Abstract
Twenty-nine imidazolones 1–29 were synthesized and were randomly screened for their in vitro anti-leishmanial potential. Compound 17 showed a good anti-leishmanial activity with an IC50 value of 12.98 ± 0.32 μg/mL. Compounds 14 and 24 were also found to be moderately active (IC50 values 28.20 ± 0.03 and 41.12 ± 0.32 μg/mL, respectively). The activity was compared with that of standard drugs, amphotericin B (IC50 = 0.12 ± 0.41 μg/mL) and pentamidine (IC50 = 2.56 ± 0.10 μg/mL).
Introduction
Leishmaniasis is a protozoan disease caused by parasites belonging to the genus Leishmania, which affects the skin, mucous membranes, and internal organs. These parasites are carried by the blood-sucking sandfly, Phlebotomus species. It also known as Kala azar, which is Hindi for “black fever”. When the parasites are transmitted to humans or animals, the host’s immune system attempts to consume the protozoa with immune cells called macrophages; these macrophages burst open, releasing the protozoa and allowing them to take over neighboring cells.
Leishmaniasis is classified into cutaneous, visceral, mucosal or mucocutaneous, and diffused cutaneous formsCitation1,Citation2. The symptoms of leishmaniasis include wounds, fever, weight loss, anorexia, change in hair color, abnormal growth and major dysfunction of the liver, damage to the spleen, bone marrow, and lymph nodes, ulcer, nasal blockage, swelling of the nose and lips with damage of the soft tissues of the oronasal cavity, wounds widely distributed on the skin, thickening of plaques, and multiplex nodules and anemia.
Leishmaniasis disease is distributed worldwide and causes considerable mortality and morbidity. It is present in approximately 88 countries ranging from Central and South America to West Asia. More than 90% of cases of visceral leishmaniasis are in Bangladesh, Brazil, India, Nepal, and Sudan. In Sudan, one epidemic lasted from 1984 to 1994 and claimed over 100,000 lives. Leishmaniasis is treatable, but existing medicines are costly. Treatment is generally with pentavalent antimonials such as Pentostam (sodium stibogluconate) or Glucantime (meglumine antimonite). Second-line drugs are amphotericin B and pentamidine; however, these are not used routinely because of toxicityCitation3–5.
Imidazolone is a five-membered heterocyclic ring system with three carbon atoms and two nitrogen atoms at positions 1 and 3. Imidazolones are carbonyl dihydro-imidazoles, also known as oxoimidazolines. In general, imidazolones are of many types, e.g. 2-, 4-, or 5-imidazolones. The number indicates the position of the carbonyl group.
The imidazolones are diverse bioactive heterocyclic compounds. They show numerous biological activities including anti-human immunodeficiency virus (HIV), antimalarial, local anesthetic, goitrogenic, antibiotic, antifungal, antiparasitic, anticonvulsant, monoamine oxidase (MAO) inhibitory, sedative, hypnotic, central nervous system (CNS) depressant, anti-inflammatory, anticancer, anti-parkinsonian, and immunomodulatory properties. Previously, the imidazolones were prepared by heating a mixture of 5-oxazolone derivatives with differently substituted aromatic or aliphatic amines in the presence of excess of pyridine for 10–15 h. The yields of the product were moderate and the reaction required a long time to completeCitation6–16.
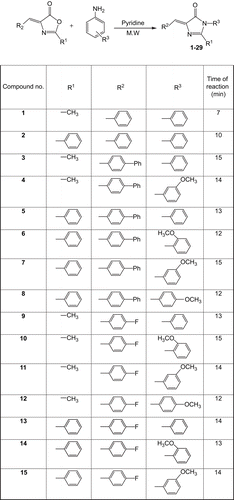
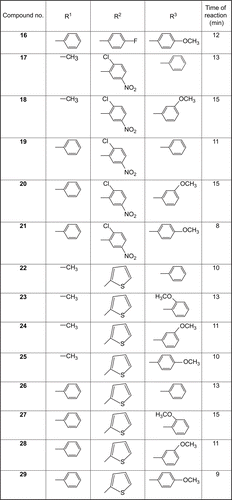
In our ongoing research on leishmaniasisCitation17–20, we synthesized 29 imidazolones, 1–29, by treating different oxazolones with varyingly substituted aromatic amines. In a typical reaction, a mixture of differently substituted E-oxazolonesCitation21–23 (1 mmol) and substituted aromatic amines (1.1 mmol) in anhydrous pyridine were irradiated by microwaves to afford compounds 1–29. Compounds 1–29 were randomly screened for their in vitro anti-leishmanial potential. Compound 17 showed a good anti-leishmanial activity, having an IC50 value of 12.98 ± 0.32 μg/mL. Compounds 14 and 24 were also found to be moderately active, having IC50 values of 28.20 ± 0.03 and 41.12 ± 0.32 μg/mL, respectively. However, compounds 1–3, 5, 6, 9, 11–13, 15, 18–23, 25, 26, 28, and 29 showed IC50 values greater than 50 μg/mL, while compounds 4, 7, 8, 10, 16, and 27 showed IC50 values greater than 100 μg/mL, and thus were considered to be inactive. The activity was compared with that of the standard drugs amphotericin B (IC50 = 0.12 ± 0.41 μg/mL) and pentamidine (IC50 = 2.56 ± 0.10 μg/mL). The structures of all the synthesized compounds were determined by spectroscopic analysis.
Material and methods
General experimental
Melting points were determined on a Büchi 434 melting point apparatus and were uncorrected. Nuclear magnetic resonance (NMR) experiments were performed on Bruker Avance AM 300 and 500 MHz spectrometers. CHN (carbon, hydrogen, and nitrogen) analysis was performed on a Carlo Erba Strumentazione-Mod-1106 elemental analyzer (Italy). Ultraviolet (UV) spectra were recorded on a PerkinElmer Lambda-5 UV/VIS spectrometer in MeOH. Infrared (IR) spectra were recorded on a Jasco IR-A-302 spectrometer as KBr (disk). Electron impact mass spectra (EI MS) were recorded on a Finnigan MAT-311A spectrometer (Germany). Reactions were carried out in a CEM Discover system, model 908010 (USA). Thin layer chromatography (TLC) was performed on pre-coated silica gel aluminum plates (Kieselgel 60, 254; E. Merck, Germany). Chromatograms were visualized under UV light at 254 and 365 nm or by using iodine vapors.
General procedure for the synthesis of compounds 1–29
A mixture of differently substituted E-oxazolonesCitation22–24 (1 mmol) and substituted aromatic amines (1.1 mmol) in anhydrous pyridine were irradiated by microwaves (CEM Discover system, model 908010) for 10–15 min at 150°C. The input power of the microwave reactor was 300 W, and the same power was used for all reactions. The reactions were performed in an open vessel. The completion of reaction was monitored by TLC, and then 5 mL of ice-cool 5% HCl in water was added and the mixture was left overnight. The resultant solids were collected and washed with water before being crystallized by ethanol, filtered, and dried to afford compounds 1–29.
