Abstract
Cathepsins are known to have many important physiological roles and provide a viable target for inhibition. Fluorobenzoyl dipeptide derivatives were synthesized and tested for biological activity in an effort to find an efficient inhibitor of the cysteine protease cathepsin L. Thirty-six novel inhibitors (1–36) were synthesized from protected amino acids via the standard DCC/HOBt coupling protocol, containing a benzyl ester or a nitrile as an electrophilic warhead. The activity of the inhibitors was evaluated against cathepsin L and IC50 values calculated. Modification of both amino acids and terminal groups afforded compounds with single digit micromolar inhibition. Results utilizing the benzoyl-L-leucine-glycine nitrile backbone are comparable to that for the commercially available inhibitor 39.
Introduction
The group of cysteine proteases known as the cathepsins have been shown to play a crucial function in diseases such as osteoporosis, rheumatoid arthritis, cancer metastasis, and infectious diseasesCitation1. Cathepsins are important targets for the development of inhibitors as therapeutic agents. Fasciolosis is caused by infection with Fasciola hepatica and Fasciola gigantica parasites. Not only is it an important human disease, but it also affects cattle and sheep worldwide, causing economic losses of approximately 2 billion dollarsCitation2. It is caused by the ingestion of vegetation or water contaminated with the encysted infectious liver fluke larvae known as metacercariaeCitation3. It has become clear that the predominant protease activity in this parasite is associated with cells of the gut epitheliumCitation4. Like many other parasitic helminths, Fasciola hepatica liver flukes release many proteolytic enzymes that belong to the group of cysteine proteases. It has been suggested that these proteases may be involved in protecting the parasite against immune attackCitation5.
Most of the current approaches to cathepsin inhibition involve molecules of a peptidic nature. The hydrolyzable amide is replaced by an electrophilic functionality and the catalytic thiol of the enzyme reacts with the inhibitor to form a covalent complex. Until recently, most potent cysteine protease inihibitors were irreversible inhibitors, with the electrophilic functionality alkylating the enzyme. Inhibitors containing electrophilic moieties such as an aldehyde, halomethyl ketone, or epoxide have been shown to be potent cysteine protease inhibitorsCitation6. Peptidic molecules containing the electrophilic benzyl ester and nitrile moieties have also been reported to be inhibitors of various cathepsinsCitation7–9.
For this study, all of the compounds (1–36) prepared are based on a dipeptidyl scaffold. Previous work in these laboratories has identified N-benzoyl-dipeptidyl derivatives as potential inhibitors of the Fasciola hepatica protease, such as N-benzoyl-L-leucine-glycine 37 (IC50 = 10.0 μM) and N-cinnamoyl-L-leucine-glycine nitrile 38 (IC50 = 11.0 μM)Citation10. The fluorobenzoyl moiety has been shown to be an effective N-terminal substituent in various potent inhibitorsCitation11.
Materials and methods
Chemistry
All chemicals were purchased from Sigma-Aldrich, Lennox Chemicals, or Fluorochem Limited, and used as received. When necessary, all solvents were purified and dried, and stored under argon. Tetrahydrofuran was distilled from Na/benzophenone and triethylamine distilled and stored over potassium hydroxide pellets. Commercial grade reagents were used without further purification procedures. Riedel-Haën silica gel was used for thin layer and column chromatography. Melting point determination was carried out using a Griffin melting point apparatus. Elemental analysis was carried out by the Microanalytical Laboratory at University College Dublin. Electrospray mass spectra were obtained on a Bruker Esquire 3000 series LC/MS (liquid chromatography/mass spectrometry) apparatus. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AC 400 NMR spectrometer operating at 400 MHz for Citation1H-NMR, 376 MHz for 19F-NMR, and 100 MHz for 13C-NMR. The 1H- and 13C-NMR chemical shifts (δ) are comparative to tetramethylsilane, and the 19F-NMR chemical shifts (δ) are relative to trifluoroacetic acid. All coupling constants (J) are in hertz (Hz).
N-2-fluorobenzoyl-l-leucine-glycine benzyl ester (1)
N-2-fluorobenzoyl-l-leucine (0.8 g, 3.16 mmol) was dissolved in dichloromethane (50 mL) with glycine benzyl ester tosylate (1.12 g, 3.16 mmol), 1-hydroxybenzotriazole (0.43 g, 3.16 mmol), and triethylamine (0.44 mL). The mixture was cooled to 0°C, and 1,3-dicyclohexylcarbodiimide (0.65 g, 3.16 mmol) was added. After 30 min the solution was raised to room temperature and the reaction was allowed to proceed for 48 h. The precipitated N,N-dicyclohexylurea was removed by filtration and the filtrate was washed with 10% potassium hydrogen carbonate, 10% citric acid, and water and dried over magnesium sulfate. The solvent was evaporated in vacuo, and recrystallization from ethyl acetate/hexane furnished 1 as a white powder (0.45 g, 37%). M.p. = 101–103°C. [α]D25 = −18.2° (c, 3.0, EtOH). Anal. calcd. for C22H25N2O4F1: C, 65.98; H, 6.29; N, 6.99%. Found: C, 65.59; H, 6.04; N, 7.12%. Mass spectrum: [M + Na]+ found 423.2. C22H25N2O4F1Na1 requires 423.44. IR (KBr): ν 3290, 2957, 1720, 1638, 1542, 752 cm−1. 1H-NMR (400 MHz, DMSO): δ 8.49 (1H, t, J = 6.0 Hz, -NH-), 8.35 (1H, dd, J = 2.8 & 8.4 Hz, -NH-), 7.62–7.66 (1H, m, -ArH 6), 7.50–7.55 (1H, m, -ArH 4), 7.24–7.36 (7H, m, -ArH 3, 5 & -PhH), 5.12 (2H, s, -OCH2-), 4.52–4.66 (1H, m, α-H), 3.99 (1H, dd, J = 6.4 & 17.2 Hz, -CH2-), 3.88 (1H, dd, J = 5.6 & 17.6 Hz, -CH2-), 1.50–1.73 (3H, m, -CH2- & -CH-), 0.89 (3H, d, J = 3.6 Hz, -CH3), 0.88 (3H, d, J = 3.6 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 172.9 (-CONH-), 169.9 (-COOCH2Ph), 164.0 (-ArCO-), 159.5 (d, -ArC 2), 136.1 (-PhC 1), 132.9 (d, -ArC 4), 130.5 (d, -ArC 6), 128.7 (-PhC 3 & 5), 128.4 (-PhC 4), 128.2 (-PhC 2 & 6), 124.7 (d, -ArC 5), 123.8 (d, -ArC 1), 116.4 (d, -ArC 3), 66.2 (-OCH2-), 51.8 (α-C), 41.1 (-CH2-), 41.0 (-CH2-), 24.6 (-CH-), 23.3 (-CH3), 21.7 (-CH3). 19F-NMR (376 MHz, DMSO): δ −38.7 to −38.8 (m).
N-3-fluorobenzoyl-l-leucine-glycine benzyl ester (2)
N-3-fluorobenzoyl-l-leucine (0.8 g, 3.16 mmol) and glycine benzyl ester tosylate (1.12 g, 3.16 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 2 as a white powder (0.55 g, 45%). M.p. = 76–78°C. [α]D25 = −21.3° (c, 3.2, EtOH). Anal. calcd. for C22H25N2O4F1: C, 65.98; H, 6.29; N, 6.99%. Found: C, 65.72; H, 6.41; N, 6.88%. Mass spectrum: [M + Na]+ found 423.1. C22H25N2O4F1Na1 requires 423.44. IR (KBr): ν 3275, 2958, 1744, 1637, 1560, 754 cm−1. 1H-NMR (400 MHz, DMSO): δ 8.63 (1H, d, J = 8.4 Hz, -NH-), 8.49 (1H, t, J = 6.0 Hz, -NH-), 7.73–7.79 (2H, m, -ArH 2 & 6), 7.49–7.53 (1H, m, -ArH 5), 7.31–7.41 (6H, m, -ArH 4 & -PhH), 5.12 (2H, s, -OCH2-), 4.54–4.58 (1H, m, α-H), 3.95 (1H, dd, J = 6.0 & 17.6 Hz, -CH2-), 3.87 (1H, dd, J = 5.6 & 17.2 Hz, -CH2-), 1.54–1.74 (3H, m, -CH2- & -CH-), 0.89 (3H, d, J = 6.4 Hz, -CH3), 0.85 (3H, d, J = 6.0 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 173.1 (-CONH-), 170.0 (-COOCH2Ph), 165.3 (d, -ArCO-), 162.2 (d, -ArC 3), 136.7 (d, -ArC 1), 136.2 (-PhC 1), 130.6 (d, -ArC 5), 128.7 (-PhC 3 & 5), 128.4 (-PhC 4), 128.2 (-PhC 2 & 6), 124.1 (d, -ArC 6), 118.5 (d, -ArC 4), 114.7 (d, -ArC 2), 66.2 (-OCH2-), 52.0 (α-C), 41.1 (-CH2-), 40.6 (-CH2-), 24.6 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO): δ −37.8 to −37.9 (m).
N-4-fluorobenzoyl-l-leucine-glycine benzyl ester (3)
N-4-fluorobenzoyl-l-leucine (0.8 g, 3.16 mmol) and glycine benzyl ester tosylate (1.12 g, 3.16 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 3 as a brown powder (0.80 g, 66%). M.p. = 108–110°C. [α]D25 = −22.2° (c, 3.2, EtOH). Anal. calcd. for C22H25N2O4F1: C, 65.98; H, 6.29; N, 6.99%. Found: C, 65.77; H, 6.31; N, 7.21%. Mass spectrum: [M + Na]+ found 423.2. C22H25N2O4F1Na1 requires 423.44. IR (KBr): ν 3285, 2957, 1742, 1632, 1502, 695 cm−1. 1H-NMR (400 MHz, DMSO): δ 8.51 (1H, d, J = 8.4 Hz, -NH-), 8.43 (1H, t, J = 6.0 Hz, -NH-), 7.92–7.98 (2H, m, -ArH 2 & 6), 7.26–7.40 (7H, m, -ArH 3, 5 & -PhH), 5.10 (2H, s, -OCH2-), 4.50–4.56 (1H, m, α-H), 3.93 (1H, dd, J = 6.0 & 17.6 Hz, -CH2-), 3.84 (1H, dd, J = 6.0 & 17.2 Hz, -CH2-), 1.52–1.71 (3H, m, -CH2- & -CH-), 0.88 (3H, d, J = 6.4 Hz, -CH3), 0.84 (3H, d, J = 6.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 173.2 (-CONH-), 170.0 (-COOCH2Ph), 165.6 (-ArCO-), 164.2 (d, -ArC 4), 136.1 (-PhC 1), 130.8 (-ArC 1), 130.6 (-ArC 2), 130.5 (-ArC 6), 128.7 (-PhC 3 & 5), 128.3 (-PhC 4), 128.2 (-PhC 2 & 6), 115.4 (-ArC 3), 115.2 (-ArC 5), 66.1 (-OCH2-), 51.9 (α-C), 41.1 (-CH2-), 40.6 (-CH2-), 24.7 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO): δ −34.1 to −34.2 (m).
