Abstract
A new series of complexes of the type [M(C24H16N4)X]X2, where M = Cr(III), Fe(III), and Mn(III), X = Cl−, NO3−, and CH3COO−, has been synthesized by template condensation of 1,8-diaminonaphthalene and glyoxal in the presence of trivalent metal salts in methanolic medium. The complexes have been characterized with the help of elemental analysis, conductance measurements, magnetic measurements, and electronic, NMR, IR, and mass spectral studies. On the basis of these studies, a five-coordinate square pyramidal geometry for all of these complexes has been proposed. All the synthesized metal complexes were also tested for their in vitro antimicrobial activities against some bacterial strains, viz. Bacillus subtilis, Bacillus stearothermophilus (gram-positive bacteria), Escherichia coli, and Pseudomonas putida (gram-negative bacteria), and some fungal strains, viz. Aspergillus flavus and Aspergillus niger. The results obtained were compared with standard antibiotics: chloramphenicol, streptomycin, and the antifungal drug cyclohexamide. Some of the tested complexes showed remarkable antimicrobial activities.
| Abbreviations: | ||
| BM | = | Bohr magneton; |
| CFU | = | colony forming unit; |
| DMF | = | N,N-dimethylformamide; |
| DMSO | = | dimethylsulfoxide; |
| IR | = | infrared; |
| MIC | = | minimum inhibitory concentration; |
| MRI | = | magnetic resonance imaging; |
| MTCC | = | Microbial Type Culture Collection; |
| NMR | = | nuclear magnetic resonance; |
| PDA | = | potato dextrose agar; |
| DOTA | = | tetra-azacyclododecane tetra-acetic acid. |
Introduction
The design and study of well-arranged metal-containing macrocycles is an interesting field of chemistryCitation1. Several synthetic and natural macrocyclic compounds have been investigatedCitation2. The chemistry of macrocyclic complexes has attracted the interest of both inorganic and bioinorganic chemists in recent yearsCitation3. The field of macrocyclic chemistry of metals is developing very rapidly because of its importance in the area of coordination chemistryCitation4. Macrocyclic compounds and their derivatives are interesting ligand systems because they are good hosts for metal anions, neutral molecules, and organic cation guestsCitation5. The metal-ion and host–guest chemistries of macrocyclic compounds are very useful in fundamental studies, e.g. phase transfer catalysis and biological studiesCitation6. The family of complexes with aza-macrocyclic ligands has remained a focus of scientific attention for many decadesCitation7. In situ one-pot template condensation reactions lie at the heart of macrocyclic chemistryCitation8. Therefore, template reactions have been widely used for the synthesis of macrocyclic complexesCitation9, where generally the transition metal ions are used as templating agentsCitation10. The metal ions direct the reaction preferentially toward cyclic rather than oligomeric or polymeric productsCitation11. There is continued interest in synthesizing macrocyclic complexes because of their potential applications in fundamental and applied sciencesCitation12, Citation13. Because of their resemblance, synthetic macrocyclic complexes mimic naturally occurring macrocycles including metalloproteins, porphyrins, and cobalamineCitation14–16. Thus, biologically active macrocyclic complexes are used in the identification of diseased and normal tissuesCitation17. Transition metal macrocyclic complexes have received much attention because of their biological activities, including antiviral, anticarcinogenicCitation16, antifertilityCitation18, antibacterial, and antifungal activitiesCitation19. Macrocyclic metal complexes of lanthanides, e.g. Gd3+, are used as MRI contrast agentsCitation20. Macrocyclic metal chelating agents (DOTA) are useful for detecting tumor lesionsCitation21. The macrocyclic metal complexes are also used as NMR shift reagentsCitation22. In a previous article we have reported the synthesis of macrocyclic complexes of Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) derived from 1,8-diaminonaphthalene and glyoxalCitation23. Prompted by this work, in the present article, the synthesis and characterization of Cr(III), Mn(III), and Fe(III) macrocyclic complexes derived from 1,8-diaminonaphthalene and glyoxal are discussed. Besides the characterization of complexes by physicochemical techniques such as IR, NMR, elemental analysis, and magnetic susceptibility and conductance measurements, the biological activities of the synthesized complexes have been examined against some bacterial strains, viz. Bacillus subtilis (MTCC 8509), Bacillus stearothermophilus (MTCC 8508), Escherichia coli (MTCC 51), and Pseudomonas putida, and some fungal strains, viz. Aspergillus flavus and Aspergillus niger. The results obtained have been compared with standard antibiotics: chloramphenicol, streptomycin, and the antifungal drug cyclohexamide.
Experimental
Reagents
All the chemicals used were of AnalaR grade. 1,8- Diaminonaphthalene and glyoxal were procured from Acros, and metal salts were purchased from Merck, Ranbaxy, and were used as received.