2-Methyl-3-phenyl-5-[(E)-phenylmethylidene]-3,5-dihydro-4H-imidazole-4-one (1) Yield: 88%; m.p.: 155°C; Rf: 0.61 (ethyl acetate/hexane, 3:7); UV (MeOH): λmax 299 (log « = 4.5) nm; IR (KBr): vmax 3020, 2917, 1708, 1635, 1250 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.21 (d, J2′,3′ = J6′,5′ = 8.2 Hz, 2H, H-2′/6′), 7.70 (d, J2″,3″ = J6″,5″ = 8.2 Hz, 2H, H-2″/6″), 7.50–7.55 (m, 3H, H-3″–5″), 7.43–7.45 (m, 3H, H-3′–5′), 7.04 (s, 1H, H-6), 2.35 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 262 (M+, 34), 144 (10), 118 (52), 77 (100); Anal. calcd for C17H14N2O: C, 77.84; H, 5.38; N, 10.68%; Found: C, 77.86; H, 5.39; N, 10.40%.
2,3-Diphenyl-5-[(E)-phenylmethylidene]-3,5-dihydro-4H-imidazole-4-one (2) Yield: 77%; m.p.: 166°C; Rf: 0.61 (ethyl acetate/hexane, 3:7); UV (MeOH): λmax 310 (log ε = 4.2) nm; IR (KBr): νmax 3019, 2823, 1708, 1655, 1267 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.46 (m, 3H, H-2′/6′), 8.21 (m, 2H, H-2″/6″), 7.88 (m, 3H, H-3′–5′), 7.57 (d, J2″′,3″′ = J6″′,5″′ = 7.8 Hz, 2H, H-2″′/6″′), 7.51 (m, 3H, H-3″′–5″′), 7.42 (m, 3H, H-3″–5″), 7.06 (s, 1H, H-6); EI MS: m/z (rel. abund. %) 324 (M+, 34), 193 (10), 118 (100), 77 (75); Anal. calcd for C22H16N2O: C, 81.46; H, 4.97; N, 8.64%; Found: C, 81.48; H, 4.99; N, 8.66%.
5-[(E)-[1,1′-Diphenyl]-4-ylmethylidene]-2-methyl-3-phe nyl-3,5-dihydro-4H-imidazole-4-one (3) Yield: 99%; m.p.: 158°C; Rf: 0.61 (ethyl acetate/hexane, 3:7); UV (MeOH): λmax 368 (log ε = 4.7) nm; IR (KBr): νmax 3027, 2923, 1708, 1645, 1267 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.26 (d, J2′,3′ = J6′,5′ = 8.4 Hz, 2H, H-2′/6′), 7.70 (d, J2″′,3″′ = J6″′,5″′ = 8.2 Hz, 2H, H-2″′/6″′), 7.64 (d, J3′,2′ = J5′,6′ = 8.3 Hz, 2H, H-3′/5′), 7.54–7.50 (m, 3H, H-3″′–5″′), 7.43–7.46 (m, 3H, H-3″–5″), 7.38 (d, J2″,3″ = J6″,5″ = 7.2 Hz, 2H, H-2″/6″), 7.07 (s, 1H, H-6), 2.36 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 238 (M+, 34), 193 (10), 118 (100), 77 (75); Anal. calcd for C23H18N2O: C, 81.63; H, 5.36; N, 8.28%; Found: C, 81.68; H, 5.39; N, 8.30%.
5-[(E)-[1,1′-Biphenyl]-4-ylmethylidene]-3-(3-meth oxyph enyl)-2-methyl-3,5-dihydro-4H-imidazole-4-one (4) Yield: 85%; m.p.: 147°C; Rf: 0.54 (ethyl acetate/hexane, 3:7); UV (MeOH): λmax 313 (log ε = 4.5) nm; IR (KBr): νmax 3028, 2977, 1643, 1601, 1270 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 8.25 (d, J2′,3′ = J6′,5′ = 8.3 Hz, 2H, H-2′/6′), 7.69 (d, J3′,2′ = J5′,6′ = 8.3 Hz, 2H, H-3′/5′), 7.45 (m, 3H, H-3″–5″), 7.68 (d, J6″′,5″′ = 8.2 Hz, 1H, H-6″′), 7.63 (m, 1H, H-5″′), 7.37 (m, 2H, H-2″/6″), 7.25 (br.s, 1H, H-2″′), 7.23 (s, 1H, H-6), 6.99 (m, 1H, H-4″′), 3.88 (s, 3H, OCH3), 2.36 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 368 (M+, 100), 193 (19.1), 148 (61), 134 (40), 77 (10.3); Anal. calcd for C24H20N2O2: C, 81.63; H, 5.36; N, 8.28%; Found: C, 81.65; H, 5.37; N, 8.31%.
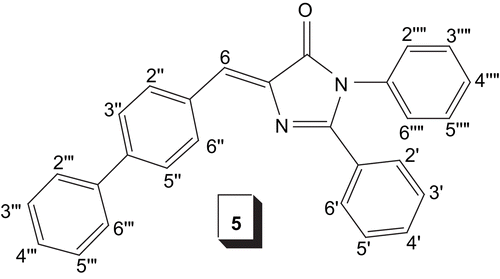
5-[(E)-[1,1′-Biphenyl]-4-ylmethylidene]-2,3-diphenyl id ene-3,5-dihydro-4H-imidazole-4-one (5) Yield: 97%; m.p.: 160°C; Rf: 0.62 (ethyl acetate/hexane, 3:7); UV (MeOH): λmax 313 (log ε = 3.45) nm; IR (KBr): νmax 3255, 3030, 1640, 1440, 1290 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.45 (m, 2H, H-2′/6′), 8.21 (m, 2H, H-2″/6″), 7.86 (m, 3H, H-3′–5′), 7.65 (d, J3″,2″= J5″,6″ = 8.4 Hz, 2H, H-3″/5″), 7.57 (d, J2″″,3″″ = J6″″,5″″ = 7.7 Hz, 2H, H-2″″/6″″), 7.50 (m, 3H, H-3″″–5″″), 7.41 (m, 3H, H-3″′–5″′), 7.37 (d, J2″′,3″′ = J6″′,5″′ = 7.2 Hz, 2H, H-2″′/6″′), 7.27 (s, 1H, H-6); EI MS: m/z (rel. abund. %) 400 (M+, 6), 390 (17), 270 (22), 167 (100), 105 (83); Anal. calcd for C28H20N2O: C, 83.98; H, 5.03; N, 7.00%; Found: C, 83.99; H, 5.12; N, 7.08% ().