N-pentafluorobenzoyl-l-leucine-glycine benzyl ester (4)
N-pentafluorobenzoyl-l-leucine (2.64 g, 8.1 mmol) and glycine benzyl ester tosylate (2.88 g, 8.1 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 4 as a white powder (1.56 g, 42%). M.p. = 165–167°C. [α]D25 = −20.8° (c, 1.5, EtOH). Anal. calcd. for C22H21N2O4F5: C, 55.93; H, 4.48; N, 5.93%. Found: C, 56.15; H, 4.62; N, 6.11%. Mass spectrum: [M + Na]+ found 495.1. C22H21N2O4F5Na1 requires 495.39. IR (KBr): ν 3287, 2966, 1745, 1662, 1546, 996 cm−1. 1H-NMR (400 MHz, DMSO): δ 9.13 (1H, d, J = 8.4 Hz, -NH-), 8.70 (1H, t, J = 6.0 Hz, -NH-), 7.31–7.38 (5H, m, -PhH), 5.13 (2H, s, -OCH2-), 4.60–4.64 (1H, m, α-H), 4.03 (1H, dd, J = 6.0 & 17.2 Hz, -CH2-), 3.84 (1H, dd, J = 6.0 & 17.6 Hz, -CH2-), 1.49–1.70 (3H, m, -CH2- & -CH-), 0.90 (3H, d, J = 6.4 Hz, -CH3), 0.89 (3H, d, J = 6.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 172.0 (-CONH-), 169.9 (-COOCH2Ph), 156.6 (-ArCO-),144.7–144.8 (m, -ArC 2), 140.3–142.8 (m, -ArC 4), 142.2 ( m, -ArC 6), 138.4–138.6 (m, -ArC 3), 136.1 (-PhC 1), 135.9–136.1 (m, -ArC 5), 128.7 (-PhC 3 & 5), 128.4 (-PhC 4), 128.3 (-PhC 2 & 6), 112.7 (t, -ArC 1), 66.2 (-OCH2-), 51.7 (α-C), 41.3 (-CH2-), 40.1 (-CH2-), 24.4 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO): δ −66.5 (dd, 2F, J = 6.7 & 22.9 Hz), −78.1 (t, J = 16.1 Hz), −86.4 to −86.5 (m, 2F).
N-2-fluorobenzoyl-l-leucine-β-alanine benzyl ester (5)
N-2-fluorobenzoyl-l-leucine (0.8 g, 3.16 mmol) and β-alanine benzyl ester tosylate (1.16 g, 3.16 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 5 as a brown powder (0.73 g, 58%). M.p. = 69–71°C. [α]D25 = −4.2° (c, 1.4, EtOH). Anal. calcd. for C23H27N2O4F1: C, 66.65; H, 6.56; N, 6.75%. Found: C, 66.60; H, 6.62; N, 6.77%. Mass spectrum: [M + Na]+ found 437.2. C23H27N2O4F1Na1 requires 437.46. IR (KBr): ν 3268, 2913, 1727, 1642, 1468, 721 cm−1. 1H-NMR (400 MHz, DMSO): δ 8.31 (1H, dd, J = 2.4 & 8.0 Hz, -NH-), 8.16 (1H, t, J = 5.6 Hz, -NH-), 7.61–7.65 (1H, m, -ArH 6), 7.50–7.55 (1H, m, -ArH 4), 7.24–7.36 (7H, m, -ArH 3, 5 & -PhH), 5.09 (2H, s, -OCH2-), 4.46–4.50 (1H, m, α-H), 3.29–3.44 (2H, m, β-CH2), 2.54 (2H, t, J = 6.8 Hz, α-CH2), 1.46–1.67 (3H, m, -CH2- & -CH-), 0.89 (3H, d, J = 2.8 Hz, -CH3), 0.87 (3H, d, J = 2.8 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 172.2 (-CONH-), 171.5 (-COOCH2Ph), 163.8 (-ArCO-), 159.5 (d, -ArC 2), 136.3 (-PhC 1), 132.8 (d, -ArC 4), 130.5 (d, -ArC 6), 128.7 (-PhC 3 & 5), 128.3 (-PhC 4), 128.2 (-PhC 2 & 6), 124.7 (d, -ArC 5), 123.9 (d, -ArC 1), 116.4 (d, -ArC 3), 65.9 (-OCH2-), 52.0 (α-C), 41.1 (-CH2-), 35.1 (β-CH2), 34.0 (α-CH2), 24.6 (-CH-), 23.3 (-CH3), 21.8 (-CH3). 19F-NMR (376 MHz, DMSO): δ −38.7 to −38.8 (m).
N-3-fluorobenzoyl-l-leucine-β-alanine benzyl ester (6)
N-3-fluorobenzoyl-l-leucine (0.8 g, 3.16 mmol) and β-alanine benzyl ester tosylate (1.16 g, 3.16 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 6 as a brown powder (0.63 g, 50%). M.p. = 63–65°C. [α]D25 = −9.1° (c, 2.4, EtOH). Anal. calcd. for C23H27N2O4F1: C, 66.65; H, 6.56; N, 6.75%. Found: C, 66.89; H, 6.90; N, 6.55%. Mass spectrum: [M + Na]+ found 437.2. C23H27N2O4F1Na1 requires 437.46. IR (KBr): ν 3324, 2960, 1736, 1655, 1542, 752 cm−1. 1H-NMR (400 MHz, DMSO): δ 8.58 (1H, d, J = 8.0 Hz, -NH-), 8.17 (1H, t, J = 5.6 Hz, -NH-), 7.67–7.78 (2H, m, -ArH 2 & 6), 7.48–7.53 (1H, m, -ArH 5), 7.28–7.39 (6H, m, -ArH 4 & -PhH), 5.07 (2H, s, -OCH2-), 4.46–4.52 (1H, m, α-H), 3.29–3.43 (2H, m, β-CH2), 2.54 (2H, t, J = 6.8 Hz, α-CH2), 1.47–1.72 (3H, m, -CH2- & -CH-), 0.88 (3H, d, J = 6.4 Hz, -CH3), 0.85 (3H, d, J = 6.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 172.6 (-CONH-), 171.5 (-COOCH2Ph), 165.3 (d, -ArCO-), 162.2 (d, -ArC 3), 136.7 (d, -ArC 1), 136.3 (-PhC 1), 130.6 (d, -ArC 5), 128.7 (-PhC 3 & 5), 128.3 (-PhC 4), 128.2 (-PhC 2 & 6), 124.0 (d, -ArC 6), 118.4 (d, -ArC 4), 114.6 (d, -ArC 2), 65.9 (-OCH2-), 52.0 (α-C), 40.7 (-CH2-), 35.1 (β-CH2), 34.0 (α-CH2), 24.7 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO): δ −37.6 to −37.7 (m).
N-4-fluorobenzoyl-l-leucine-β-alanine benzyl ester (7)
N-4-fluorobenzoyl-l-leucine (0.8 g, 3.16 mmol) and β-alanine benzyl ester tosylate (1.16 g, 3.16 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 7 as a brown powder (0.58 g, 46%). M.p. = 103–105°C. [α]D25 = -12.3° (c, 1.6, EtOH). Anal. calcd. for C23H27N2O4F1: C, 66.65; H, 6.56; N, 6.75%. Found: C, 66.29; H, 6.35; N, 6.49%. Mass spectrum: [M + Na]+ found 437.2. C23H27N2O4F1Na1 requires 437.46. IR (KBr): ν 3330, 2959, 1734, 1632, 1502, 857 cm−1. 1H-NMR (400 MHz, DMSO): δ 8.49 (1H, d, J = 8.0 Hz, -NH-), 8.14 (1H, t, J = 5.6 Hz, -NH-), 7.96–7.99 (2H, m, -ArH 2 & 6), 7.26–7.36 (7H, m, -ArH 3, 5 & -PhH), 5.07 (2H, s, -OCH2-), 4.46–4.48 (1H, m, α-H), 3.29–3.39 (2H, m, β-CH2), 2.54 (2H, t, J = 6.8 Hz, α-CH2), 1.47–1.70 (3H, m, -CH2- & -CH-), 0.88 (3H, d, J = 6.4 Hz, -CH3), 0.85 (3H, d, J = 6.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 172.6 (-CONH-), 171.5 (-COOCH2Ph), 165.5 (-ArCO-), 164.3 (d, -ArC 4), 136.4 (-PhC 1), 130.9 (-ArC 1), 130.6 (-ArC 2), 130.5 (-ArC 6), 128.7 (-PhC 3 & 5), 128.3 (-PhC 4), 128.2 (-PhC 2 & 6), 115.5 (-ArC 3), 115.3 (-ArC 5), 65.9 (-OCH2-), 52.1 (α-C), 40.7 (-CH2-), 35.1 (β-CH2), 34.0 (α-CH2), 24.7 (-CH-), 23.3 (-CH3), 21.7 (-CH3). 19F-NMR (376 MHz, DMSO): δ −34.1 to −34.2 (m).
N-pentafluorobenzoyl-l-leucine-β-alanine benzyl ester (8)
N-pentafluorobenzoyl-l-leucine (2.93 g, 9.0 mmol) and β-alanine benzyl ester tosylate (3.33 g, 9.0 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 8 as a brown oil (1.99 g, 47%). [α]D25 = –6.5° (c, 2.1, EtOH). Anal. calcd. for C23H23N2O4F5: C, 56.79; H, 4.76; N, 5.75%. Found: C, 56.61; H, 4.85; N, 6.01%. Mass spectrum: [M + Na]+ found 509.2. C23H23N2O4F5Na1 requires 509.42. IR (KBr): ν 3267, 3083, 1745, 1638, 1560, 734 cm−1. 1H-NMR (400 MHz, DMSO): δ 9.16 (1H, d, J = 8.4 Hz, -NH-), 8.35 (1H, t, J = 5.6 Hz, -NH-), 7.31–7.38 (5H, m, -PhH), 5.10 (2H, s, -OCH2-), 4.51–4.55 (1H, m, α-H), 3.30–3.44 (2H, m, β-CH2), 2.56 (2H, t, J = 6.4 Hz, α-CH2), 1.45–1.64 (3H, m, -CH2- & -CH-), 0.90 (3H, d, J = 6.4 Hz, -CH3), 0.89 (3H, d, J = 6.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 171.4 (-COOCH2Ph & -CONH-), 156.6 (-ArCO-), 144.5–144.7 (m, -ArC 2), 140.0–142.2 (m, -ArC 4), 142.2 ( m, -ArC 6), 138.2–138.4 (m, -ArC 3), 136.4 (-PhC 1), 135.8–136.0 (m, -ArC 5), 128.7 (-PhC 3 & 5), 128.3 (-PhC 4), 128.1 (-PhC 2 & 6), 112.7 (t, -ArC 1), 65.9 (-OCH2-), 51.9 (α-C), 41.3 (-CH2-), 35.1 (β-CH2), 33.9 (α-CH2), 24.4 (-CH-), 23.1 (-CH3), 21.7 (-CH3). 19F-NMR (376 MHz, DMSO): δ −66.6 (dd, 2F, J = 6.8 & 23.6 Hz), −78.4 (t, J = 21.6 Hz), −86.6 to −86.7 (m, 2F).