Isolation of complexes
All the complexes were synthesized by the template method, i.e. by condensation of 1,8-diaminonaphthalene and glyoxal in the presence of the respective trivalent metal salts. To a hot stirring methanolic solution (~50 mL) of 1,8- diaminonaphthalene (10 mmol) was added trivalent chromium, manganese, or iron salt (5 mmol) dissolved in the minimum quantity of MeOH (~20 mL). The resulting solution was refluxed for 0.5 h. After that, glyoxal (10 mmol) was added in the refluxing mixture and refluxing was continued for 8–10 h. The mixture was concentrated to half of its volume and kept in a desiccator overnight. On overnight cooling, dark colored precipitates formed, which were filtered, washed with methanol, acetone, and diethylether and dried in vacuo. A yield of ∼70–85% was obtained. The complexes were soluble in DMF and DMSO. They were found to be thermally stable in the temperature range 200–250°C, above which their decomposition began.
The template condensation of 1,8-diaminonaphthalene and glyoxal in the presence of trivalent metal salts in the molar ratio 2:2:1 is represented by .
Analytical and physical measurements
The microanalysis of C, H, and N was carried out at SAIF (Sophisticated Analytical Instrumentation Facility), Punjab University, Chandigarh. Magnetic susceptibility measurements were carried out at SAIF, IIT Roorkee. The metal contents in the complexes were determined by the literature methodCitation24. IR spectra were recorded on a Fourier transform (FT)-IR spectrophotometer (PerkinElmer) in the range 4000–200 cm−1 using Nujol mull. 1H-NMR spectra (at room temperature, in DMSO-d6) were recorded on a Bruker Avance II 400 NMR spectrometer (400 MHz) at SAIF, Punjab University, Chandigarh. Electronic spectra (in DMSO) were recorded on a Cary 14 spectrophotometer at room temperature. Fast atom bombardment (FAB) mass spectra (at room temperature) were recorded on an electrospray time-of-flight (TOF MS ES+) mass spectrometer at SAIF, Punjab University, Chandigarh. Thermogravimetric analyses were carried out at Kurukshetra University, Kurukshetra, using a PerkinElmer Diamond TG/DTA analyzer. Conductivity was measured on a digital conductivity meter (HPG system, G-3001). Melting points were determined using capillary tubes in an electrical melting point apparatus.
Biological assay
Test microorganisms
Two gram-positive bacteria Bacillus subtilis (MTCC 8509) and Bacillus stearothermophilus (MTCC 8508), two gram-negative bacteria Escherichia coli (MTCC 51) and Pseudomonas putida (MTCC 121), and two fungal strains Aspergillus flavus and Aspergillus niger were used for biological assay.
In vitro antibacterial activity
Primary screening
The antibacterial activities of the newly synthesized complexes were evaluated by the agar well diffusion assay techniqueCitation25 against two gram-positive bacteria: Bacillus subtilis (MTCC 8509) and Bacillus stearothermophilus (MTCC 8508), and two gram-negative bacteria: Escherichia coli (MTCC 51) and Pseudomonas putida (MTCC 121). The bacterial cultures were maintained on the nutrient agar medium by sub-culturing them on fresh slants every 4–6 weeks and incubating them at the appropriate temperature for 24 h. All stock cultures were stored at 4°C. For the evaluation of antimicrobial activities of the synthetic compounds, a suspension of each test microorganism was prepared. The turbidity of each suspension was adjusted to 0.5 McFarland units by suspending the cultures in sterile distilled water. The size of the final inoculum was adjusted to 5 × 107 CFU/mL. A volume of 20 mL of agar medium was poured into each Petri plate, and plates were swabbed with broth cultures of the respective microorganisms and kept for 15 min for adsorption to take place. Wells of ∼ 8 mm in diameter were punched in the seeded agar plates, and a 100 μL volume of each test compound reconstituted in DMSO was added to the wells. DMSO was used as control for all the test compounds. To allow diffusion of the compounds into the agar, the plates were held at room temperature for 2 h. After that, the plates were incubated at 37°C for 24 h. Antibacterial activities were determined by measuring inhibition zone diameters. All tests were done in triplicate, and the mean diameter of inhibition was calculated.
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) is the lowest concentration of the antimicrobial agent that prevents the development of viable growth after overnight incubationCitation26. The nutrient broth was adjusted to pH 7.0 to be used for determination of the MICCitation27. Inocula of the test microorganisms were prepared by using 16 h-old cultures, adjusted by reference to the 0.5 McFarland standard (108 cells/mL)Citation28. These cultures were further diluted up to 10-fold with nutrient broth to obtain an inoculum size of 1.2 × 107 CFU/mL. A positive control (containing inoculum but no compound) and a negative control (containing compound but no inoculum) were also prepared. A stock solution of 4 mg/mL of each compound was prepared in DMSO and further appropriately diluted to a final concentration ranging from 250 to 0.03 μg/mLCitation26. Separate flasks were taken for each test dilution. To each flask was added 100 μL of inoculum. Then, an appropriately diluted test sample was added to each flask containing broth and microbial inoculum. The contents of the flask were mixed and incubated for 24–48 h at 37°C. The test bacterial cultures were spotted in a predefined pattern by aseptically transferring 5 μL of each bacterial culture onto the surface of solidified agar plates, and incubated at 37°C for 24 h for determination of MIC values.
