Model R52; Shanghai Yarong Biochemistry Instrument Co., Shanghai, China) to a final volume of approximately 50 mL. Then the extract was freeze-dried in vacuo to powder form by a freeze dryer (Model LGJ102; Sihuan Instrument Co., Beijing, China), and stored at −36°C until further use.
Preparation of ethanol extracts of Du-zhong leaves (EEDL)
Du-zhong leaf powder (100 g) was extracted using a Soxhlet extractor for 2 h with 500 mL of ethanol under reflux conditions. The extract was vacuum-filtered through Whatman No. 2 filter paper using a Buchner funnel, the filtrates were evaporated under vacuum below 40°C to a final volume of approximately 50 mL, and then the extract was freeze-dried in vacuo to powder form, and stored at −36°C until further use.
Determination of total phenolic content
The total phenolic content of the extract was determined according to the method of Sokmen et al.Citation21. One milligram of extract was taken in a volumetric flask, 46 mL distilled water and l mL Folin–Ciocalteau reagent were added, and the flask was shaken thoroughly. After 3 min, 3 mL solution of Na2CO3 (2%, w/v) was added and the mixture was allowed to stand for 2 h with intermittent shaking. Absorbance was measure at 760 nm using a spectrophotometer (Hitachi UV-Vis model U-3110 spectrophotometer; Tokyo, Japan). The total phenolic amount was calculated as chlorogenic acid equivalents (CAE) from a calibration curve.
Determination of DPPH radical-scavenging activity
The radical-scavenging activity assay was performed as described, with some modificationsCitation22. An ethanol solution (1 mL) of Du-zhong leaf extract (0.16–10 mg/mL), or TBHQ (standard, 0.02–1.25 mg/mL), was mixed with 100 mM Tris-HCl buffer (2 mL, pH 7.4) and then added to 2 mL of 1.5 mM DPPH in ethanol. The mixture was shaken vigorously and left to stand for 20 min at room temperature in the dark. The absorbance was read using a spectrophotometer at 517 nm. The DPPH radical-scavenging effect was calculated using the following equation:
where Ablank is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the test compound. The scavenging effect of the sample was expressed as 50% effective concentration (EC50), which represented the concentration of sample having 50% DPPH radical-scavenging effect. All tests were carried out in triplicate.
Determination of Fe2+-chelating ability
The Fe2+-chelating ability was determined according to the method of Decker and WelchCitation23, described by Chou et al.Citation24. Fe2+ was monitored by measuring formation of the ferrous iron–Ferrozine complex. Du-zhong leaf extract (1.25–20 mg/mL), or TBHQ (standard, 0.02–1.25 mg/mL), was mixed with 2 mM FeCl2 and 5 mM Ferrozine at a ratio of 10:1:2. The mixture was shaken and left at room temperature for 10 min. The absorbance of the resulting solution was measured at 562 nm. A lower absorbance of the reaction mixture indicated a higher Fe2+-chelating ability. The ability to chelate the ferrous iron was calculated by the following equation:
where Ablank is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the test compound. The Fe2+-chelating ability of the sample was expressed as 50% effective concentration (EC50), which represented the concentration of sample having 50% Fe2+-chelating ability. All tests were carried out in triplicate.
Determination of lipid peroxidation in linoleic acid system
Thiobarbituric acid reactive substances (TBARS) were determined according to the modified method of McDonald and HultinCitation25, described by Chun et al.Citation26. Emulsions were prepared by homogenizing 10% linoleic acid and 5% Tween 40 in 100 mL of distilled water. One milliliter of emulsion was added to a glass tube containing 1 mL ethanol solution of Du-zhong leaf extract (0.62–20 mg/mL), or TBHQ (standard, 0.02–1.25 mg/mL), and 2 mL of phosphate buffer (0.2M, pH 7.4). Tubes were incubated at 50°C for 10 h. The reaction was terminated by adding 2 mL TCA (20%, w/v), followed by 0.5 mL TBA (2%, w/v). The mixture was vortexed and heated in a boiling water bath for 90 min. After cooling with tap water for 10 min, the solution was centrifuged for 15 min at 2000g. The absorbance of the upper layer was measured at 532 nm. The inhibition percentage of lipid peroxidation of the sample was calculated by the following equation:
where Ablank is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the test compound. The inhibition of lipid peroxidation of the sample was expressed as 50% inhibition concentration (IC50), which represented the concentration of sample having 50% inhibition effect on the lipid peroxidation of linoleic acid. All tests were carried out in triplicate.