N-2-fluorobenzoyl-l-leucine-γ-aminobutyric acid benzyl ester (9)
N-2-fluorobenzoyl-l-leucine (0.8 g, 3.16 mmol) and γ-aminobutyric acid benzyl ester tosylate (1.21 g, 3.16 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 9 as a brown oil (0.79 g, 63%). [α]D25 = −19° (c, 0.18, EtOH). Anal. calcd. for C24H29N2O4F1: C, 67.27; H, 6.82; N, 6.53%. Found: C, 67.60; H, 6.58; N, 6.32%. Mass spectrum: [M + Na]+ found 451.2. C24H29N2O4F1Na1 requires 451.49. IR (KBr): ν 3301, 2985, 1737, 1662, 1435, 728 cm−1. 1H-NMR (400 MHz, DMSO): δ 8.30 (1H, dd, J = 2.4 & 8.0 Hz, -NH-), 8.05 (1H, t, J = 5.6 Hz, -NH-), 7.61–7.65 (1H, m, -ArH 6), 7.49–7.55 (1H, m, -ArH 4), 7.24–7.38 (7H, m, -ArH 3, 5 & -PhH), 5.08 (2H, s, -OCH2-), 4.45–4.50 (1H, m, α-H), 3.13 (2H, q, J = 6.8 Hz, γ-CH2), 2.38 (2H, t, J = 7.6 Hz, α-CH2), 1.48–1.73 (5H, m, β-CH2, -CH2- & -CH-), 0.89 (6H, t, J = 6.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 172.9 (-CONH-), 172.1 (-COOCH2Ph), 163.8 (-ArCO-), 159.5 (d, -ArC 2), 136.5 (-PhC 1), 132.8 (d, -ArC 4), 130.5 (d, -ArC 6), 128.7 (-PhC 3 & 5), 128.3 (-PhC 4), 128.2 (-PhC 2 & 6), 124.7 (-ArC 5), 123.9 (d, -ArC 1), 116.3 (d, -ArC 3), 65.7 (-OCH2-), 52.2 (α-C), 41.1 (-CH2-), 38.1 (γ-CH2), 31.1 (α-CH2), 24.75 (-CH-), 24.72 (β-CH2), 23.2 (-CH3), 21.9 (-CH3). 19F-NMR (376 MHz, DMSO): δ −38.7 to −38.8 (m).
N-3-fluorobenzoyl-l-leucine-γ-aminobutyric acid benzyl ester (10)
N-3-fluorobenzoyl-l-leucine (0.8 g, 3.16 mmol) and γ-aminobutyric acid benzyl ester tosylate (1.21 g, 3.16 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 10 as a white powder (0.63 g, 50%). M.p. = 69–71°C. [α]D25 = −2.5° (c, 1.2, EtOH). Anal. calcd. for C24H29N2O4F1: C, 67.27; H, 6.82; N, 6.53%. Found: C, 67.65; H, 7.01; N, 6.38%. Mass spectrum: [M + Na]+ found 451.2. C24H29N2O4F1Na1 requires 451.49. IR (KBr): ν 3330, 2954, 1725, 1634, 1534, 754 cm−1. 1H-NMR (400 MHz, DMSO): δ 8.55 (1H, d, J = 8.0 Hz, -NH-), 8.06 (1H, t, J = 5.6 Hz, -NH-), 7.70–7.77 (2H, m, -ArH 2 & 6), 7.47–7.54 (1H, m, -ArH 5), 7.29–7.39 (6H, m, -ArH 4 & -PhH), 5.07 (2H, s, -OCH2-), 4.44–4.50 (1H, m, α-H), 3.10 (2H, q, J = 6.4 Hz, γ-CH2), 2.37 (2H, t, J = 7.2 Hz, α-CH2), 1.50–1.73 (5H, m, β-CH2, -CH2- & -CH-), 0.89 (3H, d, J = 6.0 Hz, -CH3), 0.85 (3H, d, J = 6.0 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 172.9 (-CONH-), 172.4 (-COOCH2Ph), 165.3 (d, -ArCO-), 162.2 (d, -ArC 3), 136.7 (t, -ArC 1), 136.5 (-PhC 1), 130.6 (d, -ArC 5), 128.7 (-PhC 3 & 5), 128.3 (-PhC 4), 128.2 (-PhC 2 & 6), 124.0 (d, -ArC 6), 118.4 (d, -ArC 4), 114.6 (d, -ArC 2), 65.7 (-OCH2-), 52.4 (α-C), 40.7 (-CH2-), 38.1 (γ-CH2), 31.1 (α-CH2), 24.79 (-CH-), 24.77 (β-CH2), 23.3 (-CH3), 21.7 (-CH3). 19F-NMR (376 MHz, DMSO) : δ −37.7 to −37.8 (m).
N-4-fluorobenzoyl-l-leucine-γ-aminobutyric acid benzyl ester (11)
N-4-fluorobenzoyl-l-leucine (0.8 g, 3.16 mmol) and γ-aminobutyric acid benzyl ester tosylate (1.21 g, 3.16 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 11 as a brown powder (0.73 g, 63%). M.p. = 83–85°C. [α]D25 = −2.9° (c, 1.2, EtOH). Anal. calcd. for C24H29N2O4F1: C, 67.27; H, 6.82; N, 6.53%. Found: C, 67.55; H, 7.13; N, 6.69%. Mass spectrum: [M + Na]+ found 451.2. C24H29N2O4F1Na1 requires 451.49. IR (KBr): ν 3330, 2954, 1725, 1634, 1534, 754 cm−1. 1H-NMR (400 MHz, DMSO): δ 8.49 (1H, d, J = 8.0 Hz, -NH-), 8.06 (1H, t, J = 5.6 Hz, -NH-), 7.96–8.02 (2H, m, -ArH 2 & 6), 7.25–7.39 (7H, m, -ArH 3, 5 & -PhH), 5.08 (2H, s, -OCH2-), 4.44–4.50 (1H, m, α-H), 3.11 (2H, q, J = 6.4 Hz, γ-CH2), 2.37 (2H, t, J = 7.2 Hz, α-CH2), 1.50–1.73 (5H, m, β-CH2, -CH2- & -CH-), 0.89 (3H, d, J = 6.4 Hz, -CH3), 0.86 (3H, d, J = 6.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 172.9 (-CONH-), 172.5 (-COOCH2Ph), 165.6 (-ArCO-), 164.4 (d, -ArC 4), 136.5 (-PhC 1), 130.9 (-ArC 1), 130.6 (-ArC 2), 130.5 (-ArC 6), 128.7 (-PhC 3 & 5), 128.3 (-PhC 4), 128.2 (-PhC 2 & 6), 115.4 (-ArC 3), 115.2 (-ArC 5), 65.7 (-OCH2-), 52.3 (α-C), 40.8 (-CH2-), 38.1 (γ-CH2), 31.1 (α-CH2), 24.8 (-CH-), 24.7 (β-CH2), 23.3 (-CH3), 21.7 (-CH3). 19F-NMR (376 MHz, DMSO): δ −34.1 to −34.2 (m).
N-pentafluorobenzoyl-l-leucine-γ-aminobutyric acid benzyl ester (12)
N-pentafluorobenzoyl-l-leucine (2.69 g, 8.2 mmol) and γ-aminobutyric acid benzyl ester tosylate (3.15 g, 8.2 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 12 as a white powder (1.82 g, 46%). M.p. = 54–56°C. [α]D25 = –4.2° (c, 1.2, EtOH). Anal. calcd. for C24H25N2O4F5: C, 57.59; H, 5.04; N, 5.59%. Found: C, 57.32; H, 4.77; N, 5.91%. Mass spectrum: [M + Na]+ found 523.1. C24H25N2O4F5Na1 requires 523.45. IR (KBr): ν 3329, 2962, 1736, 1655, 1508, 991 cm−1. 1H-NMR (400 MHz, DMSO): δ 9.16 (1H, d, J = 8.4 Hz, -NH-), 8.23 (1H, t, J = 5.6 Hz, -NH-), 7.31–7.37 (5H, m, -PhH), 5.10 (2H, s, -OCH2-), 4.48–4.51 (1H, m, α-H), 3.09–3.16 (2H, m, γ-CH2), 2.40 (2H, t, J = 7.2 Hz, α-CH2), 1.47–1.75 (5H, m, β-CH2, -CH2- & -CH-), 0.90 (6H, t, J = 6.0 Hz, -CH3). 13C-NMR (100 MHz, DMSO): δ 172.8 (-CONH-), 171.3 (-COOCH2Ph), 156.6 (-ArCO-), 144.5–144.7 (m, -ArC 2), 140.0–142.6 (m, -ArC 4), 142.2 ( m, -ArC 6 ), 138.2–138.5 (m, -ArC 3), 136.5 (-PhC 1), 135.8–136.0 (m, -ArC 5), 128.7 (-PhC 3 & 5), 128.3 (-PhC 4), 128.1 (-PhC 2 & 6), 112.7 (t, -ArC 1), 65.7 (-OCH2-), 52.1 (α-C), 41.3 (-CH2-), 38.1 (γ-CH2), 31.0 (α-CH2), 24.7 (β-CH2), 24.5 (-CH-), 23.1 (-CH3), 21.7 (-CH3). 19F-NMR (376 MHz, DMSO): δ −66.6 (dd, 2F, J = 6.8 & 23.3 Hz), −78.4 (t, J = 22.1 Hz), −86.6 to −86.7 (m, 2F).
N-2-fluorobenzoyl-l-leucine-glycine nitrile (13)
N-2-fluorobenzoyl-l-leucine (1.01 g, 4 mmol) and glycine nitrile hydrochloride (0.37 g, 4 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 13 as a white powder (0.81 g, 69%). M.p. = 91–92°C. [α]D25 = −4.5° (c, 2.1, EtOH). Anal. calcd. for C15H18N3O2F1: C, 61.84; H, 6.23; N, 14.42%. Found: C, 61.98; H, 6.18; N, 14.17%. IR (KBr): ν 3277, 2961, 2372, 1637, 1534, 751 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.78 (1H, t, J = 5.6 Hz, -NH-), 8.35 (1H, d, − = 7.6 Hz, -NH-), 7.60–7.65 (1H, m, -ArH 6), 7.51–7.56 (1H, m, -ArH 4), 7.26–7.34 (2H, m, -ArH 3 & 5), 4.48–4.53 (1H, m, α-H), 4.17 (2H, d, J = 5.6 Hz, -CH2-), 1.47–1.72 (3H, m, -CH2- & -CH-), 0.90 (6H, t, J = 3.6 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.9 (-CONH-), 164.2 (-ArCO-), 159.5 (d, -ArC 2), 132.8 (d, -ArC 4), 130.5 (d, -ArC 6), 124.6 (d, -ArC 5), 124.0 (d, -ArC 1), 117.9 (-CN), 116.4 (d, -ArC 3), 51.7 (α-C), 40.5 (-CH2-, −ve DEPT), 27.4 (-CH2-, −ve DEPT), 24.6 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −38.5 to −38.7 (m).