In vitro antifungal activity
Antifungal activities of the synthesized compounds were determined against two fungal strains, i.e. Aspergillus flavus and Aspergillus niger, by the agar plate techniqueCitation25. Further, the antifungal (percentage inhibition) activities of these compounds were compared with the standard drug cyclohexamide. Potato dextrose agar (PDA) medium was prepared in a flask and sterilized. To check the growth of bacterial culture in the medium, the requisite quantity of a standard antibiotic (ampicillin) was added, so as to obtain the desired final concentration of 100 μg/mL of medium. Test samples were prepared at different concentrations (10 μg, 50 μg, 100 μg per mL) in DMSO, and 200 μL of each sample was spread on PDA medium contained in sterilized Petri plates. Mycelial disks taken from the standard cultures (Aspergillus flavus and Aspergillus niger) of fungi were grown on PDA medium for 5–7 days. These cultures were used for the purpose of inoculation in sterilized Petri dishes, aseptically. Standard cultures inoculated at 28 ± 1°C were also used as controls. The efficiency of each sample was determined by measuring radial mycelial growth. The radial growth of the colony was measured in two directions at right angles to each other, and the average of two replicates was recorded in each case. Data are expressed as percentage inhibition over control according to the size of colonies. The percentage inhibition as given in was calculated using the formula:
% Inhibition = (C – T) × 100/C
where C is the diameter of the fungus colony on the control plate after 96 h incubation, and T is the diameter of the fungus colony on the test plate after the same incubation period.
Results and discussion
Chemistry
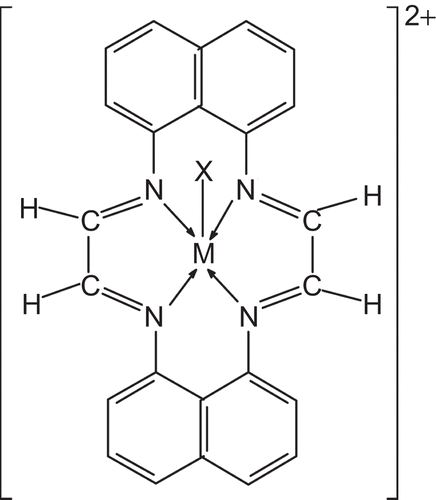
The analytical data show the formula for the macrocyclic complexes as: [M(C24H16N4)X]X2, where M = Cr(III), Fe(III), and Mn(III) and X = Cl−, NO3−, and CH3COO−. Measurements of molar conductance in DMSO showed these chelates to be 1:2 electrolytesCitation29 (conductance 160–189 ohm−1 cm2 mol−1). Tests for anions were positive before and after decomposing the chelates with concentrated HNO3, showing their presence outside as well as inside the coordination sphere. Various methods such as crystallization using mixtures of solvents and low temperature crystallization were unsuccessful in obtaining a single crystal suitable for X-ray crystallography. However, the analytical, spectroscopic, and magnetic data enabled us to predict the possible structure of the synthesized complexes. All macrocyclic complexes were dark colored solids and were soluble in DMF or DMSO. All complexes gave satisfactory elemental analysis results, as shown in .
Table 1. Analytical data for trivalent chromium, manganese, and iron complexes derived from 1,8-diaminonaphthalene and glyoxal.
IR spectra
It was noted that a pair of bands were present in the spectrum of 1,8-diaminonaphthalene at 3350 and 3390 cm−1, corresponding to the ν(NH2) group, which were absent from the IR spectra of all the complexes. Further, no strong absorption band was observed near 1715 cm−1, indicating the absence of the >C=O group of the glyoxal moiety. The disappearance of these bands and the appearance of a new, strong absorption band near 1590–1629 cm−1 confirmed condensation of the carbonyl group of glyoxal and the amino group of diaminonaphthalene and the formation of a macrocyclic Schiff baseCitation30, as these bands may be ascribed to the ν(C=N) stretching vibrationsCitation31,Citation32. The lower value of the ν(C=N) stretching vibrations may be explained on the basis of drift of the lone pair density of the azomethine nitrogen toward the metal atomCitation33,Citation34, indicating that coordination takes place through the nitrogen of the C=N group. The bands present in the range 3010–3050 cm−1 may be ascribed to the ν(C—H) stretching vibrations of the glyoxal and naphthalene moietiesCitation35. The various absorption bands in the region 1450–1588 cm−1 may be ascribed to the ν(C=C) aromatic stretching vibrations of the naphthalene ringCitation36,Citation37. The bands in the region 740–785 cm−1 may be ascribed to ν(C—H) out of plane bending of the aromatic ringCitation38,Citation39. The presence of absorption bands at 1408–1440, 1290–1320, and 1010–1030 cm−1 in the IR spectra of the Cr(III) and Fe(III) nitrato complexes suggests that the nitrate groups are coordinated to the central metal ion in an unidentate fashionCitation40,Citation41. The IR spectra of the chromium, manganese, and iron acetate complexes showed an absorption band in the region 1650–1680 cm−1, which is ascribed to ν(COO−) asymmetric (as) stretching of the acetate ion, and another in the region 1258–1290 cm−1, which can be ascribed to ν(COO−) symmetric (s) stretching vibration of the acetate ion. A difference between νas and νs of around 390–370 cm−1, greater than 144 cm−1, indicates the unidentate coordination of the acetate group with the central metal ionCitation42. The far infrared spectra showed bands in the region 420–445 cm−1, corresponding to ν(M—N) vibrationsCitation43–45. The presence of bands in all complexes in the region 420–445 cm−1 originates from (M—N) azomethine vibrational modes and identifies coordination of the azomethine nitrogenCitation46. The bands present in the range 300–320 cm−1 may be ascribed to the ν(M—Cl) vibrationCitation43–45. The bands present in the region 230–250 cm−1 in all nitrato complexes are assignable to the ν(M—O) stretching vibrationCitation43,Citation44.