5-[(E)-[1,1′-Biphenyl]-4-ylmethylidene]-3-(2-methoxyp henyl)-2-phenyl-3,5-dihydro-4H-imidazole-4-one (6) Yield: 85%; m.p.: 130°C; Rf: 0.52 (ethyl acetate/hexane, 3:7), UV (MeOH): λmax 319 (log ε = 4.69) nm; IR (KBr): νmax 3354, 3056, 1653, 1528, 1250 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.44 (d, J2′,3′ = J6′,5′ = 7.2 Hz, 2H, H-2′/6′), 8.19 (d, J2″,3″ = J6″,5″ = 7.2 Hz, 2H, H-2″/6″), 7.91 (m, 3H, H-3′–5′), 7.65 (d, J3′,2′ = J5′,6′ = 7.3 Hz, 2H, H-3″/5″), 7.57 (d, J6″′,5″′ = 8.0 Hz, 1H, H-6″′), 7.42 (m, 3H, H-3″′–5″′), 7.35 (d, J2″′,3″′ = J6″′,5″′ = 7.3 Hz, 2H, H-2″′/6″′), 7.31 (m, 2H, H-4″′, 5″′), 7.28 (s, 1H, H-6), 6.86 (d, J3″′,4″′ = 8.1 Hz, 1H, H-3″′), 3.79 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 430 (M+, 5), 326 (9.34), 123 (92), 105 (100), 77 (30); Anal. calcd for C29H22N2O: C, 80.91; H, 5.15; N, 6.51%; Found: C, 80.93; H, 5.15; N, 6.52%.
5-[(E)-[1,1′-Biphenyl]-4-ylmethylidene]-3-(3-methoxyp henyl)-2-phenyl-3,5-dihydro-4H-imidazole-4-one (7) Yield: 83%; m.p.: 175°C; Rf: 0.53 (ethyl acetate/hexane, 3:7); UV (MeOH): λmax 314 (log ε = 4.6) nm; IR (KBr): νmax 3256, 3062, 3029, 1641, 1481, 1260 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.28 (d, J2′,3′ = J6′,5′ = 8.4 Hz, 2H, H-2′/6′), 8.20 (d, J2″,3″ = J6″,5″ = 7.1 Hz, 2H, H-2″/6″), 7.90 (m, 3H, H-3′-5′), 7.70 (m, 1H, H-6″″), 7.65 (m, 1H, H-5″″), 7.52 (d, J3″,2″ = J5″,6″ = 8.5 Hz, 2H, H-3″/5″), 7.41 (m, 3H, H-3″′–5″′), 7.36 (d, J2″′,3″′ = J6″′,5″′ = 8.4 Hz, 2H, H-2″′/6″′), 7.25 (s, 1H, H-6), 7.21 (s, 1H, H-2″″), 7.05 (m, 1H, H-4″″), 3.80 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 430 (M+, 2), 327 (11), 281 (2.6), 123 (40), 105 (100), 77 (29); Anal. calcd for C29H22N2O2: C, 80.91; H, 5.15; N, 6.51%; Found: C, 80.92; H, 5.16; N, 6.53%.
5-[(E)-[1,1′-Biphenyl]-4-ylmethylidene]-3-(4-methoxyp henyl)-2-phenyl-3,5-dihydro-4H-imidazole-4-one (8) Yield: 85%; m.p.: 175°C; Rf: 0.54 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 313 (log ε = 4.65); IR (KBr): νmax 3257, 3060, 2930, 1638, 1510, 1242 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.54 (d, J3″,2″ = J5″,6″ = 7.5 Hz, 2H, H-3″/5″), 8.46 (m, 2H, H-2′/6′), 8.39 (m, 2H, H-2″/6″), 7.87 (m, 3H, H-3′-5′), 7.65 (d, J2″″,3″″ = J6″″,5″″ = 8.9 Hz, 2H, H-2″″/6″″), 7.43 (m, 3H, H-3″′–5″′), 7.40 (d, J2″′,3″′ = J6″′,5″′ = 7.7 Hz, 2H, H-2″′/6″′), 7.24 (s, 1H, H-6), 6.83 (d, J3″″,2″″ = J5″″,6″″ = 8.8 Hz, 2H, H-3″″/5″″), 3.76 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 430 (M+, 5), 326 (9.34), 123 (92), 105 (100), 77 (30); Anal. calcd for C29H22N2O2: C, 80.91; H, 5.15; N, 6.51%; Found: C, 80.94; H, 5.17; N, 6.54%.
5-[(E)-(4-Fluorophenyl)methylidene]-2-methyl-3-phenyl-3,5-dihydro-4H-imidazole-4-one (9) Yield: 70%; m.p.: 122°C; Rf: 0.59 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 346 (log ε = 4.82) nm; IR (KBr): νmax 3404, 3067, 1652, 1498, 1160 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.49 (d, J3″,2″ = J5″,6″ = 7.7 Hz, 2H, H-3′/5′), 8.19 (d, J2′,3′ = J6′,5′ = 8.6 Hz, 2H, H-2′/6′), 7.52 (m, 2H, H-2″/6″), 7.44 (m, 3H, H-3″–5″), 7.15 (s, 1H, H-6), 2.28 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 280 (M+, 31.62), 135 (10.0), 118 (98), 77 (100); Anal. calcd for C17H13FN2O: C, 72.84; H, 4.67; N, 9.99%; Found: C, 72.86; H, 4.68; N, 9.10%.
5-[(E)-(4-Fluorophenyl)methylidene]-3-(2-methoxyph eny)l-2-methyl-3,5-dihydro-4H-imidazole-4-one (10) Yield: 60%; m.p.: 124°C; Rf: 0.50 (ethyl acetate/hexane, 3:7); UV (MeOH): λmax 344 (log ε = 5.2) nm; IR (KBr): νmax 3043, 2844, 1651, 1504, 1159 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.39 (d, J3″,2″ = J5″,6″ = 8.5 Hz, 2H, H-3′/5′), 8.19 (d, J2′,3′ = J6′,5′ = 8.5 Hz, 2H, H-2′/6′), 7.43 (m, 2H, H-4″–6″), 7.24 (s, 1H, H-6), 7.03 (d, J3″,4″ = 8.5 Hz, 1H, H-3″), 3.80 (s, 3H, OCH3), 2.17 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 310 (M+, 35.2), 148 (100), 134 (53), 92 (19), 77 (29); Anal. calcd for C18H15FN2O2: C, 69.67; H, 4.87; N, 9.03%; Found: C, 69.69; H, 4.89; N, 9.05%.
5-[(E)-(4-Fluorophenyl)methylidene]-3-(3-methox yphe nyl)-2-methyl-3,5-dihydro-4H-imidazole-4-one (11) Yield: 56%; m.p.: 125°C; Rf: 0.51 (ethyl acetate/hexane, 3:7); UV (MeOH): λmax 346 (log ε = 4.75) nm; IR (KBr): νmax 3075, 3004, 2968, 1651, 1497, 1392 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.39 (d, J3′,2′ = J5′,6′ = 8.5 Hz, 2H, H-3′/5′), 8.19 (d, J2′,3′ = J6′,5′ = 8.5 Hz, 2H, H-2′/6′), 7.40 (d, J6″,5″ = 7.5 Hz, 1H, H-6″), 7.24 (s, 1H, H-2″), 7.23 (s, 1H, H-6), 6.99 (m, 2H, H-4″, 5″), 3.82 (s, 3H, OCH3), 2.30 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 310 (M+, 100), 221 (4), 148 (69), 134 (32), 77 (16); Anal. calcd for C18H15FN2O2: C, 69.67; H, 4.87; N, 9.03%; Found: C, 69.70; H, 4.90; N, 9.05%.