HPLC-MS instrumentation and conditions
Polyphenol analyses from EWEDL and EEDL were carried out on an HP 1100 HPLC system equipped with a diode array detector (DAD) (Agilent Technologies, Palo Alto, CA, USA) and interfaced with an Adilent 1100 LC/MSD Ion Trap (Agilent Technologies) mass spectrometer (MS) with an electrospray interface (ESI). Separation was carried out using a Waters C18 reverse column (250 × 4. 6 mm i.d., 5 μm; Waters, CA, USA). The samples were analyzed according to the method of Li et al., with minor modificationCitation9. Samples were dissolved in methanol, filtered through a 0.45 μm nylon filter, and injected (10 μL) into a HPLC-MS system. CH3OH-H2O-CH3COOH (20:80:1, v/v) was used as the mobile phase, the flow rate was 1 mL/min, the column was at room temperature, and the detecting wavelength was set at 240 nm.
The mass spectrometer was programmed to operate in full scan MS mode from m/z 50 to 2200. Mass spectra were acquired in negative mode with ion spray voltage at 3.5 kV, capillary temperature at 350°C, and capillary voltage at −85.5 V. Nitrogen was used as the drying gas at 5 L/min and 325°C.
In EWEDL and EEDL, quantification of epicatechin and chlorogenic acid were achieved by comparison with an external standard of known phenolic compounds and expressed as milligrams per gram of extract; standard curves were obtained for each standard. Because of the lack of geniposidic acid standard, the amount of geniposidic acid was calculated as chlorogenic acid equivalents (CAE) from a calibration curve.
Statistical analysis
All results were obtained in triplicate and data are presented as mean ± standard deviation. The mean values of data were analyzed by one-way analysis of variance (ANOVA) and the significance of the difference between means was determined by Duncan’s multiple range test (p < 0.05) using SPSS software (SPSS, version 12.0).
Results and discussion
Proton radical-scavenging action is known as an important mechanism for measuring antioxidant activity. DPPH was used to determine the proton radical-scavenging action of EWEDL, EEDL, and TBHQ, and results are shown in . Both extracts and TBHQ were capable of quenching DPPH radicals in a concentration-dependent manner. At a concentration of 0.16–10 mg/mL, the scavenging activity of EWEDL on DPPH radicals increased with increasing concentration of EWEDL. In particular, the DPPH radical-scavenging activity of EWEDL increased significantly with concentration from 0.16 to 0.31 mg/mL, and leveled off as the concentration further increased; a similar result was found for EEDL at a concentration of 0.16–2.5 mg/mL and TBHQ at a concentration of 0.02–0.16 mg/mL. These results implied that the antioxidant activity of extracts from Du-zhong leaves might be attributed to their proton-donating ability, because both EWEDL and EEDL might prevent reactive radical species from reaching biomolecules by means of hydrogen and/or electron donationCitation22.
Figure 1. Scavenging effect of enzyme-assisted water extracts (EWEDL) and ethanol extracts (EEDL) of Du-zhong leaves on α,α-diphenyl-β-picrylhydrazyl (DPPH) radicals. Values are expressed as mean (n = 3). Tertiary butylhydroxyquinone (TBHQ) was used as the standard.
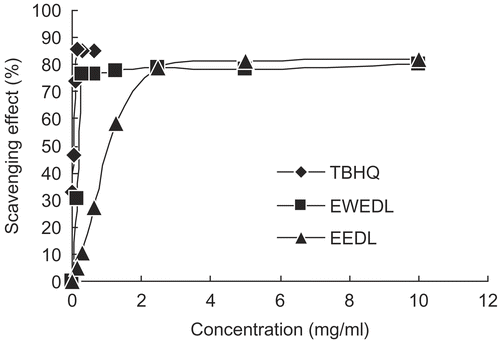
The EC50 value, i.e. the effective concentration of a sample for 50% reduction of free radicals, was determined from the plotted graph of scavenging activity against concentration of Du-zhong leaf extract. The quality of scavenging activity for EWEDL, EEDL, and TBHQ was evaluated by EC50 value as shown in . A low EC50 value indicates strong antioxidant activity in a sample. Based on , EWEDL provided a significantly lower (p < 0.05) EC50 value of 0.10 ± 0.01 mg/mL than that of EEDL (1.35 ± 0.03 mg/mL). However, when compared to the standard, TBHQ, both EWEDL and EEDL showed lower radical-scavenging activity; TBHQ had a significantly lower (p < 0.05) EC50 of 0.04 ± 0.01 mg/mL.