NMR spectra
The 1H-NMR spectrum of the zinc(II) complex showed multiplets in the region 6.62–7.32 ppm, corresponding to aromatic ring protons of the naphthalene moiety (12H)Citation47. The singlet at 7.9 ppm may be ascribed to the azomethine (HC=N) protons (4H)Citation48.
Mass spectra
The FAB mass spectra of Cr(III), Mn(III), and Fe(III) macrocyclic complexes were recorded. All the spectra exhibited parent peaks due to molecular ions [M]+. The proposed molecular formulae of these complexes were confirmed by comparing their molecular (formula) weights with m/z values. The molecular ion [M]+ peaks obtained for various complexes were as follows: (1) m/z = 516.6 (due to 35Cl) and 518.6 (due to 37Cl) [mol. wt. 518], (2) m/z = 597.4 [mol. wt. 598], (3) m/z = 587.5 [mol. wt. 589], (4) m/z = 590.5 [mol. wt. 592], (5) m/z = 521.6 (due to 35Cl) and 523.6 (due to 37Cl) [mol. wt. 522], (6) m/z = 601.3 [mol wt. 602], and (7) m/z = 592.4 [mol. wt. 593]. These data are in good agreement with the proposed molecular formulae for these complexes, i.e. [M(C24H16N4)X]X2, where M = Cr(III), Mn(III), and Fe(III), and X = Cl−, NO3−, and CH3COO−. This confirms the formation of the macrocyclic frame. In addition to the peaks due to molecular ions, the spectra exhibited peaks assignable to various fragments arising from the thermal cleavage of the complexes. The peak intensity gives an idea of the stability of the fragments.
Magnetic measurements and electronic spectra
Chromium complexes
Magnetic moments of chromium(III) complexes were found in the range of 3.98–4.30 BM at room temperature, which is close to the predicted values for the three unpaired electrons in the metal ionCitation49. The electronic spectra of the chromium complexes showed bands at ∼9010–9330 cm−1, 13,050–13, 540 cm−1, 17,450–18, 350 cm−1, 27,320–27, 880 cm−1, and 34, 820 cm−1. The spectral bands are consistent with those of five-coordinated Cr(III) complexes, whose structures have been confirmed with the help of X-ray measurementsCitation50. On the basis of the analytical data, spectral studies, and electrolytic nature of these complexes, a five-coordinate square pyramidal geometry may be ascribed for these complexes. Thus, assuming the symmetry C4V for these complexesCitation51–53, the various spectral bands may be assigned as: 4B1→4Ea, 4B1→4B2, 4B1→4A2, and 4B1→4Eb.
Manganese complex
The magnetic moment of the manganese(III) complex was 4.90 BM, which indicates the high spin d4 system49. The electronic spectrum of the manganese complex showed three d-d bands which lay in the range 12,250–12,590, 16,050–18,920, and 35,440–35, 750 cm−1. The higher energy band at 35,440–35, 750 cm−1 may be assigned to charge transfer transitions. The spectrum resembled those reported for five-coordinate square pyramidal manganese porphyrinsCitation51–53. This idea is further supported by the presence of the broad ligand field band at 20, 400 cm−1 diagnostic of C4V symmetry, and thus the various bands may be assigned as follows: 5B1→5A1, 5B1→5B2, and 5B1→5E, respectively. The band assignment in single electron transition may be made as: dz2→dx2–y2, dxy→dx2–y2, and dxz, dyz→dx2–y2, respectively, in order of increasing energy.
Iron complexes
The magnetic moment of iron(III) complexes lay in the range 5.84–5.90 BM, corresponding to five unpaired electrons, which are close to the predicted high spin values for these metal ionsCitation49. The electronic spectra of iron(III) complexes showed various bands in the ranges 9850–9980, 15,530–15,565, and 27,600–27, 750 cm−1, consistent with the ranges of spectral bands reported for five-coordinate square pyramidal iron(III) complexesCitation52–54. Assuming C4V symmetry for these complexes, the various bands can be assigned as: dxy→dxz, dyz and dxy→dzCitation2. Any attempt to make accurate assignment is difficult due to interactions of the metal–ligand π-bond systems, lifting the degeneracy of the dxz and dyz pair.