N-4-fluorobenzoyl-l-leucine-glycine nitrile (14)
N-4-fluorobenzoyl-l-leucine (1.01 g, 4 mmol) and glycine nitrile hydrochloride (0.37 g, 4 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 14 as a white powder (0.75 g, 64%). M.p. = 138–139°C. [α]D25 = −8.6° (c, 2.2, EtOH). Anal. calcd. for C15H18N3O2F1: C, 61.84; H, 6.23; N, 14.42%. Found C, 61.72; H, 6.27; N, 14.29%. IR (KBr): ν 3286, 3070, 2346, 1674, 1634, 1505, 854 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.76 (1H, t, J = 5.6 Hz, -NH-), 8.43 (1H, d, J = 8.0 Hz, -NH-), 7.99–8.02 (2H, m, -ArH 2 & 6), 7.28–7.32 (2H, m, -ArH 3 & 5), 4.48–4.54 (1H, m, α-H), 4.14 (2H, d, J = 5.6 Hz, -CH2-), 1.52–1.76 (3H, m, -CH2- & -CH-), 0.90 (3H, d, J = 6.4 Hz, -CH3), 0.86 (3H, d, J = 6.0 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 173.3 (-CONH-), 165.7 (-ArCO-), 164.3 (d, -ArC 4), 130.7 (-ArC 1), 130.66 (-ArC 2), 130.62 (-ArC 6), 117.9 (-CN), 115.5 (-ArC 3), 115.2 (-ArC 5), 51.9 (α-C), 40.2 (-CH2-, −ve DEPT) 27.4 (-CH2-, −ve DEPT), 24.7 (-CH-), 23.3 (-CH3), 21.5 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −33.8 to −34.0 (m).
N-2-fluorobenzoyl-l-alanine-glycine nitrile (15)
N-2-fluorobenzoyl-l-alanine (0.84 g, 4 mmol) and glycine nitrile hydrochloride (0.37 g, 4 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 15 as a white powder (0.55 g, 55%). M.p. = 109–111°C. [α]D25 = −12.2° (c, 1.6, EtOH). Anal. calcd. for C12H12N3O2F1: C, 57.83; H, 4.85; N, 16.86%. Found: C, 57.91; H, 4.77; N, 16.56%. IR (KBr): ν 3336, 2944, 2364, 1677, 1638, 1534, 758 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.71 (1H, t, J = 5.6 Hz, -NH-), 8.51 (1H, d, J = 6.4 Hz, -NH-), 7.67–7.71 (1H, m, -ArH 6), 7.52–7.57 (1H, m, -ArH 4), 7.27–7.31 (2H, m, -ArH 3 & 5), 4.45–4.52 (1H, m, α-H), 4.18 (2H, d, J = 5.6 Hz, -CH2-), 1.33 (3H, d, J = 7.2 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 173.0 (-CONH-), 163.8 (-ArCO-), 159.7 (d, -ArC 2), 133.0 (d, -ArC 4), 130.7 (d, -ArC 6), 124.7 (d, -ArC 5), 123.7 (d, -ArC 1), 117.9 (-CN), 116.4 (d, -ArC 3), 49.0 (α-C), 27.5 (-CH2-, −ve DEPT), 18.0 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −38.4 to −38.6 (m).
N-pentafluorobenzoyl-l-alanine-glycine nitrile (16)
N-pentafluorobenzoyl-l-alanine (1.13 g, 4 mmol) and glycine nitrile hydrochloride (0.37 g, 3.16 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 16 as a white powder (0.54 g, 42%). M.p. = 150–151°C. [α]D25 = −16.8° (c, 2.4, EtOH). Anal. calcd. for C12H8N3O2F5: C, 44.87; H, 2.51; N, 13.08%. Found: C, 45.04; H, 2.44; N, 12.93%. IR (KBr): ν 3293, 3061, 2258, 1662, 1523, 999 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 9.30 (1H, d, J = 7.2 Hz, -NH-), 8.86 (1H, t, J = 5.6 Hz, -NH-), 4.47–4.54 (1H, m, α-H), 4.20 (2H, d, J = 5.6 Hz, -CH2-), 1.30 (3H, d, J = 7.2 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.2 (-CONH-), 156.6 (-ArCO-), 144.7–144.8 (m, -ArC 2), 140.2–142.7 (m, -ArC 4), 142.2 ( m, -ArC 6), 138.2–138.5 (m, -ArC 3), 135.8–136.0 (m, -ArC 5), 117.8 (-CN), 112.5 (t, -ArC 1), 48.9 (α-C), 27.4 (-CH2-, −ve DEPT), 18.1 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −66.3 (dd, 2F, J = 6.8 & 22.9 Hz), −77.9 (t, J = 15.4 Hz), −86.4 to −86.6 (m, 2F).
N-2-fluorobenzoyl-l-leucine-β-alanine nitrile (17)
N-2-fluorobenzoyl-l-leucine (1.01 g, 4 mmol) and β-alanine nitrile (0.28 g, 4 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 17 as a white powder (0.83 g, 68%). M.p. = 84–85°C. [α]D25 = −8.3° (c, 1.2, EtOH). Anal. calcd. for C16H20N3O2F1: C, 62.94; H, 6.60; N, 13.76%. Found: C, 63.31; H, 6.98; N, 13.40%. IR (KBr): ν 3293, 2960, 2247, 1655, 1542, 755 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.41 (1H, t, J = 5.6 Hz, -NH-), 8.37 (1H, d, J = 7.6 Hz, -NH-), 7.63–7.67 (1H, m, -ArH 6), 7.50–7.55 (1H, m, -ArH 4), 7.25–7.30 (2H, m, -ArH 3 & 5), 4.48–4.54 (1H, m, α-H), 3.27–3.42 (2H, m, β-CH2), 2.66 (2H, t, J = 6.0 Hz, α-CH2), 1.51–1.71 (3H, m, -CH2- & -CH-), 0.92 (3H, d, J = 6.0 Hz, -CH3), 0.90 (3H, d, J = 6.0 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.6 (-CONH-), 163.9 (-ArCO-), 159.5 (d, -ArC 2), 132.8 (d, -ArC 4), 130.6 (d, -ArC 6), 124.6 (d, -ArC 5), 124.0 (d, -ArC 1), 119.4 (-CN), 116.4 (d, -ArC 3), 52.0 (α-C), 41.1 (-CH2-, −ve DEPT), 35.2 (-CH2, −ve DEPT), 24.6 (-CH-), 23.3 (-CH3), 21.8 (-CH3), 17.8 (α-CH2, −ve DEPT). 19F-NMR (376 MHz, DMSO-d6): δ −38.6 to −38.7 (m).
N-4-fluorobenzoyl-l-leucine-β-alanine nitrile (18)
N-4-fluorobenzoyl-l-leucine (1.01 g, 4 mmol) and β-alanine nitrile (0.28 g, 4 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 18 as a white powder (0.64 g, 53%). M.p. = 119–120°C. [α]D25 = −17.5° (c, 1.5, EtOH). Anal. calcd. for C16H20N3O2F1: C, 62.94; H, 6.60; N, 13.76%. Found: C, 63.14; H, 6.86; N, 13.62%. IR (KBr): ν 3326, 2957, 2249, 1649, 1604, 1502, 847 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.53 (1H, d, J = 8.4 Hz, -NH-), 8.36 (1H, t, J = 8.0 Hz, -NH-), 7.97–8.01 (2H, m, -ArH 2 & 6), 7.27–7.32 (2H, m, -ArH 3 & 5), 4.45–4.50 (1H, m, α-H), 3.25–3.36 (2H, m, β-CH2), 2.64 (2H, t, J = 6.0 Hz, α-CH2), 1.52–1.74 (3H, m, -CH2- & -CH-), 0.90 (3H, d, J = 6.0 Hz, -CH3), 0.87 (3H, d, J = 6.0 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 173.1 (-CONH-), 165.6 (-ArCO-), 164.3 (d, -ArC 4), 130.8 (d, -ArC 1), 130.6 (-ArC 2), 130.5 (-ArC 6), 119.5 (-CN), 115.5 (-ArC 3), 115.3 (-ArC 5), 52.1 (α-C), 40.7 (-CH2-, −ve DEPT) 35.1 (β-CH2, −ve DEPT), 24.7 (-CH-), 23.3 (-CH3), 21.6 (-CH3), 17.8 (α-CH2, −ve DEPT). 19F-NMR (376 MHz, DMSO-d6): δ −34.0 to −34.1 (m).
N-2-fluorobenzoyl-l-alanine-β-alanine nitrile (19)
N-2-fluorobenzoyl-l-alanine (0.84 g, 4 mmol) and β-alanine nitrile (0.28 g, 4 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 19 as a white powder (0.51 g, 49%). M.p. = 127–128°C. [α]D25 = −11.3° (c, 1.8, EtOH). Anal. calcd. for C13H14N3O2F1: C, 59.31; H, 5.36; N, 15.96%. Found: C, 59.72; H, 5.67; N, 15.69%. IR (KBr): ν 3376, 3278, 2250, 1677, 1648, 1542, 901 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.36–8.39 (2H, m, -NH-), 7.68–7.72 (1H, m, -ArH 6), 7.51–7.56 (1H, m, -ArH 4), 7.27–7.31 (2H, m, -ArH 3 & 5), 4.44–4.48 (1H, m, α-H), 3.27–3.41 (2H, m, β-CH2), 2.66 (2H, t, J = 6.4 Hz, α-CH2), 1.33 (3H, d, J = 7.2 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.7 (-CONH-), 163.4 (-ArCO-), 159.7 (d, -ArC 2), 133.0 (d, -ArC 4), 130.7 (d, -ArC 6), 124.7 (d, -ArC 5), 123.6 (d, -ArC 1), 119.4 (-CN), 116.4 (d, -ArC 3), 49.1 (α-C), 35.1 (β-CH2, −ve DEPT), 18.6 (-CH3), 17.8 (α-CH2, −ve DEPT). 19F-NMR (376 MHz, DMSO-d6): δ −38.5 to −38.6 (m).