5-[(E)-(4-Fluorophenyl)methylidene]-3-(4-methoxyphe nyl)-2-methyl-3,5-dihydro-4H-imidazole-4-one (12) Yield: 99%; m.p.: 122°C Rf: 0.52 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 342 (log ε = 4.69) nm; IR (KBr): νmax 3056, 2940, 1644, 1511, 1159 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.41 (d, J3′,2′ = J5′,6′ = 8.5 Hz, 2H, H-3′/5′), 8.19 (d, J2′,3′ = J6′,5′ = 8.5 Hz, 2H, H-2′/6′), 7.65 (d, J2″,3″ = J6″,5″ = 8.9 Hz, 1H, H-2″/6″), 7.23 (s, 1H, H-6), 7.00 (d, J3″,2″ = J5″,6″ = 8.8 Hz, 1H, H-3″/5″), 3.83 (s, 3H, OCH3), 2.28 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 311 (M+, 52), 148 (100), 134 (76), 77 (44). Anal. calcd for C18H15FN2O2: C, 69.67; H, 4.87; N, 9.03%; Found: C, 69.68; H, 4.88; N, 9.04%.
5-[(E)-(4-Fluorophenyl)methylidene]-2,3-diphenyl-3,5- dihydro-4H-imidazole-4-one (13) Yield: 40%; m.p.: 242°C; Rf: 0.63 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 282 (log ε = 4.46) nm; IR (KBr): νmax 3271, 3135, 3062, 1648, 1474, 1326 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.39 (d, J3″,2″ = J5″,6″ = 8.5 Hz, 2H, H-3″/5″), 8.26 (m, 2H, H-2′/6′), 8.20 (d, J2″,3″ = J6″,5″ = 8.5 Hz, 2H, H-2″/6″), 7.86 (m, 3H, H-3′–5′), 7.56 (d, J2″′,3″′ = J6″′,5″′ = 7.7 Hz, 2H, H-2″′/6″′), 7.48 (m, 3H, H-3″′–5″′), 7.22 (s, 1H, H-6); EI MS: m/z (rel. abund. %) 343 (M+, 3), 267 (9), 135 (4), 105 (100), 93 (66), 77 (46); Anal. calcd for C22H15FN2O: C, 77.18; H, 4.42; N, 8.18%; Found: C, 77.00; H, 4.03; N, 8.20%.
5-[(E)-(4-Fluorophenyl)methylidene]-3-(2-methoxyphe nyl)-2-methyl-3,5-dihydro-4H-imidazole-4-one (14) Yield: 80%; m.p.: 196°C; Rf: 0.57 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 290 (log ε = 4.6) nm; IR (KBr): νmax 3219, 3062, 2923, 1643, 1462, 1227 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.39 (d, J3″,2″ = J5″,6″ = 7.8 Hz, 2H, H-3″/5″), 8.25 (m, 2H, H-2′/6′), 8.19 (d, J2″,3″ = J6″,5″ = 7.8 Hz, 2H, H-2″/6″), 7.88 (m, 3H, H- 3′–5′), 6.84 (d, J3″′,4″′ = 8.0 Hz, 1H, H-3″′), 7.55 (d, J5″′,6″′ = 7.5 Hz, 1H, H-6″′), 7.46 (m, 2H, H-4″′, 5″′), 7.22 (s, 1H, H-6), 3.77 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 309 (M+, 42), 177 (4.1), 105 (100), 77 (22); Anal. calcd for C23H17FN2O2 C, 74.18; H, 4.60; N, 7.52%; Found: C, 74.22; H, 4.63; N, 7.56%.
5-[(E)-(4-Fluorophenyl)methylidene]-3-(3-methoxyphe nyl)-2-methyl-3,5-dihydro-4H-imidazole-4-one (15) Yield: 75%; m.p.: 204°C; Rf: 0.59 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 288 (log ε = 4.8) nm; IR (KBr): νmax 3152, 3083, 2933, 1648, 1415, 1227 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.39 (d, J3″,2″ = J5″,6″ = 7.8 Hz, 2H, H-3″/5″), 8.26 (m, 2H, H-2′/6′), 8.19 (d, J2″,3″ = J6″,5″ = 8.3 Hz, 2H, H-2″/6″), 7.88 (m, 3H, H-3′–5′), 7.43 (m, 1H, H-6″′), 7.36 (s, 1H, H-2″′), 7.23 (s, 1H, H-6), 7.04 (m, 2H, H-4″′, 5″′), 3.79 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 373 (M+, 3), 269 (17), 242 (5), 123 (62), 105 (100), 77 (45); Anal. calcd for C23H17FN2O2 C, 74.18; H, 4.60; N, 7.52%; Found: C, 74.21; H, 4.62; N, 7.55%.
5-[(E)-(4-Fluorophenyl)methylidene]-3-(4-methoxyphe nyl)-2-methyl-3,5-dihydro-4H-imidazole-4-one (16) Yield: 98%; m.p.: 185°C; Rf: 0.58 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 282 (log ε = 4.7) nm; IR (KBr): νmax 3131, 3069, 2958, 1650, 1478, 1241 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.39 (d, J3″,2″ = J5″,6″ = 8.3 Hz, 2H, H-3″/5″), 8.25 (m, 2H, H-2′/6′), 8.18 (d, J2″,3″ = J6″,5″ = 8.3 Hz, 2H, H-2″/6″), 7.85 (m, 3H, H-3′-5′), 7.46 (d, J2″,3″ = J6″′,5″′ = 8.9 Hz, 2H, H-2″′/6″′), 7.23 (s, 1H, H-6), 6.85 (d, J3″′,2″′ = J5″′,6″′ = 8.9 Hz, 2H, H-3″′/5″′), 3.78 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 373 (M+, 2), 267 (6), 123 (100), 105 (84), 77 (37); Anal. calcd for C23H17FN2O2: C, 74.18; H, 4.60; N, 7.52%; Found: C, 74.20; H, 4.61; N, 7.53%.
5-[(E)-(4-Fluorophenyl)methylidene]-2-methyl-3-phenyl-3,5-dihydro-4H-imidazole-4-one (17) Yield: 88%; m.p.: 118°C; Rf: 0.55 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 276 (log ε = 4.5) nm; IR (KBr): νmax 3374, 3097, 1642, 1523, 1265 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.13 (s, 1H, H-6′), 8.12 (d, J4′,3′ = 8.8 Hz, 1H, H-4′), 8.09 (d, J3′,4′ = 8.8 Hz, 1H, H-3′), 7.58 (d, J2″,3″ = J6″,5″ = 8.8 Hz, 2H, 2″/6″), 7.48 (m, 3H, H-3″–5″), 7.25 (s, 1H, H-6), 2.32 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 341 (M+, 14), 306 (65), 276 (42), 118 (73), 77 (63); Anal. calcd for C17H12ClN3O3: C, 59.75; H, 3.54; N, 12.30%; Found: C, 59.76; H, 3.55; N, 12.31%.