Table 1. Antioxidant activities of extracts from Du-zhong leaves and tertiary butylhydroxyquinone (TBHQ) as expressed by IC50 or EC50.
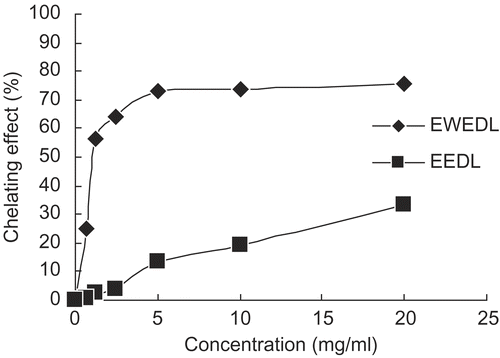
Food is often contaminated with transition metal ions, which may be introduced by manufacturing methods. Bivalent transition metal ions play an important role as catalysts of oxidative processes, leading to the formation of hydroxyl radicals and hydroperoxide decomposition reactions via Fenton chemistry; these processes can be delayed by iron chelation and deactivationCitation27,28. Since Fe2+ has also been shown to cause the production of oxyradicals and lipid peroxidation, minimizing the Fe2+ concentration in the Fenton reaction affords protection against oxidative damage. Therefore, the ability of Du-zhong leaf extracts to chelate Fe2+ was evaluated, and the result is presented in . EWEDL showed 25.06–75.76% ability to chelate Fe2+ at a concentration of 0.62–20 mg/mL, and its chelating ability increased with concentration of the extract; however, the highest chelating ability of EEDL was only 33.43% in the same concentration range. The chelating ability of EWEDL was about 2.5–50 times that of EEDL at the same concentration level. The result showed that EWEDL had a better Fe2+-chelating ability, and EEDL possessed limited chelating effects under the experimental conditions. In the case of Du-zhong leaves, the result implied that enzyme-assisted water extraction might be a better extraction method than ethanol extraction for extracting Fe2+-chelating agent. However, TBHQ in the range of 0.02–0.62 mg/mL showed no detectable Fe2+-chelating ability. Chou et al. reported a similar result of Fe2+-chelating ability for butylated hydroxytoluene (BHT) and α-tocopherol using the same methodCitation24. EWEDL in our research showed an EC50 of 1.45 ± 0.03 mg/mL (), but EC50 values for EEDL and TBHQ were not obtained in the Fe2+-chelating ability assay.
Figure 2. Chelating effect of EWEDL and EEDL on Fe2+. Values are expressed as mean (n = 3). TBHQ was used as the standard.
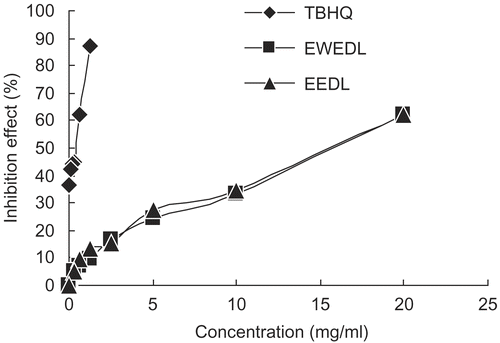
In the present study, the effect of the Du-zhong leaf extracts on the inhibition of lipid peroxidation in linoleic acid was determined by the TBA method, in which the amount of thiobarbituric acid reactive substances (TBARS), namely malonaldehhyde (MDA), a secondary lipid peroxidation product, was determined by measuring the absorbance at 532 nmCitation29. From , it can be seen that, in the range 0.31–20 mg/mL, EWEDL and EEDL exhibited 5.31–62.12% and 5.16–62.35% inhibition effect of linoleic acid oxidation, respectively. This result indicated that EWEDL and EEDL showed moderate inhibition activity in the linoleic acid peroxidation system, compared to the DPPH radical-scavenging activity assay. The IC50 value () for EWEDL was 16.62 ± 0.10 mg/mL, which was slightly lower than the IC50 value for EEDL (16.74 ± 0.09 mg/mL), but no significant difference existed between them (p < 0.05). The highest inhibition activity in the linoleic acid peroxidation system was found to be exhibited by TBHQ, as positive control, which had a significantly lower (p < 0.05) IC50 value at 0.76 ± 0.02 mg/mL than those of EWEDL and EEDL.
Figure 3. Inhibition effect of EWEDL and EEDL on linoleic acid peroxidation. Values are expressed as mean (n = 3). TBHQ was used as the standard.