N-pentafluorobenzoyl-l-alanine-β-alanine nitrile (20)
N-pentafluorobenzoyl-l-alanine (1.13 g, 4 mmol) and β-alanine nitrile (0.28 g, 4 mmol) were used. Recrystallization from ethyl acetate/hexane furnished 20 as a white powder (0.71 g, 53%). M.p. = 150–152°C. [α]D25 = −13.6° (c, 2.3, EtOH). Anal. calcd. for C13H10N3O2F5: C, 46.57; H, 3.01; N, 12.54%. Found: C, 46.92; H, 3.23; N, 12.33%. IR (KBr): ν 3365, 3274, 2252, 1672, 1626, 1522, 992 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 9.19 (1H, d, J = 7.6 Hz, -NH-), 8.48 (1H, t, J = 5.6 Hz, -NH-), 4.45–4.53 (1H, m, α-H), 3.26–3.45 (2H, m, β-CH2), 2.62–2.70 (2H, m, α-CH2), 1.31 (3H, d, J = 7.2 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 171.9 (-CONH-), 156.5 (-ArCO-), 144.6–144.8 (m, -ArC 2), 140.1-142.6 (m, -ArC 4), 142.1–142.3 (m, -ArC 6), 138.3–138.5 (m, -ArC 3), 135.8–136.0 (m, -ArC 5), 119.4 (-CN), 112.7 (t, -ArC 1), 49.2 (α-C), 35.1 (β-CH2, −ve DEPT), 18.7 (-CH3), 17.8 (α-CH2, −ve DEPT). 19F-NMR (376 MHz, DMSO-d6): δ −66.4 (dd, 2F, J = 6.8 & 23.3 Hz), −78.2 (t, J = 15.8 Hz), −86.6 to −86.7 (m, 2F).
N-3-fluorobenzoyl-l-leucine-glycine nitrile (21)
N-3-fluorobenzoyl-l-leucine (2.59 g, 10.24 mmol) and glycine nitrile hydrochloride (0.95 g, 10.24 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 21 as a white powder. (1.72 g, 57%). M.p. 131–134°C. [α]D25 = −10.0° (c, 2.1, EtOH). Anal. calcd. for C15H18N3O2F1: C, 61.84; H, 6.23; N, 14.42%. Found: C, 61.88; H, 6.16; N, 14.35%. IR (KBr): ν 3329, 2927, 2106, 1637, 1552, 1493, 1050, 842 cm−1. Mass spectrum: [M + Na]+ found 314.1. C15H18N3O2F1Na1 requires 314.31. 1H-NMR (400 MHz, DMSO-d6): δ 8.75 (1H, t, J = 5.6 Hz, -NH-), 8.69–8.71 (1H, d, J = 8 Hz, -NH-), 7.72–7.79 (2H, m, -ArH 2 & 6), 7.51–7.56 (1H, m, -ArH 5), 7.40–7.43 (1H, m, -ArH 4), 4.48–4.53 (1H, m, α-H), 4.14 (2H, d, J = 5.6 Hz, -CH2-), 1.48–1.75 (3H, m, -CH2- & -CH-), 0.86–0.92 (6H, dd, J = 6.4 & 18.8 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 173.2 (-CONH-), 165.5 (-ArCO-), 163.45 (d, -ArC 3), 136.5 (d, -ArC 1), 130.7 (d, -ArC 5), 124.2 (d, -ArC 6), 118.6 (d, -ArC 4), 118.0 (-CN), 114.8 (d, -ArC 2), 52.0 (α-C), 40.3 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.8 (-CH-), 23.4 (-CH3), 21.5 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −36.94 to −37.01 (m).
N-2,3-difluorobenzoyl-l-leucine-glycine nitrile (22)
N-2,3-difluorobenzoyl-l-leucine (1.33 g, 4.9 mmol) and glycine nitrile hydrochloride (0.45 g, 4.9 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 22 as a white powder (0.73 g, 48%). M.p. 103–105°C. [α]D25 = −13.0° (c, 2.1, EtOH). Anal. calcd. for C15H17N3O2F2: C, 58.25; H, 5.54; N, 13.59%. Found: C, 57.95; H, 5.52; N, 13.36%. IR (KBr): ν 3280, 2928, 2198, 1637, 1548, 1483, 1272, 1050, 803 cm−1. Mass spectrum: [M + Na]+ found 332.2 C15H17N3O2F2Na1 requires 332.3. 1H-NMR (400 MHz, DMSO-d6): δ 8.82–8.85 (1H, t, J = 5.4 Hz, -NH-), 8.74–8.76 (1H, d, J = 8 Hz, -NH-), 7.57–7.60 (1H, m, -ArH 6), 7.43–7.46 (1H, m, -ArH 5), 7.31–7.35 (1H, m, -ArH 4), 4.53–4.55 (1H, m, α-H), 4.21 (2H, d, J = 4 Hz, -CH2-, −ve DEPT), 1.49–1.82 (3H, m, -CH2- & -CH-), 0.94–0.96 (6H, t, J = 8.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.8 (-CONH-), 163.2 (-ArCO-), 151.2 (dd, -ArC 3), 146.3 (dd, -ArC 2), 126.5 (d, -ArC 1), 125.5 (d, -ArC 4), 125.1 (d, -ArC 5), 119.6 (d, -ArC 6), 117.9 (-CN), 51.8 (α-C), 40.5 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.6 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −62.40 to −62.51 (m), −64.0 to −64.10 (m).
N-2,4-difluorobenzoyl-l-leucine-glycine nitrile (23)
N-2,4-difluorobenzoyl-l-leucine (1.47 g, 5.4 mmol) and glycine nitrile hydrochloride (0.5 g, 5.4 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 23 as a white powder (1.1 g, 66%). M.p. 108–111°C. [α]D25 = −10.9° (c, 2.1, EtOH). Anal. calcd. for C15H17N3O2F2: C, 58.25; H, 5.54; N, 13.59%. Found: C, 57.96; H, 5.50; N, 13.37%. IR (KBr): ν 3201, 2987, 2110, 1640, 1507, 1301, 1089, 775 cm−1. Mass spectrum: [M + Na]+ found 332.2 C15H17N3O2F2Na1 requires 332.3. 1H-NMR (400 MHz, DMSO-d6): δ 8.77–8.79 (1H, t, J = 4 Hz, -NH-), 8.50–8.52 (1H, d, J = 9.2 Hz, -NH-), 7.71–7.73 (1H, m, -ArH 6), 7.31–7.40 (1H, m, -ArH 5), 7.17–7.25 (1H, m, -ArH 3), 4.45–4.55 (1H, m, α-H), 4.17 (2H, d, J = 5.2 Hz, -CH2-), 1.46–1.78 (3H, m, -CH2- & -CH-), 0.90–0.92 (6H, t, J = 6.8 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.9 (-CONH-), 163.4 (d, -ArC 4), 162.5 (-ArCO-), 158.9 (dd, -ArC 2), 132.4 (dd, -ArC 6), 120.8 (dd, -ArC 1), 117.9 (-CN), 111.8 (dd, -ArC 3), 104.7 (t, -ArC 5), 51.8 (α-C), 40.6 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.6 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −30.29 to −30.38 (m), −32.90 to −32.98 (m).
N-2,5-difluorobenzoyl-l-leucine-glycine nitrile (24)
N-2,5-difluorobenzoyl-l-leucine (2.1 g, 7.75 mmol) and glycine nitrile hydrochloride (0.72 g, 7.75 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 24 as a white powder (1.43 g, 60%). M.p. 101–104°C. [α]D25 = −9.3° (c, 2.1, EtOH). Anal. calcd. for C15H17N3O2F2: C, 58.25; H, 5.54; N, 13.59%. Found: C, 58.28; H, 5.56; N, 13.40%. IR (KBr): ν 3290, 2911, 2102, 1651, 1437, 1078, 985m−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.75–8.78 (1H, t, J = 5.6 Hz, -NH-), 8.62–8.64 (1H, d, J = 7.6 Hz, -NH-), 7.36–7.46 (3H, m, -ArH 3, 4 & 6), 4.46–4.8 (1H, m, α-H), 4.17 (2H, d, J = 5.6 Hz, -CH2-), 1.60–1.68 (3H, m, -CH2- & -CH-), 0.89–0.92 (6H, t, J = 6.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.3 (-CONH-), 162.7 (-ArCO-), 157.5 (d, -ArC 2), 155.5 (d, -ArC 5), 125.1 (dd, -ArC 1), 118.9 (dd, -ArC 6), 117.9 (dd, -ArC 3), 117.5 (-CN), 116.3 (dd, -ArC 4), 51.5 (α-C), 39.9 (-CH2-, −ve DEPT), 27.1 (-CH2-, −ve DEPT), 24.2 (-CH-), 22.9 (-CH3), 21.3 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −42.38 to −42.48 (m), −43.38 to −43.45 (m).
N-2,6-difluorobenzoyl-l-leucine-glycine nitrile (25)
N-2,6-difluorobenzoyl-l-leucine (2.23 g, 8.23 mmol) and glycine nitrile hydrochloride (0.76 g, 8.23 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 25 as a white powder (0.98 g, 39%). M.p. 159–163°C. [α]D25 = −20.3° (c, 2.1, EtOH). Anal. calcd. for C15H17N3O2F2: C, 58.25; H, 5.54; N, 13.59%. Found: C, 58.18; H, 5.49; N, 13.51%. IR (KBr): ν 3190, 2875, 2055, 1675, 1688, 1402, 1075, 626 cm−1. Mass spectrum: [M + Na]+ found 332.1. C15H17N3O2F2Na1 requires 332.3. 1H-NMR (400 MHz, DMSO-d6): δ 9.02 (1H, d, J = 8.4 Hz, -NH-), 8.85–8.88 (1H, t, J = 5.6 Hz, -NH-), 7.10–7.55 (1H, m, -ArH 4), 7.13–7.17 (2H, m, -ArH 3 & 5), 4.40–4.57 (1H, m, α-H), 4.18 (2H, d, J = 2.4 Hz, -CH2-), 1.41–1.78 (3H, m, -CH2- & -CH-), 0.89–0.92 (6H, dd, J = 4.4 & 2.5 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.5 (-CONH-), 160.45 (d, -ArC 2), 159.96 (-ArCO-), 157.95 (d, -ArC 6), 131.91 (-ArC 4), 117.9 (-CN), 115.5 (t, -ArC 1), 112.1 (dd, -ArC 3 & 5), 51.5 (α-C), 40.8 (-CH2-, −ve DEPT), 27.4 (-CH2-, −ve DEPT), 24.5 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −37.54 to −37.58 (m).
N-3,4-difluorobenzoyl-l-leucine-glycine nitrile (26)
N-3,4-difluorobenzoyl-l-leucine (2.3 g, 8.5 mmol) and glycine nitrile hydrochloride (0.79 g, 8.5 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 26 as a white powder (0.52 g, 20%). M.p. 139–142°C. [α]D25 = −19.6° (c, 2.1, EtOH). Anal. calcd. for C15H17N3O2F2: C, 58.25; H, 5.54; N, 13.59%. Found: C, 58.05; H, 5.51; N, 13.29%. IR (KBr): ν 3198, 2654, 2063, 1641, 1567, 1246, 1098, 803 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.76–8.82 (1H, t, J = 11.4 Hz, -NH-), 8.71–8.73 (1H, d, J = 7.7 Hz, -NH-), 7.97–8.00 (1H, m, -ArH 6), 7.81–7.84 (1H, m, -ArH 2), 7.54–7.61 (1H, m, -ArH 5), 4.47–4.52 (1H, m, α-H), 4.14 (2H, d, J = 5.6 Hz, -CH2-), 1.59–1.75 (3H, m, -CH2- & -CH-), 0.85–0.92 (6H, dd, J = 6.4 & 19.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 173.1 (-CONH-), 164.64 (-ArCO-), 151.8 (dd, ArC 4), 149.3 (dd, -ArC 3), 131.55 (t, -ArC 1), 125.52 (dd, -ArC 2), 117.9 (-CN), 117.8 (d, -ArC 5), 117.3 (d, -ArC 6), 52.0 (α-C), 40.3 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.7 (-CH-), 23.3 (-CH3), 21.5 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −58.33 to −58.41 (m), −62.09 to −62.20 (m).