Thermogravimetric analyses
The thermogravimetric analysis/differential thermogravimetry (TGA/DTG) thermograms () of two metal complexes [Cr(C24H16N4)Cl]Cl2 and [Fe(C24H16N4)Cl]Cl2 were recorded in a dynamic N2 atmosphere over the temperature range 100–800°C at a heating rate of 10°C/min. TGA thermograms showed two stages of thermal decomposition for both complexes. Thermal degradation of the metal complexes is an exothermic event as there are two exothermic peaks corresponding to the respective thermal decomposition stages. The TGA thermogram of the Cr(III) complex showed two stages of thermal decomposition (). The first step runs over the temperature range 246–378°C. The second step runs over the temperature range 378–600°C and corresponds to decomposition of the remaining organic moiety. The TGA thermogram of the Fe(III) complex also showed two stages of thermal decomposition (). The first step runs over the temperature range 200–405°C. The second step runs over the temperature range 405–600°C and corresponds to decomposition of the remaining organic moiety. The first step in the decomposition of both complexes corresponds to removal of the coordinated chloride ions, and after that the organic moiety is decomposed, with the final residues as oxides of the metals.
Proposed structure of complexes
Based on various studies including elemental analysis, conductance measurements, magnetic susceptibilities, and IR, NMR, electronic, and mass spectral studies, a five-coordinate square pyramidal geometry as shown in may be proposed for all the complexes.
Biological results and discussion
The newly synthesized compounds (1–7) were tested in the present investigation for their in vitro antibacterial as well as antifungal activities. The antibacterial activities were studied against the two gram-positive bacteria, i.e. Bacillus subtilis (MTCC 8509), Bacillus stearothermophilus (MTCC 8508), and two gram-negative bacteria, i.e. Escherichia coli (MTCC 51), Pseudomonas putida (MTCC 121). The minimum inhibitory concentrations (MICs) of all the complexes against gram-positive and gram-negative bacteria were determined by the National Committee for Clinical Laboratory Standards (NCCLS) methodCitation26 and are given in . In the whole series, compound (4) showed the highest MIC, 2 μg/mL, against B. subtilis, P. putida, and E. coli (). Compound (2) possessed an MIC of 4 μg/mL against B. subtilis and an MIC of 8 μg/mL against B. stearothermophilus and E. coli. Compound (6) showed an MIC of 4 μg/mL against the bacteria B. subtilis and an MIC of 8 μg/mL against B.stearothermophilus and E. coli. Further, the antibacterial activities of these complexes were compared with standard antibiotics, viz. chloramphenicol and streptomycin (). In general, all the synthesized compounds showed antibacterial activities, but they were found to be more potent inhibitors against gram-negative as compared to gram-positive bacteria (, ). Some compounds were found to be more potent than the standard antibiotics against some species of bacteria. Compound (4) was found to be more potent than the commercial antibiotics chloramphenicol and streptomycin against the bacterial strain P. putida, showing an MIC of 2 μg/mL (, ). Further, the MIC (2 μg/mL) shown by compound (4) is equal to the MIC shown by standard antibiotics chloramphenicol and streptomycin against the bacterial strain B. subtilis and antibiotic chloramphenicol against the bacterial strain E. coli.
Figure 3. Comparison of MIC of compounds with standard antibiotics up to the concentration 32 μg/mL. a, Bacillus subtilis (MTCC 8509); b, Bacillus stearothermophilus (MTCC 8508); c, Pseudomonas putida (MTCC 121); d, Escherichia coli (MTCC 51). Chloramphenicol, streptomycin: standard antibiotics.
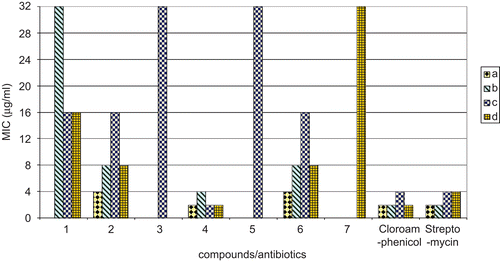
Table 2. Minimum inhibitory concentration (MIC) of complexes against test bacteria using agar dilution assay.
The antifungal activities of the complexes were determined against two fungal strains, i.e. Aspergillus flavus and Aspergillus niger, and then compared with the standard drug cyclohexamide (, ). The antifungal activities (percentage inhibition) are given in . In the whole series, compound (2) showed the highest percentage inhibition against both fungal strains, whereas none of the tested compounds restricted fungal growth excellently (). However, the tested compounds (4), (5), and (6) showed a moderate capability to check the growth of these fungal species ().
Figure 4. Comparison of percentage inhibition of compounds against fungal strains with standard drug cyclohexamide. f, Aspergillus flavus; g, Aspergillus niger. Cyclohexamide: standard drug.