Due to the differences observed in the antioxidant activities of EWEDL and EEDL, their phenolic compounds were studied by HPLC in a reversed-phase column coupled with a diode array detector and mass spectrometer (HPLC-DAD-MS). Identification of the main phenolic compounds in EWEDL and EEDL was carried out by comparing HPLC retention time, ultraviolet (UV) absorption, and MS fragment pattern with those of the standards and literature data. The chromatograms of EWEDL and EEDL are shown in , while and show retention times, MS spectral data, and identification results for peaks numbered in the chromatogram.
Figure 4. High performance liquid chromatography-diode array detection (HPLC-DAD) chromatograms of (A) ethanol extracts of Du-zhong leaves (EEDL) and (B) enzyme-assisted water extracts of Du-zhong leaves (EWEDL). Peaks: 1, geniposidic acid; 2, epicatechin; 3, chlorogenic acid.
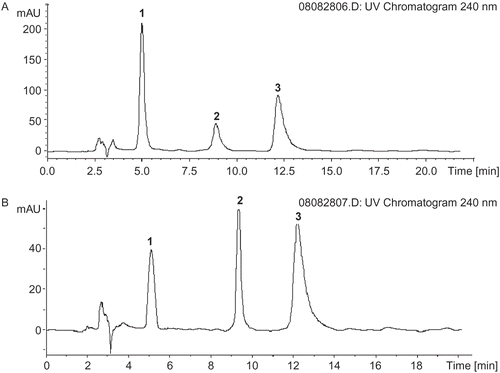
Figure 5. Mass spectra of peak 1 (A), peak 2 (B), and peak 3 (C) in HPLC chromatograms for EWEDL and EEDL.
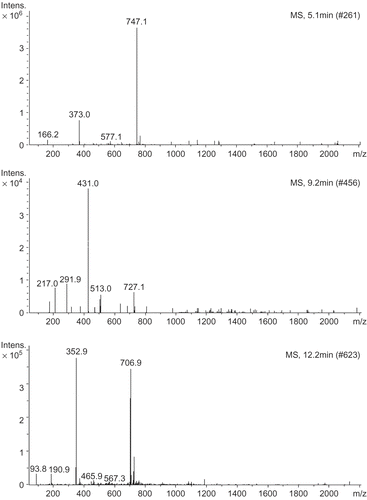
Table 2. Identification of phenolic compounds contained in enzyme-assisted water extracts of Du-zhong leaves (EWEDL) and ethanol extracts of Du-zhong leaves (EEDL).
Peaks 2 and 3 were identified as epicatechin and chlorogenic acid, respectively, by comparing to the HPLC retention times and mass spectra of authentic standards and literature dataCitation30–32. Du-zhong leaves have been reported to contain epicatechin and chlorogenic acidCitation10,18, and thus, peak 2 of the HPLC chromatograms for EWEDL and EEDL (), having an [M–H]− ion at m/z 292, could be epicatechin, which has a molecular weight (MW) of 290. Also, peak 3 may be chlorogenic acid, which has an MW of 354. Because of the lack of corresponding reference compound, peak 1was tentatively identified as geniposidic acid (fragment m/z 373) by comparing to MS spectra of literature dataCitation32,33. It should be noted that peaks 1, 2, and 3 had signals with higher molecular weights at 747, 431, and 707 m/z (), respectively, in their MS spectra. One possible explanation for this result is that the main phenolic compounds in EWEDL and EEDL were present as their glycosidic compounds. Glycosidic compounds are common in plant material. Tong et al. reported that geniposidic acid was present as linking to the aglycone form in Du-zhong leaves and barkCitation33. As shown in and , the main phenolic compounds (geniposidic acid, epicatechin, and chlorogenic acid) identified in EWEDL and EEDL were similar. Several studies have reported the same phenolic compounds identified in Du-zhong leaf extractsCitation3,10,18. This result showed that there was no difference in main phenolic compounds extracted by the enzyme-assisted water method, compared to ethanol extraction, in Du-zhong leaves; however, differences were found in contents of the three main phenolic compounds (). Hence, differences observed in antioxidant activity could be explained by differences of the main phenolic contents, not the main phenolic compounds in EWEDL and EEDL.
Table 3. Concentrations of main phenolic compounds in EWEDL and EEDL (mg/g of extract).