N-3,5-difluorobenzoyl-l-leucine-glycine nitrile (27)
N-3,5-difluorobenzoyl-l-leucine (2.3 g, 8.5 mmol) and glycine nitrile hydrochloride (0.79 g, 8.5 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 27 as a white powder (0.62 g, 22%). M.p. 150–153°C. [α]D25 = −16.0° (c, 2.1, EtOH). Anal. calcd. for C15H17N3O2F2: C, 58.25; H, 5.54; N, 13.59%. Found: C, 58.94; H, 5.37; N, 12.83%. IR (KBr): ν 3281, 2967, 2146, 1641, 1543, 1298, 1023, 754 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.78–8.80 (2H, m, -NH-), 7.64–7.67 (2H, m, -ArH 2 & 6), 7.53–7.45 (1H, m, -ArH 4), 4.47–4.53 (1H, m, α-H), 4.14 (2H, d, J = 5.6 Hz, -CH2-), 1.55–1.74 (3H, m, -CH2- & -CH-), 0.86–0.92 (6H, dd, J = 6.4 & 20 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.99 (-CONH-), 164.27 (-ArCO-), 163.73 (d, -ArC 3), 161.27 (d, -ArC 5), 137.62 (t, -ArC 1), 117.94 (-CN), 111.35 (dd, -ArC 2 & 5), 107.22 (t, -ArC 4), 52.1 (α-C), 40.2 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.7 (-CH-), 23.3 (-CH3), 21.5 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −32.61 to −32.68 (m).
N-2,3,6-trifluorobenzoyl-l-leucine-glycine nitrile (28)
N-2,3,6-trifluorobenzoyl-l-leucine (1.05 g, 3.63 mmol) and glycine nitrile hydrochloride (0.34 g, 3.63 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 28 as a white powder (0.28 g, 24%). M.p. 157–160°C. [α]D25 = −27.2° (c, 2.1, EtOH). Anal. calcd. for C15H16N3O2F3: C, 55.05; H, 4.93; N, 12.84%. Found: C, 55.13; H, 4.98; N, 12.67%. IR (KBr): ν 3276, 2961, 2252, 1660, 1541, 1492, 1025, 814 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 9.13–9.15 (1H, d, J = 8.2 Hz, -NH-), 8.90–8.93 (1H, t, J = 11.8 Hz, -NH-), 7.55–7.64 (1H, m, -ArH 4), 7.18–7.24 (1H, m, -ArH 5), 4.51–4.57 (1H, m, α-H), 4.20 (2H, d, J = 3.2 Hz, -CH2-), 1.41–1.78 (3H, m, -CH2- & -CH-), 0.90–0.92 (6H, dd, J = 5.4 & 6.3 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.3 (-CONH-), 158.77 (-ArCO-), 154.5 (d, -ArC 2), 147.9 (d, -ArC 6), 145.4 (t, -ArC 3), 118.70 (dd, -ArC 4), 117.87 (-CN), 117.0 (t, -ArC 1), 112.4 (d, -ArC 5), 51.5 (α-C), 40.8 (-CH2-, −ve DEPT), 27.4 (-CH2-, −ve DEPT), 24.5 (-CH-), 23.2 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −42.18 to −42.25 (m), −61.096 to −61.18 (m), −66.48 to −66.62 (m).
N-2,3,4-trifluorobenzoyl-l-leucine-glycine nitrile (29)
N-2,3,4-trifluorobenzoyl-l-leucine (1.17 g, 4 mmol) and glycine nitrile hydrochloride (0.37 g, 4 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 29 as a white powder (0.58 g, 44%). M.p. 116–119°C. [α]D25 = −4.2° (c, 2.1, EtOH). Anal. calcd. for C15H16N3O2F3: C, 55.05; H, 4.93; N, 12.84%. Found: C, 54.43; H, 5.95; N, 12.58%. IR (KBr): ν 3287, 2964, 2249, 1657, 1547, 1496, 1045, 828 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.85–8.87 (1H, t, J = 5.6 Hz, -NH-), 8.75–8.77 (1H, d, J = 8 Hz, -NH-), 7.41–7.55 (2H, m, -ArH 5 & 6), 4.52–4.56 (1H, m, α-H), 4.21 (2H, d, J = 5.6 Hz, -CH2-), 1.51–1.75 (3H, m, -CH2- & -CH-), 0.93–0.97 (6H, dd, J = 6.4 & 10.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.7 (-CONH-), 162.53 (-ArCO-), 151.7 (dd, ArC 4), 148.8 (dd, -ArC 2), 139.3 (dt, -ArC 3), 125.02 (dd, -ArC 5), 122.24 (t, -ArC 1), 117.89 (-CN), 113.02 (dd, -ArC 6), 51.8 (α-C), 40.5 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.6 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −55.67 to −55.79 (m), −58.92 to −59.02 (m), −84.95 to −85.08 (m).
N-2,4,5-trifluorobenzoyl-l-leucine-glycine nitrile (30)
N-2,4,5-trifluorobenzoyl-l-leucine (1.94 g, 6.7 mmol) and glycine nitrile hydrochloride (0.62 g, 6.7 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 30 as a white powder (1.20 g, 55%). M.p. 104–108°C. [α]D25 = −5.1° (c, 2.1, EtOH). Anal. calcd. for C15H16N3O2F3: C, 55.05; H, 4.93; N, 12.84%. Found: C, 54.75; H, 4.90; N, 12.63%. IR (KBr): ν 3282, 2959, 2261, 1657, 1537, 1465, 1031, 824 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.81–8.84 (1H, t, J = 5.4 Hz, -NH-), 8.65–8.67 (1H, d, J = 8 Hz, -NH-), 7.68–7.80 (2H, m, -ArH 3 & 6), 4.50–4.53 (1H, m, α-H), 4.20 (2H, d, J = 5.6 Hz, -CH2-), 1.55–1.73 (3H, m, -CH2- & -CH-), 0.94–0.96 (6H, t, J = 6.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.7 (-CONH-), 162.3 (-ArCO-), 155.2 (dd, ArC 2), 150.8 (dd, -ArC 5), 146.0 (dd, -ArC 4), 120.85 (d, -ArC 1), 118.55 (d, -ArC 6), 117.90 (-CN), 107.20 (dd, -ArC 3), 51.9 (α-C), 40.6 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.6 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −37.97 to −38.07 (m), −54.57 to −54.70 (m), −66.76 to −66.90 (m).
N-3,4,5-trifluorobenzoyl-l-leucine-glycine nitrile (31)
N-3,4,5-trifluorobenzoyl-l-leucine (2 g, 7 mmol) and glycine nitrile hydrochloride (0.65 g, 7 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 31 as a white powder (1.20 g, 52%). M.p. 146–149°C. [α]D25 = −0.2° (c, 2.1, EtOH). Anal. calcd. for C15H16N3O2F3: C, 55.05; H, 4.93; N, 12.84%. Found: C, 54.83; H, 4.87; N, 12.73%. IR (KBr): ν 3247, 2943, 2253, 1653, 1529, 1476, 1040, 794 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.77–8.81 (2H, m, -NH-), 7.87–7.91 (2H, m, -ArH 2 & 6), 4.45–4.52 (1H, m, α-H), 4.14 (2H, d, J = 6 Hz, -CH2-), 1.53–1.73 (3H, m, -CH2- & -CH-), 0.86–0.92 (6H, dd, J = 6 & 20.8 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.9 (-CONH-), 163.6 (-ArCO-), 150.3 (dd, ArC 3&6), 141.2 (dt, -ArC 4), 130.43 (-ArC 1), 117.91 (-CN), 113.1 (dd, -ArC 2 & 6), 52.2 (α-C), 40.3 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.7 (-CH-), 23.3 (-CH3), 21.5 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −58.22 to −58.30 (m), −80.76 to −80.91 (m).
N-2,3,4,5-tetrafluorobenzoyl-l-leucine-glycine nitrile (32)
N-2,3,4,5-tetrafluorobenzoyl-l-leucine (1.54 g, 5 mmol) and glycine nitrile hydrochloride (0.46 g, 5 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 32 as a white powder (0.72 g, 42%). M.p. 97–101°C. [α]D25 = −2.2° (c, 2.1, EtOH). Anal. calcd. for C15H15N3O2F4: C, 52.18; H, 4.38; N, 12.17%. Found: C, 52.02; H, 4.33; N, 12.06%. IR (KBr): ν 3294, 2962, 2252, 1648, 1521, 1482, 1361, 1238, 1031, 881 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.80–8.84 (2H, m, -NH-), 7.61–7.63 (1H, m, -ArH 6), 4.44–4.50 (1H, m, α-H), 4.17 (2H, d, J = 5.6 Hz, -CH2-), 1.48–1.73 (3H, m, -CH2- & -CH-), 0.88–0.93 (6H, dd, J = 6.4 & 17.6 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.5 (-CONH-), 161.5 (-ArCO-), 146.9 (d, -ArC 2), 144.5 (d, ArC 5), 142.0 (d, -ArC 4), 138.91–139.95 (d, -ArC 3), 120.64 (-ArC 1), 117.87 (-CN), 112.13 (d, -ArC 6), 51.9 (α-C), 40.6 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.6 (-CH-), 23.3 (-CH3), 21.6 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −63.08 to −63.34 (m, 2F), −76.97 to −77.12 (m, 1F), −79.43 to −79.54 (t, 1F).
N-2,3,4,5,6-pentafluorobenzoyl-l-leucine-glycine nitrile (33)
N-2,3,4,5,6-pentafluorobenzoyl-l-leucine (1.52 g, 6.6 mmol) and glycine nitrile hydrochloride (0.6 g, 6.6 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 33 as a white powder (1.20 g, 51%). M.p. 131–133°C. [α]D25 = −13.0° (c, 2.1, EtOH). Anal. calcd. for C15H14N3O2F5: C, 49.60; H, 3.88; N, 11.57%. Found: C, 49.70; H, 3.87; N, 11.40%. IR (KBr): ν 3265, 3077, 2962, 2252, 1654, 1554, 1521, 1343, 1115, 991 cm−1. Mass spectrum: [M + Na]+ found 386.1. C15H14N3O2F5Na1 requires 386.29. 1H-NMR (400 MHz, DMSO-d6): δ 9.26–9.28 (1H, d, J = 7.2 Hz, -NH-), 8.96–8.99 (1H, t, J = 5.6 Hz, -NH-), 4.51–4.57 (1H, m, α-H), 4.20 (2H, d, J = 2.8 Hz, -CH2-), 1.60–1.68 (3H, m, -CH2- & -CH-), 0.89–0.93 (6H, t, J = 6.8 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.1 (-CONH-), 156.9 (-ArCO-), 135.95-144.69 (m, -ArC 2, 3, 4, 5, & 6), 117.81 (-CN), 112.6 (t, -ArC 1), 51.7 (α-C), 40.8 (-CH2-, −ve DEPT), 27.4 (-CH2-, −ve DEPT), 24.5 (-CH-), 23.2 (-CH3), 21.5 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ −65.65 to −66.11 (m, 2F), −76.99 to −77.11 (m, 1F), −85.50 to −86.71 (m, 2F).