5-[(E)-(2-Chloro-5-nitrophenyl)methylidene]-3-(3-meth oxyphenyl)-2-methyl-3,5-dihydro-4H-imidazole-4-one (18) Yield: 99%; m.p.: 130°C; Rf: 0.52 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 208 (log ε = 4.8) nm; IR (KBr): νmax 3378, 3091, 1602, 1525, 1270 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.14 (s, IH, H-6′), 8.12 (d, J4′,3′ = 8.8 Hz, 1H, H-4′), 8.10 (d, J3′,4′ = 8.8 Hz, 1H, H-3′), 7.58 (d, J6″,5″ = 8.8 Hz, 1H, H-6″), 7.41 (t, J5″,4″ = J5″,6″ = 8.8 Hz, 1H, 5″), 7.24 (s, 1H, H-6), 7.01 (d, J4″,5″ = 8.8 Hz, 1H, 4″), 6.99 (s, 1H, H-2″), 3.82 (s, 3H, OCH3), 2.34 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 371 (M+, 20), 336 (100), 148 (34), 77 (14); Anal. calcd for C18H14ClN3O4: C, 58.15; H, 3.80; N, 11.30%; Found: C, 58.19; H, 3.84; N, 11.33%.
5-[(E)-(2-Chloro-5-nitrophenyl)methylidene]-2,3-diphenyl-3,5-dihydro-4H-imidazole-4-one (19) Yield: 87%; m.p.: 192°C; Rf: 0.56 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 204 (log ε = 4.8) nm; IR (KBr): νmax 3277, 3070, 1642, 1474, 1263 cm–1; 1H-NMR (400 MHz, CDCl3) δ: 8.16 (s, 1H, H-6″), 8.14 (m, 1H, H-4″), 8.12 (m, 1H, H-3″), 7.89 (d, J2′,3′ = J6′,5′ = 7.5 Hz, 2H, H-2′/6′), 7.79 (m, 3H, H-3′–5′), 7.64 (d, J2″,3″ = J6″,5″ = 7.5 Hz, 2H, H-2″/6″), 7.46 (m, 3H, H-3″–5″), 7.34 (s, 1H, H-6); EI MS: m/z (rel. abund. %) 403 (M+, 2), 367 (14), 273 (25), 180 (22), 105 (94), 93 (9), 77 (100), 121 (100); Anal. calcd. for C22H14ClN3O3: C, 65.43; H, 3.49; N, 10.41%; Found: C, 65.44; H, 3.50; N, 10.42%.
5-[(E)-(2-Chloro-5-nitrophenyl)methylidene]-3-(3-meth oxyphenyl)-2-phenyl-3,5-dihydro-4H-imidazole-4-one (20) Yield: 53%; m.p.: 135°C; Rf: 0.54 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 207 (log ε = 5.0) nm; IR (KBr): νmax 3369, 3078, 2937, 1604, 1345, 1284 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.13 (d, J4″,5″ = 8.8 Hz, 1H, H-4″), 8.12 (s, 1H, H-6″), 8.00 (d, J3″,4″ = 8.8 Hz, 1H, H-3″), 7.92 (d, J2′,3′ = J6′,5′ = 7.6Hz, 2H, H-2′/6′), 7.77 (m, 3H, H-3′–5′), 7.64 (d, J6″′,5″′ = 7.4 Hz, 1H, H-6″′), 7.45 (t, J5″′,4″′ = J5″′,6″′ = 7.6 Hz, 1H, H-5″′), 7.20 (s, 1H, H-6), 7.07 (s, 1H, H-2″′), 6.99 (d, J4″′,5″′ = 7.5 Hz, 1H, H-4″′), 3.82 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 433 (M+, 2.0), 398 (5), 292 (8), 123 (21), 105 (100), 77(56); Anal. calcd for C23H16ClN3O4: C, 63.67; H, 3.72; N, 9.69%; Found: C, 63.68; H, 3.76; N, 9.74%.
5-[(E)-(2-Chloro-5-nitrophenyl)methylidene]-3-(4-meth oxyphenyl)-2-phenyl-3,5-dihydro-4H-imidazole-4-one (21) Yield: 62%; m.p.: 198°C; Rf: 0.55 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 203 (log ε = 4.9); IR (KBr): νmax 3282, 3004, 2839, 1641, 1468, 1251 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.13 (s, 1H, H-6″), 8.11 (d, J4″,5″ = 8.8 Hz, 1H, H-4″), 8.03 (d, J3″,4″ = 8.8 Hz, 1H, H-3″), 7.84 (d, J2′,3′ = J6′,5′ = 7.6Hz, 2H, H-2′/6′), 7.72 (m, 3H, H-3′–5′), 7.63 (d, J2″′,3″′ = J6″′,5″′ = 7.5 Hz, 2H, H-2″′/6″′), 7.34 (s, 1H, H-6), 6.80 (d, J3″′,2″′ = J5″′,6″′ = 7.5 Hz, 2H, 3″′/5″′), 3.82 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 433 (M+, 2), 415 (11), 293 (9), 210 (14), 123 (50), 105 (100), 77 (41); Anal. calcd for C23H16ClN3O4: C, 63.67; H, 3.72; N, 9.69%; Found: C, 63.68; H, 3.73; N, 9.70%.
2-Methyl-3-phenyl-5-[(E)-2-thienylmethylidene]-3,5-dihydro-4H-imidazol-4-one (22) Yield: 70%; m.p.: 151°C; Rf: 0.48 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 373 (log ε = 4.5) nm; IR (KBr): νmax 3397, 3071, 2923, 1640, 1387, 1263 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 7.66 (d, J5′,4′ = 4.7 Hz, 1H, H-5′), 7.54 (d, J3′,4′ = 3.5 Hz, 1H, H-3′), 7.49 (d, J2″,3″ = J6″,5″ = 7.7 Hz, 2H, H-2″/6″), 7.43 (m, 3H, H-3″–5″), 7.42 (s, 1H, H-6), 7.12 (dd, J4′,3′ = 4.7, J4′,5′ = 3.5 Hz, 1H, H-4′), 2.37 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 268, (M+, 31), 118 (88), 77 (100), 51 (41); Anal. calcd for C15H12N2OS: C, 67.14; H, 4.51; N, 10.44%; Found: C, 67.15; H, 52.00; N, 10.45%.
