Abstract
Trioxsalen (TRX) is a 4,5′,8-trimethylated psoralen analog presenting interesting biological activities when irradiated with UVA light. A series of TRX derivatives, which where obtained by its chemical modification and incorporation of a variety of unsaturated functions at position 4′ of the psoralen ring-system, were evaluated for their antioxidant activity and their inhibitory activity on soybean lipoxygenase (LOX) and lipid peroxidation. The reducing properties of the compounds were evaluated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay and found to be very low, in the range 0–14%, with the exception of the hydroxamic acid 6 which showed almost identical activity to BHT. TRX derivative 3 significantly inhibited LOX, with IC50 9.4 μM. With the exception of TRX, all tested analogs inhibited lipid peroxidation in the range of 35–91%. The most potent compound, namely TRX derivative 3, was studied for its anti-inflammatory activity in vivo on rat paw edema induced by carrageenan, and was found to be of almost identical activity to indomethacin. The results of the biological tests are discussed in terms of structural characteristics.
Introduction
Psoriasis is a chronic inflammatory skin condition. The cause of psoriasis is not known. Several factors are thought to aggravate psoriasis. Japanese researchers believe that they have identified a role for lipoxygenase (LOX) in interferon (IFN)-γ-induced inflammatory processes in psoriasis. Both molecules are known to have inflammatory functions, with LOX involved in the biochemical pathway in which arachidonic acid is metabolized into the proinflammatory products leukotrienes, while IFN-γ has already been implicated in psoriasisCitation1.
Lipoxygenases play an essential role in the biosynthesis of the leukotrienes (LTs). LTs are potent biological mediators in the pathophysiology of inflammatory diseases and host defense reactionsCitation2. These properties imply a significant role for LTB4 in the pathogenesis of inflammatory diseases, such as asthmaCitation3, psoriasisCitation4, atherosclerosisCitation5, and cancerCitation6,Citation7.
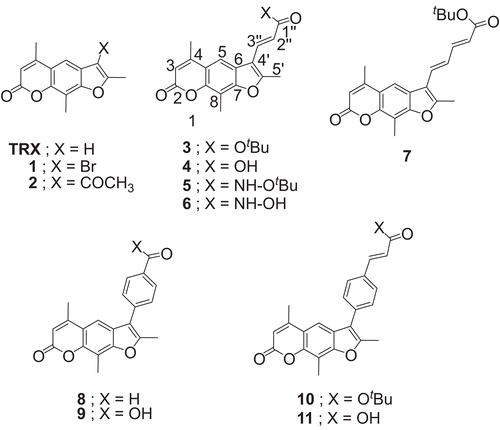
Psoralen is the parent compound in a family of natural products known as furocoumarins. Psoralens are naturally occurring or synthetic compounds presenting strong photobiological activities. Psoralens, such as trioxsalen (TRX, ), are commonly used in combination with ultraviolet A (UVA) light for systemic treatment by photochemotherapy (psoralen + UVA = PUVA) of skin hyperproliferative diseases such as psoriasisCitation8,Citation9. However, PUVA is associated with important acute and chronic effectsCitation10. In an effort to obtain psoralens with superior photoreactivity with nucleic acids compared with the parent compound TRX, we have recently synthesized a series of C-4′ psoralen derivativesCitation11, some of which bear structural characteristics of another well-known antipsoriatic drug, acitretinCitation10,Citation12. Since psoralens show cyclooxygenase-2 (COX-2)– 5-LOX dual inhibitory activityCitation13, we considered it interesting to examine the possibility that TRX and some of the aforementioned derivatives, that is compounds 1–4 and 7–11 (), act as inhibitors of lipoxygenase (LOX) activity and of the peroxidation of biological membranes as well as present anti-inflammatory properties. It is worth noting that TRX derivatives such as 4 and 11 incorporate structural characteristics of cinnamic acids. For the sake of completion of the present study, we have now prepared compound 6, the hydroxamic acid analog of carboxylic acid 4, and its corresponding tert-butyl ester 5. Through these compounds, we could hopefully determine the effect of the following structural elements: (1) the nature of the substitutent at position 4′ of the psoralen nucleus, (2) the length of the unsaturated side-chain (analogs 3 and 7), (c) the type of substituent at the end of the unsaturated side-chain (analogs 3–6), and (d) the type of substituent on position 4 of the phenyl ring, itself attached on the C-4′ of the psoralen nucleus (analogs 8–11), on the biological activity.