N-2-trifluoromethylbenzoyl-l-leucine-glycine nitrile (34)
N-2-trifluoromethylbenzoyl-l-leucine (2.27 g, 7.5 mmol) and glycine nitrile hydrochloride (0.70 g, 7.5 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 34 as a white powder (1.30 g, 51%). M.p. 163–166°C. [α]D25 = −4.0° (c, 2.1, EtOH). Anal. calcd. for C16H18N3O2F3: C, 56.30; H, 5.32; N, 12.31%. Found: C, 56.07; H, 5.28; N, 12.17%. IR (KBr): ν 3292, 2958, 2247, 1642, 1547, 1341, 1283, 1173, 1121, 1068, 692 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.79–8.81 (1H, d, J = 8 Hz, -NH-), 8.75–8.77 (1H, t, J = 5.6 Hz, -NH-), 7.73–7.79 (2H, m, -ArH 4 & 5), 7.66–7.68 (1H, d, J = 7.6 Hz, -ArH 3), 7.58–7.60 (1H, d, J = 7.2 Hz, -ArH 6), 4.41–4.55 (1H, m, α-H), 4.19 (2H, d, J = 5.6 Hz, -CH2-), 1.44–1.79 (3H, m, -CH2- & -CH-), 0.89–0.92 (6H, dd, J = 2.8 & 6.4 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 172.9 (-CONH-), 167.5 (-ArCO-), 136.2 (-ArC 1) 132.6 (-ArC 6), 130.2 (-ArC 4), 129.2 (-ArC 3), 126.5 (t, -ArC 5), 125.8 (d, -ArC 2), 122.7 (d, -CF3), 117.9 (-CN), 51.6 (α-C), 40.4 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.5 (-CH-), 23.3 (-CH3), 21.4 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ +18.08 (3F).
N-3-trifluoromethylbenzoyl-l-leucine-glycine nitrile (35)
N-3-trifluoromethylbenzoyl-l-leucine (2.27 g, 7.5 mmol) and glycine nitrile hydrochloride (0.70 g, 7.5 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 35 as a white powder (0.9 g, 36%). M.p. 104–107°C. [α]D25 = −33.5° (c, 2.1, EtOH). Anal. calcd. for C16H18N3O2F3: C, 56.30; H, 5.32; N, 12.31%. Found: C, 56.21; H, 5.34; N, 12.11%. IR (KBr): ν 3295, 2962, 2246, 1637, 1540, 1333, 1278, 1178, 1119, 1073, 696 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.94–8.96 (1H, d, J = 7.4 Hz, -NH-), 8.82–8.85 (1H, t, J = 5.6 Hz, -NH-), 8.33 (1H, s, -ArH 2); 8.26–8.27 (1H, d, J = 7.6 Hz, -ArH 4), 7.94–7.96 (1H, d, J = 8 Hz, -ArH 6), 7.75–7.79 (1H, t, J = 7.6 Hz, -ArH 5), 4.55–4.62 (1H, m, α-H), 4.19 (2H, d, J = 5.6 Hz, -CH2-), 1.58–1.82 (3H, m, -CH2- & -CH-), 0.90–0.96 (6H, dd, J = 6.4 & 18 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 173.1 (-CONH-), 165.4 (-ArCO-), 135.05(-ArC 1), 132.2 (-ArC 4), 129.9 (-ArC 5), 129.4 (d, -ArC 3), 128.3 (d, -ArC 6), 124.55 (d, -ArC 2), 123.0 (-CF3), 117.9 (-CN), 52.0 (α-C), 40.3 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.7 (-CH-), 23.3 (-CH3), 21.5 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ +14.94 (3F).
N-4-trifluoromethylbenzoyl-l-leucine-glycine nitrile (36)
N-4-trifluoromethylbenzoyl-l-leucine (1.52 g, 5 mmol) and glycine nitrile hydrochloride (0.46 g, 5 mmol) were used. Isolation by column chromatography and recrystallization from ethyl acetate/hexane furnished 36 as a white powder (0.5 g, 28%). M.p. 153–157°C. [α]D25 = −26.0° (c, 2.1, EtOH). Anal. calcd. C16H18N3O2F3: C, 56.30; H, 5.32; N, 12.31%. Found: C, 56.19; H, 5.32; N, 12.17%. IR (KBr): ν 3291, 2968, 2249, 1635, 1544, 1335, 1281, 1176, 1112, 1074, 691 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ 8.90–8.92 (1H, d, J = 8 Hz, -NH-), 8.82–8.85 (1H, t, J = 5.6 Hz, -NH-), 8.16 (2H, d, J = 8 Hz, -ArH 2 & 6), 7.91 (2H, d, J = 8.4 Hz, -ArH 3 & 5), 4.54–4.63 (1H, m, α-H), 4.19 (2H, d, J = 5.6 Hz, -CH2-), 1.57–1.83 (3H, m, -CH2- & -CH-), 0.91–0.97 (6H, dd, J = 6.4 & 17.2 Hz, -CH3). 13C-NMR (100 MHz, DMSO-d6): δ 173.1 (-CONH-), 165.7 (-ArCO-), 137.99 (-ArC 1), 131.62 (d, -ArC 4), 128.89 (-ArC 2 & 6), 125.6 (q, -ArC 3 & 5), 122.9 (-CF3), 117.9 (-CN), 52.0 (α-C), 40.3 (-CH2-, −ve DEPT), 27.5 (-CH2-, −ve DEPT), 24.8 (-CH-), 23.3 (-CH3), 21.5 (-CH3). 19F-NMR (376 MHz, DMSO-d6): δ +14.71 (3F).
Biology
Enzyme assays with Fasciola hepatica cysteine cathepsin L endoprotease
Inactivated Fasciola hepatica cysteine cathepsin L endoprotease was isolated as previously reportedCitation12. The protease was activated by the addition of dithiothreitol, ethylenediaminetetraacetic acid (EDTA), and sodium acetate followed by incubation at 37°C for 2 h. Proteinase activity was measured fluorometrically using Z-Phe-Arg-NHMec as substrate. The substrate was stored as a 1 mM stock solution in dimethyl sulfoxide (DMSO). Assays were carried out using a final concentration of 10 μM substrate in 0.1 M sodium acetate containing 0.1 M dithiothreitol and 0.5 M EDTA. The enzyme and inhibitor were incubated together for 15 min at 37°C, at which stage the substrate was added. The mixture was incubated at 37°C for 30 min. The reaction was stopped by the addition of 150 μL of a 10% acetic acid solution. The amount of 7-amino-4-methylcoumarin (-NHMec) released was measured using a PerkinElmer fluorescence spectrophotometer with excitation set at 370 nm and emission at 440 nm.
Results and discussion
Chemistry
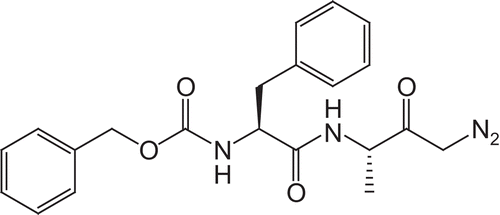
The fluorobenzoyl amino acids were prepared using a Schotten–Baumann reaction furnishing the N-fluorobenzoyl amino acids in quantative yields. The dipeptidyl derivatives were synthesized using the standard DCC/HOBt protocol ()Citation13–15. Chiral integrity can be preserved by the use of 1-hydroxybenzotriazole (HOBt). HOBt acts as an auxiliary nucleophile and reduces the lifetime of the O-acylisourea intermediate, thus decreasing the occurrence of racemization. An acylating agent of lower potency is formed which is still reactive to aminolysis, but is less susceptible to racemization or other side reactions. 1-Hydroxybenzotriazole also offers an acidic hydrogen that can be more easily removed by bases than the proton at the chiral center.
Scheme 1. Reagents and conditions: (a) l-Leu or l-Ala, NaOH; (b) C-protected amino acid, DCC, HOBt, TEA, DCM.
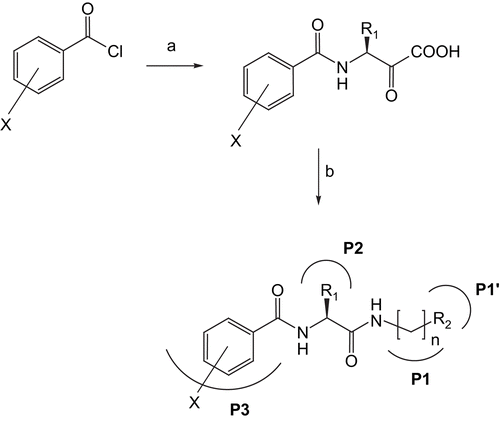
The initial series of compounds synthesized concentrated on modification of the P1 position, with benzyl ester protection of the C-terminus (1–12). At this position, the methylene unit of the C-terminal amino acid glycine was increased in length. Using glycine benzyl ester, β-alanine benzyl ester, and γ-aminobutyric acid benzyl ester the chain length was extended. The optimal amino acid at the P2 position of the dipeptide was shown to be l-leucineCitation8,Citation10. Compounds 1–12 were synthesized with the aim of optimizing the dipeptide backbone structure. The ortho-, meta-, and para-fluorinated derivatives were prepared along with the pentafluoro, for each of the three P1 amino acids.
The second series of inhibitors prepared involved modification of both the P1 and P2 positions of the dipeptide backbone. The fluorinated P3 positions were the same as the benzyl ester series. A nitrile moiety was introduced at the C-terminal. Compounds 13–20 were prepared by coupling either fluorinated benzoyl-l-leucine or benzoyl-l-alanine to glycine nitrile or β-alanine nitrile.
Biological results (see below) from this investigation showed compounds 13 and 14, the leucine-glycine nitriles, to be the most active. A complete structure–activity relationship (SAR) study of these compounds was performed. By altering the number and position of fluorines at the P3 phenyl ring position, compounds 21–36 were synthesized by coupling the relevant fluorinated benzoyl-l-leucine with 2-aminoacetonitrile.