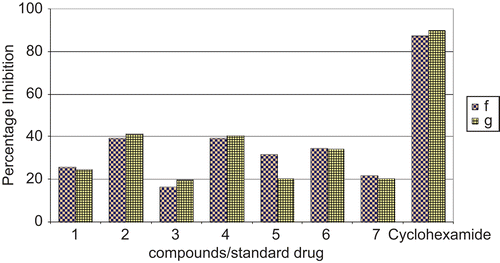
Table 3. Antifungal (percentage inhibition) activities of complexes against fungal strains (for concentration 100 μg/mL).
Conclusions
It has been suggested that chelation/coordination reduces the polarity of the metal ion mainly because of partial sharing of its positive charge with the donor group within the whole chelate ring systemCitation55. This process of chelation thus increases the lipophilic nature of the central metal atom, which in turn, favors its permeation through the lipid layer of the membrane, hence causing the metal complex to cross the bacterial membrane more effectively, thus increasing the activity of the complex. Besides this, many other factors such as solubility, dipole moment, and conductivity influenced by the metal ion may be possible reasons for the remarkable antibacterial activities of these complexesCitation56.
Acknowledgements
One of the authors (D.P.S.) thanks the University Grants Commission, New Delhi for financial support in the form of a Major Research Project (MRP-F. No. 32-196/2006(SR)) and Krishan Kumar for the award of Project Fellowship under the above. Mr. Mahender Kumar, Department of Chemistry, KUK, is acknowledged for providing the TGA/DTG facility. Thanks are also due to the authorities of NIT, Kurukshetra, for providing necessary research facilities.
Declaration of interest: The authors report no conflicts of interest.
References
- Chandra S, Gupta LK, Agrawal S. Modern spectroscopic and biological approach in the characterization of a novel 14-membered [N4] macrocyclic ligand and its transition metal complexes. Transit Met Chem 2007;32:240–5.
- Hanafy AI, Maki ABKT, Mostafa MM. Synthesis, structural and catalytic activity of binaphthyl macrocyclic complexes. Transit Met Chem 2007;32:960–6.
- Fernandez MC, Basitida R, Macias A, Valencia L, Lourida PP. Different nuclearities of M(II) nitrate complexes (M = Co, Ni, Cu, and Cd) with a tetrapyridyl pendant-armed hexaazamacrocyclic ligand. Polyhedron 2006;25:783–92.
- Ilhan S, Temel H. Synthesis and characterization of a new macrocyclic Schiff base derived from 2,6-diaminipyridine and 1,10-bis(2-formylphenyl)-1,4,7,10-tetraoxadecane and its Cu(II), Ni(II), Pb(II), Co(III), La(III) complexes. Transit Met Chem 2007;32:1039–46.
- Chandra S, Gautum A, Tyagi M. Synthesis and spectroscopic characterization of transition metal complexes of a 12-membered tetraaza [N4] macrocyclic ligand and their biological activity. Transit Met Chem 2007;32:1079–84.
- Bozic LT, Marotta E, Traldi P. Efficient solid-state microwave-promoted complexation of a mixed dioxa-diaza macrocycle of a sodium ethyl 4-benzeneazophosphonate complex. Polyhedron 2007;26:1663–8.
- Lindoy LF. The Chemistry of Macrocyclic Ligand Complexes. Cambridge: Cambridge University Press, 1989.
- Curtis NF. Macrocyclic coordination compounds formed by condensation of metal-amine complexes with aliphatic carbonyl compounds. Coord Chem Rev 1968;3:3–47.
- Niasari MS, Davar F. In situ one-pot template synthesis (IOPTS) and characterization of copper(II) complexes of 14-membered hexaaza macrocyclic ligand 3,10-dialkyl-dibenzo-1,3,5,8,10,12-hexaazcyclo tetradecane. Inorg Chem Commun 2006;9:175–9.
- Prasad RN, Gupta S, Jangir S. Synthesis and characterization of MgII, CaII, SrII and BaII complexes of 2,13-dimethyl-3,14-diethyl/propyl/phenyl-1,4,12,15-tetraazacyclodocosa-1,3,12,14-tetraene. J Indian Chem Soc 2007;84:1191–4.
- Khan TA, Hasan SS, Mohamed AK, Shakir M. Template synthesis and spectroscopic studies of 13-membered oxotetraaza macrocyclic complexes. Indian J Chem 1998;37A:1123–5.
- Kumar DS, Alexander V. Synthesis of lanthanide(III) complexes of chloro and bromo substituted 18-membered tetraaza macrocycles. Polyhedron 1999;18:1561–8.
- Ilhan S, Temel H, Ziyadanogullari R, Sekerci M. Synthesis and spectral characterization of macrocyclic Schiff base by reaction of 2,6-diaminipyridine and 1,4-bis(2-carboxyaldehydephenoxy) butane and its Cu(II), Ni(II), Pb(II), Co(III), La(III) complexes. Transit Met Chem 2007;32:584–90.