3-(2-Methoxyphenyl)-2-methyl-5-[(E)-2-thienylmethylid ene]-3,5-dihydro-4H-imidazol-4-one (23) Yield: 78%; m.p.: 151°C; Rf: 0.43 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 368 (log ε = 4.61) nm; IR (KBr): νmax 3072, 2838, 1640, 1391, 1247 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 7.66 (d, J5′,4′ = 4.7 Hz, 1H, H-5′), 7.54 (d, J3′,4′ = 3.5 Hz, 1H, H-3′), 7.45 (d, J6″,5″ = 8.3 Hz, 1H, H-6″), 7.42 (s, 1H, H-6), 7.42 (m, 2H, H-3″, 4″), 7.14 (d, J2″,3″ = 8.8 Hz, 1H, H-2″), 7.12 (dd, J4′,3′ = 4.7, J4′,5′ = 3.5 Hz, 1H, H-4′), 3.80 (s, 3H, OCH3), 2.31 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 298 (M+, 53), 283 (4), 148 (100), 134 (24), 92 (38), 77 (68); Anal. calcd for C16H14N2O2S: C, 64.41; H, 4.73; N, 9.39%; Found: C, 64.45; H, 4.77; N, 9.42%.
3-(3-Methoxyphenyl)-2-methyl-5-[(E)-2-thienylmethylid ene]-3,5-dihydro-4H-imidazol-4-one (24) Yield: 65%; m.p.: 170°C; Rf: 0.44 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 372 (log ε = 5.2) nm; IR (KBr): νmax 3072, 3001, 2935, 1639, 1320, 1241 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 7.66 (d, J5′,4′ = 4.7 Hz, 1H, H-5′), 7.54 (d, J3′,4′ = 3.5 Hz, 1H, H-3′), 7.42 (s, 1H, H-6), 7.42 (d, J6″,5″ = 8.1 Hz, 1H, H-6″), 7.22 (s, 1H, H-2″), 7.12 (dd, J4′,3′ = 4.7, J4′,5′ = 3.5 Hz, 1H, H-4′), 7.20 (t, J5″,4″ = J5″′6″ = 8.1 Hz, 1H, H-5″), 6.81 (d, J4″,5″ = 8.7 Hz, 1H, H-4″), 3.85 (s, 3H, OCH3), 2.50 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 298 (M+, 57), 148 (100), 134 (19), 107 (12); Anal. calcd for C16H14N2O2S: C, 64.41%; H, 4.73; N, 9.39; Found: C, 64.46; H, 4.78; N, 9.43%.
3-(4-Methoxyphenyl)-2-methyl-5-[(E)-2-thienylmethylid ene]-3,5-dihydro-4H-imidazol-4-one (25) Yield: 78%; m.p.: 109°C; Rf: 0.45 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 372 (log ε = 4.67) nm; IR (KBr): νmax 3039, 2966, 1635, 1510, 1247 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 7.66 (d, J5′,4′ = 4.7 Hz, 1H, H-5′), 7.63 (d, J2″,3″ = J6″,5″ = 8.7 Hz, 2H, H-2″/6″), 7.54 (d, J3′,4′ = 3.5 Hz, 1H, H-3′), 7.42 (s, 1H, H-6), 7.12 (dd, J4′,3′ = 4.7, J4′,5′ = 3.5 Hz, 1H, H-4′), 6.99 (d, J3″,4″ = J5″,6″ = 8.8 Hz, 2H, H-3″/5″), 3.83 (s, 3H, OCH3), 2.30 (s, 3H, CH3); EI MS: m/z (rel. abund. %) 298, (M+, 64), 148 (100), 134 (56), 92 (28), 77 (44); Anal. calcd for C16H14N2O2S: C, 64.41; H, 4.73; N, 9.39%; Found: C, 64.43; H, 4.75; N, 9.40%.
2,3-Diphenyl-5-[(E)-2-thienylmethylidene]-3,5-dihydro-4H-imidazol-4-one (26) Yield: 96%; m.p.: 205°C; Rf: 0.49 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 318 (log ε = 4.37) nm; IR (KBr): νmax 3254, 3131, 3063, 1649, 1473, 1280 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 8.32 (d, J2′,3′ = J6′,5′ = 8.7 Hz, 2H, H-2′/6′), 8.30 (m, 3H, H-3′–5′), 7.63 (d, J5″,4″ = 4.7 Hz, 1H, H-5″), 7.61 (d, J2″′,3″′ = J6″′,5″′ = 7.3 Hz, 2H, H-2″′/6″′), 7.55 (d, J3″,4″ = 3.5 Hz, 1H, H-3″), 7.54 (m, 3H, H-3″′–5″′), 7.32 (dd, J4′,3′ = 4.7, J4′,5′ = 3.5 Hz, 1H, H-4″), 7.30 (s, 1H, H-6); EI MS: m/z (rel. abund. %) 330 (M+, 3.4), 255 (17.35), 227 (9.2), 105 (100), 77 (56); Anal. calcd for C20H14N2OS C27H18O6: C, 72.70; H, 4.27; N, 8.48%; Found: C, 72.72; H, 4.26; N, 8.86%.
3-(2-Methoxyphenyl)-2-phenyl-5-[(E)-2-thienylmethylid ene]-3,5-dihydro-4H-imidazol-4-one (27) Yield: 90%; m.p.: 171°C; Rf: 0.47 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 323 (log ε = 4.8) nm; IR (KBr): νmax 3285, 3072, 2936, 1671, 1462, 1248 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 7.90 (d, J2′,3′ = J6′,5′ = 7.5 Hz, 2H, H-2′/6′), 7.79 (m, 3H, H-3′–5′), 7.63 (d, J5″,4″ = 4.7 Hz, 1H, H-5″), 7.61 (d, J6″′,5″′ = 7.3 Hz, 1H, H-6″′), 7.56 (d, J3″,4″ = 3.5 Hz, 1H, H-3″), 7.45 (m, 2H, H-4″′–5″′), 7.33 (dd, J4′,3′ = 4.7, J4′,5′ = 3.5 Hz, 1H, H-4″), 7.33 (s, 1H, H-6), 6.98 (d, J3″′,4″′ = 7.6 Hz, 1H, H-3″′), 3.37 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 360 (M+, 10.6), 210 (13.50), 173 (100), 145 (42), 105 (41), 69 (63), 57 (65); Anal. calcd for C21H16N2O2S: C, 69.98; H, 4.47; N, 7.77%; Found: C, 69.20; H, 4.50; N, 7.80%.
3-(3-Methoxyphenyl)-2-phenyl-5-[(E)-2-thienylmethylid ene]-3,5-dihydro-4H-imidazol-4-one (28) Yield: 82%; m.p.: 189°C; Rf: 0.48 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 319 (log ε = 4.5) nm; IR (KBr): νmax 3144, 3073, 2958, 1643, 1478, 1267 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 7.92 (d, J2′,3′ = J6′,5′ = 7.5 Hz, 2H, H-2′/6′), 7.77 (m, 3H, H-3′-5′), 7.64 (d, J6″′,5″′ = 7.4 Hz, 1H, H-6″′), 7.63 (d, J5″,4″ = 4.7 Hz, 1H, H-5″), 7.56 (d, J3″,4″ = 3.5 Hz, 1H, H-3″), 7.45 (t, J5″′,4″′ = J5″″,6″′ = 7.6 Hz, 1H, H-5″′), 7.33 (dd, J4′,3′ = 4.7, J4′,5′ = 3.5 Hz, 1H, H-4″), 7.32 (s, 1H, H-6), 7.07 (s, 1H, H-2″′), 6.99 (d, J4″′,5″′ = 7.5 Hz, 1H, H-4″′), 3.37 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 360 (M+, 10), 257 (4), 123 (38), 105 (100), 7 (52); Anal. Calcd for C21H16N2O2S: C, 69.98; H, 4.47; N, 7.77%; Found: C, 69.22; H, 4.52; N, 7.83%.