Materials and methods
Materials
Melting points were determined with a Buchi SMP-20 apparatus and are uncorrected. Infrared (IR) spectra were recorded for KBr pellets on a PerkinElmer 16PC Fourier transform (FT)-IR spectrophotometer. 1H nuclear magnetic resonance (NMR) spectra were obtained at 400.13 MHz and 13C NMR spectra at 100.62 MHz on a Bruker DPX spectrometer; tetramethylsilane (TMS) was used as reference. Electrospray ionization mass spectra (ESI-MS) were recorded at 30 V on a Micromass-Platform LC spectrometer using MeOH as solvent. Microanalyses were performed on a Carlo Erba EA 1108 CHNS (carbon, hydrogen, nitrogen, sulfur) elemental analyzer in the Laboratory of Instrumental Analysis of the University of Patras. Flash column chromatography was performed on Merck silica gel 60 (230–400 mesh) and thin layer chromatography (TLC) on Merck 60F254 films (0.2 mm) precoated on Al foil. Spots were visualized with UV light at 254 nm and charring agents.
All the chemicals used were of analytical grade and commercially available from Merck. 1,1-Diphenyl-2- picrylhydrazyl (DPPH), butylated hydroxyl toluene (BHT), and caffeic acid (CA) were purchased from Aldrich Chemical Co. (Milwaukee, WI, USA). Soybean lipoxygenase (type I-B), linoleic acid sodium salt, and indomethacin were obtained from Sigma Chemical Co. (St. Louis, MO, USA) and carrageenan, type K, was commercially available. For the in vivo experiments, male and female Fischer-344 rats (180–240 g) were used. TRX was purchased from CHEMOS GmbH. The TRX derivatives 1–4 and 7–11 were synthesized according to the methods described in reference 11. The tert-butyl ester 5 and hydroxamic acid 6 were obtained as described below.
(E)-N-tert-butoxy-3-(trioxsalen-4′-yl)acrylamide (5).
To an ice-cold suspension of acid 4 (0.60 g, 2 mmol) and HCl.H2NOtBu (0.28 g, 2.2 mmol) in anhydrous DMF (4 mL) was added Et3N (0.96 mL, 6.9 mmol) and O-(benzotriazol- 1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (0.87 g, 2.3 mmol). The resulting reaction mixture was stirred at 0°C for 5 min and at ambient temperature for 15 min and then diluted with CHCl3. The resulting solution was washed sequentially with a 5% aqueous citric acid solution, H2O, a 5% aqueous NaHCO3 solution, and H2O, then dried (Na2SO4) and finally evaporated to leave a solid residue. The latter was subjected to flash column chromatography using toluene/ethyl acetate (1:1) as the eluent. That way, pure ester 5 was obtained as a white solid [0.61 g (82% yield)]; m.p. 195–198°C; Rf (toluene/ethyl acetate: 1/1) = 0.19; IR (KBr, cm−1): 3270, 1738, 1686, 1664, 1638, 1614, 1598; 1H NMR (d6-DMSO): δ (ppm) 1.23 (s, 9H, C(CH3)3), 2.40 (s, 3H, CH3), 2.48 (s, 3H, CH3), 2.57 (s, 3H, CH3), 6.31 (s, 1 H, H-3), 6.58 (d, J = 16 Hz, 1H, H-2′′), 7.51 (d, J = 16 Hz, 1H, H-3′′), 7.69 (s, 1H, H-5), 10.58 (s, 1H, NH); 13C NMR (d6-DMSO): δ (ppm) 8.5, 12.9, 19.1, 26.9, 81.4, 108.5, 112.5, 112.9, 113.0, 116.4, 118.7, 122.3, 129.1, 148.9, 153.8, 154.1, 159.5, 160.1, 164.7; ESI-MS (30 eV): m/z 761.16 (2M + Na), 739.32 (2M + H), 392.22 (M + Na), 370.07 (MH), 314.05 (MH-C4H8); Anal. Calcd. for C21H23NO5: C, 68.28; H, 6.28; N, 3.79. Found: C, 68.45; H, 6.05; N, 3.62%.
(E)-N-hydroxy-3-(trioxsalen-4′-yl)acrylamide (6).