- Shakir M, Khatoon S, Parveen S, Azim Y. Synthesis and spectral studies of a 12-membered tetraimine macrocyclic ligand and its complexes. Transit Met Chem 2007;32:42–6.
- Chandra S, Sharma S. Synthesis and spectral studies of transition metal complexes with dibenzo-[b,i]-8,10,19,21-tetramethyl-[1,5,8,12]-tetraazatetradeca-1,3,5,7,10,12,14,16,18,21-decane a fourteen membered tetradentate macrocyclic ligand. J Indian Chem Soc 2006;83:988–92.
- Chandra S, Pundir M. Spectroscopic characterization of chromium (III), manganese(II) and nickel(II) complexes with a nitrogen donor tetradentate, 12-membered azamacrocyclic ligand. Spectrochim Acta A 2008;69:1–7.
- Prasad RN, Upadhyay A. Chromium(III), iron(III) and cobalt(II) complexes of 14 and 16-membered tetraazamacrocycles. J Indian Chem Soc 2006;83:857–60.
- Chandra S, Gupta R, Gupta N, Bawa SS. Biologically relevant macrocyclic complexes of copper: spectral, magnetic, thermal and antibacterial approach. Transit Met Chem 2006;31:147–51.
- Chandra S, Gupta LK, Agrawal S. Synthesis spectroscopic and biological approach in the characterization of novel [N4] macrocyclic ligand and its transition metal complexes. Transition Met Chem 2007;32:558–63.
- Singh DP, Kumar R, Malik V, Tyagi P. Template synthesis, spectroscopic studies and biological activities of macrocyclic complexes derived from thiocarbohydrazide and glyoxal. J Enzyme Inhib Med Chem 2007;22:177–82.
- Kosmos C, Snook D, Gooden CS, Courtenay Luck NS, McCalla MJ, Meares CF, et al. Development of humoral immune responses against a macrocyclic chelating agent (DOTA) in cancer patients receiving radioimmunoconjugates for imaging and therapy. Cancer Res 1992;52:904–11.
- Dong W, Yang R, Yan L. Rare earth complexes of a new Schiff base macrocyclic ligand derived from 2,2’-(ethylenedioxy) bis-benzaldehyde and 2,2’-(ethylenedioxy) bis-benzylamine. Indian J Chem 2001;40A:202–6.
- Singh DP, Kumar K, Dhiman SS, Sharma J. Biologically active macrocyclic complexes derived from diaminonaphthalene and glyoxal: template synthesis and spectroscopic approach. J Enzyme Inhib Med Chem 2008 Dec 2:1. [Epub ahead of print]
- Vogel AI. A Text Book of Qualitative Chemical analysis. 5th edition. Longman, London1989.
- Collee JG, Miles RS, Watt B. Laboratory control of antimicrobial therapy. In: David W Hecht, Practical Medical Microbiology, 14th ed. New York: Churchill Livingstone, 1996; M11–A7.
- NCCLS. Method for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically, Approved Standards, 5th ed. Villanova, PA: National Committee for Clinical Laboratory Standards, 2000.
- Greenwood D, Slack R, Peutherer J. Medical Microbiology: A Guide to Microbial Infections: Pathogenesis Immunity, Laboratory Diagnosis and Control, 15th ed. Churchill Livingstone: Edinburgh; London, 1997.
- McFarland J. The nephelometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J Am Med Assoc 1907;49:1176–8.
- Kumar R, Singh R. Chromium(III) complexes with different chromospheres macrocyclic ligand: synthesis and spectroscopic studies. Turk J Chem 2006;30:77–87.
- Singh DP, Kumar R, Tyagi P. Template synthesis, spectroscopic studies and biological screening of macrocyclic complexes derived from thiocarbohydrazide and benzil. Transit Met Chem 2006;31:970–3.
- Siddiqi ZA, Khan M, Khalid M, Kumar S. Spectral and electrochemical characterization of bimetallic complexes of a novel 32-membered unsymmetrical [N12] macrocycle. Transit Met Chem 2007;32:927–35.
- Singh DP, Shishodia N, Yadav BP, Rana VB. Synthesis and characterization of bivalent metal complexes of a tetradentate [N6] macrocyclic ligand. Polyhedron 1997;16:2229–32.
- Chandra S, Sharma SD. Chromium(III), manganese(II), cobalt(II), nickel(II), copper(II) and palladium(II) complexes of a 12-membered tetraaza [N4] macrocyclic ligand. Transit Met Chem 2002;27:732–5.
- Lodeiro C, Basitida R, Bertolo E, Macias A, Rodriguez R. Synthesis and characterization of four novel NxOy-Schiff base macrocyclic ligands and their metal complexes. Transit Met Chem 2003;28:388–94.
- Singh DP, Kumar R, Malik V, Tyagi P. Synthesis and characterization of complexes of Co(II), Ni(II), Cu(II), Zn(II), Cd(II) with macrocycle 3,4,11,12-tetraoxo-1,2,5,6,9,10,13,14-octaazacyclohexadeca-6,8,14,16-tetraene and their biological screening. Transit Met Chem 2007;32:1051–5.