3-(4-Methoxyphenyl)-2-phenyl-5-[(E)-2-thienylmethylid ene]-3,5-dihydro-4H-imidazol-4-one (29) Yield: 90%; m.p.: 192°C; Rf: 0.48 (ethyl acetate/hexane, 3:7); UV (MeOH) λmax 400 (log ε = 2.15) nm; IR (KBr): νmax 3132, 3071, 2951, 1634, 1511, 1248 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 7.84 (d, J2′,3′ = J6′,5′ = 7.6 Hz, 2H, H-2′/6′), 7.79 (m, 3H, H-3′-5′), 7.63 (d, J5″,4″ = 4.7 Hz, 1H, H-5″), 7.63 (d, J2″′,3″′ = J6″′,5″′ = 7.5 Hz, 2H, H-2″′/6″′), 7.55 (d, J3″,4″ = 3.5 Hz, 1H, H-3″), 7.32 (dd, J4′,3′ = 4.7, J4′,5′ = 3.5 Hz, 1H, H-4″), 7.30 (s, 1H, H-6), 6.80 (d, J2″′,3″′ = J6″′,5″′ = 7.5 Hz, 2H, H-3″′/5″′), 3.77 (s, 3H, OCH3); EI MS: m/z (rel. abund. %) 360 (M+, 100), 210 (37), 105 (77), 77 (16); Anal. calcd for C21H16N2O2S: C, 69.98; H, 4.47; N, 7.77%; Found: C, 69.99; H, 4.49; N, 7.78%.
Assay for leishmaniasisCitation24
Leishmania major were grown in bulk in modified Novy–MacNeal–Nicolle (NNN) biphasic medium by using normal physiological saline. Leishmania promastigotes were cultured with RPMI-1640 medium, supplemented with 10% heat inactivated fetal bovine serum (FBS). Parasites at the log phase were centrifuged at 2000 rpm for 10 min, and washed three times with saline at the same speed and for the same time. Parasites were diluted with fresh culture medium to a final density of 106 cells/mL.
In a 96-well microtiter plate, 180 μL of medium was added in the first row and 100 μL of medium was added in other wells; 20 μL of the experimental compound was added in the medium and serially diluted; 100 μL of parasite culture was added to all wells. Two rows were left for negative and positive controls. Negative controls received only the medium, while the positive control contained a varying concentration of standard anti-leishmanial drugs, amphotericin B and pentamidine. The plate was incubated at 21–22°C for 72 h. The culture was examined microscopically on an improved Neubauer counting chamber, and IC50 values of compounds were calculated by Software Ezfit 5.03 (Perella Scientific). All assays were performed in triplicate.
Results and discussion
Chemistry
Imidazolones 1–29 were synthesized from differently substituted E-oxazolonesCitation21–23 (1 mmol) and substituted aromatic amines (1.1 mmol) in anhydrous pyridine through microwave irradiation (). The structures of imidazolones 1–29 were determined using different spectroscopic techniques, including 1H-NMR, EI, IR, and UV, and by elemental analysis.
Biology
All the synthesized imidazolones 1–29 were evaluated for their leishmanicidal activity according to the literature protocolCitation24.
Compounds 1–29 demonstrated a varying degree of in vitro anti-leishmanial activities, with IC50 values in the range of 12.98 ± 0.32–96.17 ± 0.11 μg/mL, and compared with standard drugs amphotericin B and petamidine (IC50 = 0.12 ± 0.41 and 2.56 ± 0.10 μg/mL, respectively). Compound 17 (IC50 = 12.98 ± 0.32 μg/mL) was found to be the most active member of the series. Compounds 14 and 24 also showed good anti-leishmanial activities, with IC50 values of 28.20 ± 0.03 and 41.12 ± 0.32 μg/mL, respectively. Compounds 9, 13, 15, 18, 19, and 21 showed only moderate activity against Leishmania major, with IC50 values 57.00 ± 0.21, 60.00 ± 0.13, 54.44 ± 0.05, 57.30 ± 0.04, 51.00 ± 0.03, and 51.32 ± 0.33 μg/mL, respectively. Comp ounds 1–3, 5, 6, 11, 12, 20, 22, 23, 25, 26, 28, and 29 showed IC50 values greater than 60 μg/mL. Compounds 4, 7, 8, 16, and 27 showed IC50 values more than 100 μg/mL and were considered to be inactive ().
Table 1. Results for anti-leishmanial activity of compounds 1–29.
Compound 17 (IC50 = 12.98 ± 0.32 μg/mL) proved to be the most active anti-leishmanial molecule among the screened compounds. Limited structure–activity relationship (SAR) study suggests that the activity of the tested compounds mainly depends upon the substitution on the imidazolone moiety. Compound 17, having R1, R2, and R3 groups phenyl, methyl, and 2-chloro,5-nitrophenyl, respectively, showed the highest degree of anti-leishmanial activity; however, closely related compound 19 (IC50 = 51.00 ± 0.03 μg/mL), having a phenyl residue instead of methyl, was found to be less active than most compounds of the series. Nonetheless, when R1 in compound 18 was changed for 3- methoxyphenyl, a decrease in activity (IC50 = 57.30 ± 0.04 μg/mL) was observed. In compound 21, where R1 was 4-methoxyphenyl and R2 was phenyl, a slight increase in activity (IC50 = 51.32 ± 0.33 μg/mL) occurred, and in compound 20, where R1 was 3- methoxyphenyl and R2 was phenyl, a sharp decline in activity was observed. This clearly indicates that substitution on the imidazolone plays a vital role for leishmanicidal activity. 5-[(E)-(4- Fluorophenyl)methylidene]-3-(2-methoxyphenyl)-2- methyl-3,5-dihydro-4H-imidazole-4-one (14) was found to be the second most active compound of the series, with an IC50 value of 28.20 ± 0.03 μg/mL. When the position of the methoxy group of R1 was changed from C-2 to C-3 in a closely related molecule, 5-[(E)-(4-fluorophenyl)methylidene]-3-(3-methoxyphenyl)-2-methyl-3,5-dihydro-4H-imidazole-4-one (15), the activity decreased sharply, with an IC50 value 54.44 ± 0.05 μg/mL. If the activity of compounds 14 and 15 was compared, it was concluded that a slight change in position of the methoxy group increased the activity of compound 14. In addition, surprisingly, if the methoxy group of R1 was placed at position 4 of the phenyl ring as in compound 16, complete loss in activity was observed. In compounds 1–8 and 9–13 containing nearly the same R1, R2, and R3 groups, only slight variation in activity was observed.