Phenolic compounds in plants are powerful antioxidants, which may significantly contribute to the overall antioxidant activity. Total phenolic contents were determined for both Du-zhong leaf extracts (). The total phenolic content for EWEDL was 13.84 ± 0.11 mg chlorogenic acid equivalents (CAE) in each gram of extract, which was slightly lower than that of EEDL (14.72 ± 0.14 mg CAE in each gram of extract); however, no significant difference existed between EWEDL and EEDL (p < 0.05). This result implied that enzyme-assisted water extraction might also be an effective extracting method for phenolic compounds, compared to ethanol extraction, in Du-zhong leaves. Several studies have reported a significant positive correlation between total phenolic content and antioxidant activity of extracts from plant materials, such as mushroom and Sorbus domestica fruitsCitation34,35. Nevertheless, our study showed no positive correlation between total phenolic content and scavenging ability of EWEDL and EEDL on DPPH radicals. This finding was the same as results obtained from the Fe2+-chelating assay and lipid peroxidation assay. The result could be due to the fact that the total phenolic content did not include all the antioxidantsCitation17; EWEDL and EEDL could possess different antioxidant compounds from phenolic compounds, such as β-carotene and tocopherolCitation4.
Table 4. Total phenolic content (TPC) of extracts from Du-zhong leaves, mg/g of extract, as chlorogenic acid equivalents (CAE).
In conclusion, the extracting method and the evaluation method both significantly affected the antioxidant ability of Du-zhong leaves, based on enzyme-assisted water extraction and ethanol extraction results. Compared to EEDL, EWEDL showed higher antioxidant activity when evaluated by DPPH radical-scavenging assay and Fe2+-chelating ability assay. EWEDL and EEDL showed moderate antioxidant activity when determined by lipid peroxidation assay. By the three antioxidant methods used in our research, the extracts from Du-zhong leaves could not be comprehensively evaluated for antioxidant activity; however, they may serve as potential dietary sources of natural antioxidants for human nutrition and health. In EWEDL and EEDL, there was no significant difference in total phenolic yield. Nevertheless, enzyme-assisted water extraction has some advantages, such as moderate extracting conditions, without organic solvent residues and so on, compared to ethanol extraction. These advantages are important in terms of natural antioxidants applied to the food industry; therefore, enzyme-assisted water extraction is a possible effective way of bioactive component extraction from plant materials.
Acknowledgments
The authors are thankful to Professor Jing Zhang for generously providing laboratory facilities. This work was financially supported by the Ministry of Education of P. R. China (Project No. 00118).
Declaration of interest: The authors report no conflicts of interest.
References
- Lee MK, Kim MJ, Cho SY, Park SA, Park KK, Jung UJ, et al. Hypoglycemic effect of Du-zhong (Eucommia ulmoides Oliv.) leaves in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract 2005;67:22–8.
- Hsieh CL, Yen GC. Antioxidant actions of Du-zhong (Eucommia ulmoides Oliv.) toward oxidative damage in biomolecules. Life Sci 2000;66:1387–400.
- Kulomaa A, Siren H, Riekkola ML. Identification of antioxidative compounds in plant beverages by capillary electrophoresis with the marker index technique. J Chromatogr A 1997;781:523–32.
- Zhang KJ, Wang L, Zhang FY, Chen R. A comparison between active component contents in the bark and leaves of Eucommia ulmoides Oliv. J Northw Forestry Coll 1996;11(2):42–6 ( in Chinese).
- Nakamura T, Nakazawa Y, Onizuka S, Satoh S, Chiba A, Sekihashi K, et al. Antimutagenicity of Tochu tea (an aqueous extract of Eucommia ulmoides leaves): 1. The clastogen-suppressing effects of Tochu tea in CHO cells and mice. Mutat Res 1997;388:7–20.
- Kwan CY, Chen CX, Deyama T, Nishibe S. Endothelium-dependent vasorelaxant effects of the aqueous extracts of the Eucommia ulmoides Oliv. leaf and bark: implications on their antihypertensive action. Vasc Pharmacol 2004;40:229–35.
- Hung MY, Timothy YCF, Shih PH, Lee CP, Yen GP. Du-zhong (Eucommia ulmoides Oliv.) leaves inhibit CCl4-induced hepatic damage in rats. Food Chem Toxicol 2006;44:1424–31.
- Matsud E, Yoshizawa Y, Yokosawa Y, Watanabe N, Kawaii S, Murofushi N. Effects of Eucommia ulmoides Oliv. leaf extract on 3T3-L1 differentiation into adipocytes. J Nat Med 2006; 60:126–9.
- Li H, Chen B, Zhang ZH, Yao SZ. Focused microwave-assisted solvent extraction and HPLC determination of effective constituents in Eucommia ulmoides Oliv. (E. ulmoides). Talanta 2004;63:659–65.