- Prasad RN, Mathur M, Upadhayay A. Synthesis and spectroscopic studies of Cr(III), Fe(III) and Co(III) complexes of hexaaza macrocycles. J Indian Chem Soc 2007;84:1202–4.
- Costamagna J, Ferraudi G, Villagran M, Wolcan E. Ligand luminescence and photoinduced charge separation in bis(naphthalene) substituted fourteen-membered tetraaza-macrocyclic complexes of Cu(II) and Ni(II). J Chem Soc Dalton Trans 2000;(15):2631–7.
- Chandra S, Gupta LK. Designing and synthesis of macrocyclic Schiff base ligand: study of interaction with Mn(II), Co(II), Ni(II) and Cu(II) and biological screening. J Indian Chem Soc 2005;82:454–8.
- Singh DP, Shishodia N, Yadav BP, Rana VB. Trivalent transition metal ion directed macrocycles. J Indian Chem Soc 2004;81:287–90.
- Chandra S, Gupta LK. Spectroscopic characterization of tetradentate macrocyclic ligand: its transition metal complexes. Spectrochim Acta A 2004;60:2767–74.
- Shakir M, Varkey SP, Hameed PS. Divalent, cobalt, nickel, copper and zinc complexes of tetraaza macrocycles bearing polyamide groups: synthesis and characterization. Polyhedron 1993;12:2775–80.
- Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. New York: Wiley Interscience Publication, 1978.
- Shakir M, Nasman OSM, Varkey SP. Binuclear [N6] 22-membered macrocyclic transition metal complexes: synthesis and characterization. Polyhedron 1996;15:309–14.
- Shakir M, Islam KS, Mohamed AK, Shagufa M, Hasan SS. Macrocyclic complexes of transition metals with divalent polyaza units. Transition Met Chem 1999;24:577–80.
- Chandra S, Kumar R. Synthesis and spectral studies on mononuclear complexes of chromium(III) and manganese(II) with 12-membered tetradentate N2O2, N2S2 and N4 donor macrocyclic ligand. Transit Met Chem 2004;29:269–75.
- Rana VB, Singh DP, Singh P, Teotia MP. Trivalent chromium, manganese, iron and cobalt chelates of a tetradentate [N6] macrocyclic ligand. Transit Met Chem 1982;7:174–7.
- Jiang H, Sun H, Zhang S, Hua R, Xu Y, Jin S, et al. NMR investigations of inclusion complexes between β-cyclodextrin and naphthalene/anthraquinone derivatives. J Incl Phenom 2007;58:133–8.
- Boghaei DM, Mohebi S. Synthesis, characterization and study of vanadyl tetradentate Schiff base complexes as catalyst in aerobic selective oxidation of olefins. J Mol Catal A Chem 2002;179:41–51.
- Figgis BN, Lewis J. Progress in Inorganic Chemistry, Wiley Interscience, New York, 1964;6:37.
- Wood JS. Stereochemical electronic structural aspects of five coordination. Prog Inorg Chem 1972;16:227.
- Singh DP, Rana VB. Binuclear chromium(III), manganese(III), iron(III) and cobalt(III) complexes bridged by diaminopyridine. Polyhedron 1995;14:2901–6.
- Singh DP, Kumar R. Trivalent metal ion directed synthesis and characterization of macrocyclic complexes. J Serb Chem Soc 2007;72:1069–1074.
- Singh DP, Kumar R, Singh J. Antibacterial activity and spectral studies of trivalent chromium, manganese, iron macrocyclic complexes derived from oxalyldihydrazide and glyoxal. J Enzyme Inhib Med Chem 2008 Oct 27;1. [Epub ahead of print]
- Lever ABP. Inorganic Electronic Spectroscopy. Amsterdam: Elsevier, 1968.
- Chohan ZH, Pervez H, Rauf A, Khan KM, Supuran CT. Isatin-derived antibacterial and antifungal compounds and their transition metal complexes. J Enzyme Inhib Med Chem 2004;19:417–23.
- Chohan ZH, Scozzafava A, Supuran CT. Unsymmetrical 1,1′-disubstituted ferrocenes: synthesis of Co(II), Cu(II), Ni(II) and Zn(II) chelates of ferrocenyl-1-thiadiazolo-1′-tetrazole,-1-thidiazolo-1′-triazole and -1-tetrazolo-1′-triazole with antimicrobial properties. J Enzyme Inhib Med Chem 2002;17:261–6.
![Figure 1. Thermogravimetric analysis curves. (a) TGA/DTG curve for [Cr(C24H16N4)Cl]Cl2 complex. (b) TGA/DTG curve for [Fe(C24H16N4)Cl]Cl2 complex.](/cms/asset/33efa5b1-d63c-4446-b25c-1b4ad2ef73d9/ienz_a_393447_f0002_b.gif)