Biology
Protease inhibitory assays
Proteolytic enzymes of the liver fluke Fasciola hepatica have been associated with cells of the gut epithelium. Accordingly these enzymes have been implicated in diverse functions such as feeding, tissue degradation, and protection of the parasite against attack. The first of the Fasciola hepatica secreted cysteine proteases (FhCL1) was isolated using gel filtration and ion exchange chromatographyCitation16. NH2-terminal sequencing and substrate specificity studies revealed that the enzyme was a cathepsin L protease (FhCL1). Dowd et al. purified the second major secreted protease to homogeneityCitation17. This enzyme is now termed cathepsin L2 (FhCL2). The involvement of these enzymes in key parasite functions renders them potential targets at which to direct novel antiparasite chemotherapy.
The exploitation of the leucine-glycine dipeptidyl scaffold has resulted in numerous cathepsin protease inhibitorsCitation18. The incorporation of an aromatic substituent at the N-terminus has been imperative to both the selectivity and potency of dipeptidyl inhibitorsCitation19. Previous work in these laboratories has identified dipeptidyl ester derivatives as inhibitors of Fasciola hepatica cathepsin L proteaseCitation10. This prompted us to investigate the effect that fluorine substitution would have on the bioactivity of these compounds. The benefits of introducing fluorine into a biomolecule have been well documentedCitation20. It was also anticipated that modification of the P1 side chain by one, two, and three methylene units would furnish more potent inhibitors of Fasciola hepatica cathepsin L endoproteases. Further studies involved the two other sites of possible modification of the dipeptide structure (P2 and P3). All the synthesized compounds underwent biological evaluation. The inhibitory activities of these compounds against the Fasciola hepatica cysteine cathepsin L endoproteases were determined using the fluorescent substrate Z-Phe-Arg-NHMec.
The inhibitory activities of compounds 1–12 are shown in . The γ-aminobutyric acid (ABA) derivatives were found to be the most effective inhibitors. The incorporation of three methylene units into the P1 position gave inhibitors with activity up to two-fold greater than their β-alanine analogs and as much as seven-fold greater than the glycine derivatives. The most active compound in this series was found to be N-2-fluorobenzoyl-l-leucine-γ-aminobutyric acid benzyl ester 9, with an IC50 value of 15.3 μM, closely followed by its 4-fluorobenzoyl analog 11 that recorded an IC50 value of 19.3 μM. The P3 substitutions demonstrated a significant influence on the overall activity of these derivatives. In the case of the β-alanine and γ-ABA derivatives, mono-substitution of the benzoyl ring gave the most potent results, but in contrast, the incorporation of a pentafluorobenzoyl moiety furnished the best results with the glycine derivatives.
Table 1. In vitro inhibitory activity of N-fluorobenzoyl dipeptidyl benzyl esters 1–12, and compounds 37 and 38, with the Fasciola hepatica cysteine cathepsin L endoprotease FhCL1.
The inhibitory activities of the dipeptidyl nitriles 13–20 are shown in . The pentafluoro and 4-fluoro derivatives were shown to be more potent derivatives than the 2-fluoro analogs. A reduction of the P2 substituent size by using l-alanine resulted in a decrease in activity of up to 30-fold. By increasing the number of methylene units from one to two at position P1, the inhibitory activity was shown to decrease by up to eight-fold. This clearly shows that a glycine residue in the P1 position and a leucine residue in the P2 position are essential for effective inhibition.
Table 2. In vitro inhibitory activity of N-fluorobenzoyl dipeptidyl nitriles 13–20, and compounds 37 and 38, with the Fasciola hepatica cysteine cathepsin L endoprotease FhCL1.
The biological results of the completed SAR study (21–36) are outlined in . The IC50 of the commercial diazomethane inhibitor Z-Phe-Ala-CHN2 39 () is 3.0 μMCitation10, which is comparable to the inhibitory concentrations found for the majority of the N- fluorobenzoyl-l-leucine-glycine nitrile derivatives. From the variable concentration assays performed, it can be seen that the commercially available inhibitor 39 is more potent at lower concentrations up to about 50% inhibition. Inhibitors 21, 27, and 36 showed comparable results which are displayed in a representative bar chart (). It is clear that the introduction of fluorine atom(s) increases the biological activity of the compounds employed in this study. However, a direct correlation between number and position of the fluorine atoms on the aromatic ring with biological activity was not observed.
Figure 2. Bar chart illustration of percent inhibition of 39, 21, 27, and 36 toward cathepsin L at varying concentrations (μM).
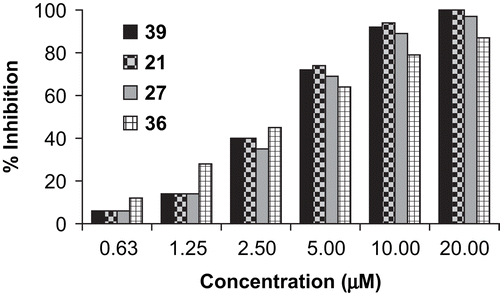
Table 3. In vitro inhibitory activity of N-fluorobenzoyl dipeptidyl nitriles 21–36, and compounds 37 and 38, with the Fasciola hepatica cysteine cathepsin L endoprotease FhCL1.
Conclusion
In summary, we have identified active inhibitors of the liver fluke cysteine protease cathepsin L. It has been shown that increasing the number of methylene units in the P1 position or reducing the size of the P2 substituent reduces the inhibitory activity of these compounds. It has also been shown that fluorobenzoyl dipeptidyl benzyl ester derivatives have the ability to be inhibitors of cathepsin L but are not as effective as the L-Leu-Gly nitrile motif. From the protease inhibition assays it has been found that at the N-terminal position P3, the location of the fluorine isostere may not be of major importance to the activity of the inhibitor. The number of fluorine substituents attached to the benzoyl group also gives little if any improvement to the biological activity. Despite this, it is evident that almost all of the fluorobenzoyl-l- leucine-glycine nitrile derivatives offer real inhibitory potency against the cathepsin L endoprotease. It is hoped that with further studies, greater improvements in bioactivity can be made. The inhibitory potency of the nitrile compounds such as N-3,5-difluorobenzoyl-l- leucine-glycine nitrile 27 relative to the commercial inhibitor Z- Phe-Ala-CHN2 39 shows that such benzoyl dipeptidyl derivatives may have a role to play as commercial inhibitors of Fasciola hepatica cathepsin L endoproteases. Subsequent reports will detail efforts to improve the activity of similar dipeptidyl derivatives with further modifications at the P3 position.
Acknowledgements
We wish to thank the National Institute for Cellular Biotechnology and Dublin City University for grants in aid of research and particularly under the Irish Government PRTLI cycle 3 initiative 2001–2006.
Declaration of interest: The authors report no conflicts of interest.
References
- Otto HH, Schirmeister T. Cysteine proteases and their inhibitors. Chem Rev 1997;97:133–71.
- Harmsen MM, Cornelissen JBWJ, Buijs HECM, Boersma WJA, Jeurissen SHM, van Milligen FJ. Identification of a novel Fasciola hepatica cathepsin L protease containing protective epitopes within the propeptide. Int J Parasitol 2004;34:675–82.
- Dalton JP, O’Neill S, Stack C, Collins P, Walshe A, Sekiya M, et al. Fasciola hepatica cathepsin L-like proteases: biology, function, and potential in the development of first generation liver fluke vaccines. Int J Parasitol 2003;33:1173–81.
- Howell RM. Collagenase activity of immature Fasciola hepatica. Nature 1966;209:713–14.
- Dalton JP, Heffernan M. Thiol proteases released in vitro by Fasciola hepatica. Mol Biochem Parasitol 1989;35:161–6.
- Leung D, Abbenante G, Fairlie DP. Protease inhibitors: current status and future prospects. J Med Chem 2000;43:305–41.
- Löser R, Schilling K, Dimmig E, Gütschow M. Interaction of papain-like cysteine proteases with dipeptide-derived nitriles. J Med Chem 2005;48:7688–707.
- Yadav MR, Shinde AK, Chouhan BS, Giridhar R, Menard R. Peptidomimetic 2-cyanopyrrolidines as potent selective cathepsin L inhibitors. J Enzyme Inhib Med Chem 2008;23:190–7.
- Gour-Salin BJ, Lachance P, Bonneau PR, Storer AC, Kirschke H, Broemme PR. E-64 analogs as inhibitors of cathepsin L and cathepsin S: importance of the S2–P2 interactions for potency and selectivity. Bioorg Chem 1994;22:227–41.
- Sheehy MJ. The design and synthesis of novel peptide derivatives as malarial protease inhibitors and electrochemical anion sensing receptors. PhD Thesis, Dublin City University, Ireland, 1999.
- Lim IT, Meroueh SO, Lee M, Heeg MJ, Mobashery S. Strategy in inhibition of cathepsin B, a target in tumor invasion and metastasis. J Am Chem Soc 2004;126:10271–7.
- Collins PR, Stack CM, O’Neill SM, Doyle S, Ryan T, Brennan GP, et al. Cathepsin L1, the major protease involved in liver fluke (Fasciola hepatica) virulence: propeptide cleavage sites and autoactivation of the zymogen secreted from gastrodermal cells. J Biol Chem 2004;279:17038–46.
- Goel A, Kenny PTM. Formation of b1-1 and the corresponding a1-1 product ions in the mass spectra of N-{para-(ferrocenyl)-benzoyl} dipeptide esters. Rapid Commun Mass Spectrom 2008;22:2398–401.
- Corry AJ, Goel A, Alley SR, Kelly PN, O’Sullivan D, Savage D, et al. N- ortho-Ferrocenyl benzoyl dipeptide esters: synthesis, structural characterization and in vitro anti-cancer activity of N-{ortho-(ferrocenyl)-benzoyl}-glycine-L-alanine ethyl ester and N-{ortho-(ferrocenyl)benzoyl}- L-alanine-glycine ethyl ester. J Organomet Chem 2007;692:1405–10.
- Kelly PN, Pretre A, Devoy S, O’Rielly I, Devery R, Goel A, et al. Synthesis, structural characterization and biological activity of novel N-(ferrocenylmethyl)benzene-carboxamide derivatives. J Organomet Chem 2007;692:1327–31.
- Smith AM, Dowd AJ, McGonigle S, Keegan PS, Brennan G, Trudgett A, et al. Purification of a cathepsin L-like proteinase secreted by adult Fasciola hepatica. Mol Biochem Parasitol 1993;62:1–8.
- Dowd AJ, Smith AM, McGonigle S, Dalton JP. Purification and characterisation of a second cathepsin L proteinase secreted by the parasitic trematode Fasciola hepatica. Eur J Biochem 1994;223:91–8.
- Robichaud J, Oballa R, Prasit P, Falgueyret JP, Percival MD, Wesolowski G, et al. A novel class of nonpeptidic biaryl inhibitors of human cathepsin K. J Med Chem 2003;46:3709-727.
- Greenspan PD, Clark KL, Cowen SD, McQuire LW, Tomassi RA, Farley DL, et al. N–arylaminonitriles as bioavailable peptidomimetic inhibitors of cathepsin B. Bioorg Med Chem Lett 2003;13:4121–4.
- Smart BE. Fluorine substituent effects (on bioactivity). J Fluorine Chem 2001;109:3–11.