- Li H, Chen B, Yao SZ. Application of ultrasonic technique for extracting chlorogenic acid form Eucommia ulmoides Oliv. (E. ulmoides). Ultrason Sonochem 2005;12:295–300.
- Çinar İ. Effects of cellulase and pectinae concentrations on the colour yield of enzyme extracted plant carotenoids. Process Biochem 2005; 40:945–9.
- Kotcharian A, Kunzek H, Dongowski G. The influence of variety on the enzymatic degradation of carrots and on functional and physiological properties of the cell wall materials. Food Chem 2004;87:231–45.
- Çinar İ. Stability studies on the enzyme extracted sweet potato carotenoproteins. Food Chem 2005;89:397–401.
- Rosenthal A, Pyle DL, Niranjan K, Gilmour S, Trinca L. Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enzym Microb Technol 2001;28:499–509.
- Zúñiga ME, Soto C, Mora A, Chamy R, Lema JM. Enzymic pre-treatment of Guevina avellana mol oil extraction by pressing. Process Biochem 2003;39:51–7.
- Rosenthal A, Pyle DL, Niranjan K. Aqueous and enzymatic processes for edible oil extraction. Enzym Microb Technol 1996;19:402–20.
- Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chem 2005;90:743–9.
- Yen GC, Hsieh CL. Reactive oxygen species scavenging activity of Du-zhong (Eucommia ulmoides Oliv.) and its active compounds. J Agric Food Chem 2000;48:3431–6.
- Yen GC, Hsieh CL. Antioxidant activity of extracts from Du-zhong (Eucommia ulmoides) toward various lipid peroxidation models in vitro. J Agric Food Chem 1998;46:3952–7.
- Yen GC, Hsieh CL. Inhibitory effects of Du-zhong (Eucommia ulmoides Oliv.) against low-density lipoprotein oxidative modification. Food Chem 2002;77:449–56.
- Sokmen M, Angelova M, Krumova E, Pashova S, Ivanchwva S, Sokmen A, et al. In vitro antioxidant activity of polyphenol extracts with antiviral properties form Geranium sanguineum L. Life Sci 2005;76:2981–93.
- Lai LS, Chous ST, Chou WW. Studies on the antioxidative activities of Hsian-tsao (Mesona procumbens Hemsl) leaf gum. J Agric Food Chem 2001;49:963–8.
- Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem 1990;38:674–7.
- Chou ST, Chou WW, Chung YC. Antioxidative activity and safety of 50% ethanolic red bean extract (Phaseolus radiatus L. var. Aurea). J Food Sci 2003;68(1):21–5.
- McDonald RE, Hultin HO. Some characteristics of the enzymic lipid peroxidation systems in the microsomal fraction of flounder muscle. J Food Sci 1987;52:15–21.
- Chun SS, Vattem DA, Lin YT, Shetty K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem 2005;40:809–16.
- Hinneburg I, Dorman HJD, Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem 2006;97:122–9.
- Halliwell B. Antioxidants: the basics – what they are and how to evaluate them. Adv Pharmacol 1997;38:3–20.
- Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem 2000;69:167–74.
- Ayaz FA, Sema HA, Gruz J, Novak O, Strnad M. Separation, characterization, and quantitation of phenolic acid in a little-known blueberry (vaccinium arctostaphylos L.) fruit by HPLC-MS. J Agric Food Chem 2005;53:8116–22.
- Pelillo M, Biguzzi B, Bendini A, Toschi G, Vanzini M, Lercker G. Preliminary investigation into development of HPLC with UV and MS-electrospray detection for the analysis of tea catechins. Food Chem 2002;78:369–74.
- Ni Y, Peng Y, Kokot S. Fingerprint anaysis of Eucommia bark by LC-DAD and LC-MS with the aid of chemometrics. Chromatographis 2008;67:211–17.
- Tong L, Wang Y, Xiong J, Cui Y, Zhou Y, Yi L. Selection and fingerprints of the control substances for plant drug Eucommia ulmodies Oliver by HPLC and LC-MS. Talanta 2008;76:80–4.
- Cheung LM, Cheung PCK, Ooi VC. Antioxidant activity and total phenolic of edible mushroom extracts. Food Chem 2003;81:249–55.
- Termentzi A, Kefalas P, Kokkalou E. Antioxidant activities of various extracts and fractions of Sorbus domestica fruits at different maturity stages. Food Chem 2006;98:599–608.