3-(3-Methoxyphenyl)-2-methyl-5-[(E)-2-thienylme th ylidene]-3,5-dihydro-4H-imidazol-4-one (24) was found to be the third most active compound of the series with an IC50 value of 41.12 ± 0.32 μg/mL. When the position of the methoxy group of R1 was changed from 3 to 2 or 3 to 4 in closely related molecules 23 and 25, the activity was sharply decreased, with IC50 values 61.43 ± 0.31 and 96.17 ± 0.11 μg/mL, respectively. However, the remaining compounds 22, 26, 28, and 29 were found to be moderately active.
During the current study, varyingly substituted imidazolones were randomly screened for their anti-leishmanial activity, and compounds 14, 17, and 24 were found to be the active members of the series. Apparently, substitution on imidazolone plays a vital role, in addition to the imidazolone ring itself. Compounds 14 and 17 may therefore serve as lead compounds for further studies on leishmanicidal imidazolones. However, compound 24 is the best candidate in the thiophenic series but really needs improvements to become a lead.
Acknowledgements
This work was financially supported by the Pakistan Telecommunication Company Limited (PTCL) through a research grant (“Synthesis of Leishmanicidal Chemotherapeutic Agents”).
Declaration of interest: The authors report no conflicts of interest.
References
- Tropical Disease Research. Progress 1999-2000. Geneva: World Health Organization, 2001.
- Olliaro PL, Bryceson ADM. Practical progress and new drugs for changing patterns of leishmaniasis. Parasitol Today 1993;9:323–8.
- Ram VJ, Nath M. Progress in chemotherapy of leishmaniasis. Curr Med Chem 1996;3:303–28.
- Croft SL, Yardley V. Chemotherapy of leishmaniasis. Curr Pharm Design 2002;8:319–42.
- Tempone AG, Perez D, Rath S, Vilarinho AL, Mortara RA, Andrade HF Jr. Targeting Leishmania (L.) chagasi amastigotes through macrophage scavenger receptors: the use of drugs entrapped in liposomes containing phosphatidylserine. J Antimicrob Chemother 2004;54:60–8.
- Siddiqui SA, Bhusare SR, Jarikote DV, Pawar RP, Vibhute YB. New novel synthesis and antibacterial activity of 1-(substituted phenyl)-2-phenyl-4-(3′-halo, 4′-hydroxy 5′-methoxy benzylidene)-imidazole-5-one. Bull Korean Chem Soc 2001;22:1033–6.
- Wright WB, Brabander JH. The rearrangement and cyclization of Ethyl N-(methylaminoalkyl) carbanilates and 1,1-dimethyl-3-methylaminoalkyl-3-phenylureas. J Org Chem 1961;26:4051–7.
- Niedbalia V, Buettcher I. Imidazole derivatives for pharmaceutical preparations. Chem Abstr 1981;94:15732.
- Pande K, Kalsi KR, Bhalla TN, Barthwal JP. Some newer imidazolones and their anti-inflammatory activity. Pharmazie 1987;42:269.
- Wright WB, Brabander HS, Hardy RA, Osterberg AC. Central nervous system depressants I. 1-Aminoalkyl-3-aryl derivatives of 2-imidazolidinone, 2-imidazolidinethione, and tetrahydro-2(1H)-pyrimidinone. J Med Chem 1966;9:852–6.
- Lingi A, Alfonso M, Pierluigi R, Afro G, Enzo Z, Nicola DT, et al. Derivatives of imidazole. III. Synthesis and pharmacological activities of nitriles, amides, and carboxylic acid derivatives of imidazo[1,2-a]pyridine. J Med Chem 1969;12:122–6.
- Brik F, Godefrot, Reatge J. DL-1-(α-Methylbenzyl)-2-methylimidazole-5-carboxylate esters. Synthesis and pharmacological properties. J Med Chem 1972;15:336–7.
- Verma M, Charturvedi AK, Chaudhary A, Parmar SS. Monoamine oxidase inhibitory and anticonvulsant properties of 1, 2, 4-trisubstituted 5-imidazolones. J Pharm Sci 1974;463:1740–4.
- Upadhyay P, Pandya A, Parekh H. Possible anticonvulsant imidazloinones. Synthesis and anticonvulsant activity of 1N-(γ′-picolinyl)-4-subsituted-benzylidene-2-methyl/phenyl-5-imidazolinone. J Indian Chem Soc 1991;68:296–8.
- Bhalla M, Naithani PK, Bhalla TN, Saxena AK, Shanker K. Novel imidazole congeners as anti-inflammatory agents. J Indian Chem Soc 1992;69:594–5.
- Mesaik MA, Khan KM, Zia-Ullah, Rahat S, Choudhary MI, Murad S, et al. Immunomodulatory properties of synthetic imidazolone derivatives. Lett Drug Des Discov 2005;2:490–6.
- Salma U, Mazhar M, Imtiaz-ud-Din, Ali S, Khan KM. In vitro antileishmanial activities of germatarnyl and silicon incorporated diorganotin derivatives: synthesis and spectroscopic properties. J Enzyme Inhib Med Chem 2009;23:413–19.
- Khan KM, Mughal UR, Samreen, Perveen S, Choudhary MI. Schiff bases of isatin: potential anti-leishmanial agents. Lett Drug Des Discov 2008;5:243–9.
- Khan KM, Ahmed S, Khan ZA, Zia-Ullah, Rani M, Choudhary MI, et al. In vitro leishmanicidal activity of 3-substituted isocoumarins: synthesis and structure-activity relationship. Med Chem 2008;4:163–9.
- Khan KM, Rasheed M, Zia-Ullah, Hayat S, Kaukab F, Choudhary MI, et al. Synthesis and in vitro leishmanicidal activity of some hydrazides and their analogs. Bioorg Med Chem 2003;11:1381–7.
- (a) A ratio of 1:10 of oxazolone to PPA was used to get E-isomer; (b) Rao YS. A new stereospecific synthesis of the E isomers of 2-phenyl-4-arylmethylene-2-oxazolin-5-ones. J Org Chem 1976;41:722–725.
- Khan KM, Mugal UR, Khan MTH, Zia-ullah, Perveen S, Choudhary MI. Oxazolones: new tyrosinase inhibitors; synthesis and their structure-activity relationships. Bioorg Med Chem 2006;14:6027–33.
- Khan KM, Mugal UR, Lodhi MA, Choudhary MI. Synthesis and chymotrypsin inhibitory activity of substituted oxazolones. Lett Drug Des Discov 2008;5:52–6.
- Atta-ur-Rahman, Choudhary MI, Thomsen WJ. Bioassay Techniques for Drug Development. Amsterdam: Harwood Academic Publishers, 2001:60–5.