Ester 5 (0.6 g, 1.6 mmol) was dissolved in ice-cold trifluoroacetic acid (2 mL) and allowed to stand at 0°C for 30 min and at ambient temperature for 1.5 h. Evaporation of the volatile components under reduced pressure left a residue which was triturated with Et2O and cooled overnight. Pure hydroxamic acid 6 was obtained, as a white solid, by filtration, washing with ice-cold Et2O, and drying under vacuum (0.43 g, 82%); m.p. 205–208°C (decn.); Rf (CHCl3/MeOH: 9/1) = 0.1; IR (KBr, cm−1): 3448–3270, 3230, 1734, 1718, 1700, 1686, 1676, 1656, 1626, 1598; 1H NMR (d6-DMSO): δ (ppm) 2.37 (s, 3H, CH3), 2.45 (s, 3H, CH3), 2.55 (s, 3H, CH3), 6.29 (s, 1 H, H-3), 6.51 (d, J = 16 Hz, 1H, H-2′′), 7.45 (d, J = 16 Hz, 1H, H-3′′), 7.65 (s, 1H, H-5), 9.11 (br. s, 1H, OH), 10.78 (s, 1H, NH); 13C NMR (d6-DMSO): δ (ppm) 8.9, 13.6, 19.7, 109.2, 111.3, 113.1, 113.4, 114.9, 117.5, 121.8, 139.3, 149.8, 154.7, 155.1, 160.5, 163.3, 164.2; ESI-MS (30 eV): m/z 314.95 (MH), 282.94 (MH-NHOH); Anal. Calcd. for C17H15NO5: C, 65.17; H, 4.83; N, 4.47. Found: C, 65.35; H, 4.67; N, 4.33%.
Biological assays
In vitro assays
For the in vitro tests a Lambda 20 (PerkinElmer) UV-Vis double beam spectrophotometer was used. Each in vitro experiment was performed at least in triplicate and the standard deviation of absorbance was less than 10% of the mean.
Determination of reducing activity of stable radical 1, 1-diphenyl-2-picrylhydrazyl (DPPH)Citation14.
To an ethanolic solution of DPPH (0.05 mM) in absolute ethanol an equal volume of the compound dissolved in dimethylsulfoxide (DMSO) was added. The mixture was shaken vigorously and allowed to stand for 20 min or 60 min; absorbance at 517 nm was determined spectrophotometrically and the percentage of activity was calculated. All tests were undertaken on three replicates and the results were averaged ().
Table 1. Percent interaction with DPPH (reducing activity, DPPH %); in vitro inhibition of soybean lipoxygenase (LOX) (IC50 or I %); percent inhibition of lipid peroxidation (AAPH %); and percent inhibition of carrageenan-induced rat paw edema (ICPE %).
Soybean lipoxygenase inhibition study in vitroCitation14.
The tested compounds dissolved in DMSO were incubated at room temperature with sodium linoleate (0.1 mL) and 0.2 mL of enzyme solution (1/9 × 10−4 w/v in saline). The conversion of sodium linoleate to 13-hydroperoxylinoleic acid at 234 nm was recorded and compared with the appropriate standard inhibitor.
Inhibition of linoleic acid lipid peroxidationCitation16.
Production of conjugated diene hydroperoxide by the oxidation of linoleic acid in an aqueous dispersion was monitored at 234 nm. 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH) was used as a free radical initiator. This assay can be used to follow oxidative changes and to understand the contribution of each tested compound.
Azo compounds generating free radicals through spontaneous thermal decomposition are useful for in vitro studies of free radical production. The water-soluble azo compound AAPH has been extensively used as a clean and controllable source of thermally produced alkylperoxyl free radicals. Ten microliters of the 16 mM linoleic acid dispersion were added to the UV cuvette containing 0.93 mL of 0.05 M phosphate buffer, pH 7.4, prethermostated at 37°C. The oxidation reaction was initiated at 37°C under air by the addition of 50 μL of 40 mM AAPH solution. Oxidation was carried out in the presence of aliquots (10 μL) in the assay without antioxidant; lipid oxidation was measured in the presence of the same level of DMSO. The rate of oxidation at 37°C was monitored by recording the increase in absorption at 234 nm caused by conjugated diene hydroperoxides.
In vivo assays
Inhibition of carrageenin-induced edemaCitation14.
Edema was induced in the right hindpaw of Fischer 344-rats (150–200 g) by the intradermal injection of 0.1 mL of 2% carrageenan in water. Both sexes were used. Pregnant females were excluded. Each group was composed of 6–15 animals. The animals, which were bred in our laboratory, were housed under standard conditions and received a diet of commercial food pellets and water ad libitum during the maintenance period, but they were entirely fasted during the experimental period. Our studies were in accordance with recognized guidelines on animal experimentation. The tested compounds, 0.01 mmol/kg body weight, were diluted in water and were given intraperitoneally, simultaneously with the carrageenan injection. The rats were euthanized 3.5 h after carrageenan injection. Both hindpaws were severed above the ankle joint and were immediately weighed in a very sensitive analytical balanceCitation17. The difference between the weights of injected and uninjected paws was calculated for each animal. The change in paw weight was compared with that in control animals (treated with water) and expressed as percent inhibition of edema (% ICPE) (). Indomethacin was tested as a reference compound in 0.01 mmol/kg (47%). Values of % ICPE are the mean from two different experiments with a standard error of the mean less than 10%.
Biological studies
In vitro biological studies
In this investigation all compounds were studied in order to gain insight into their biological response. The C-4′ substituted trioxsalens and the parent molecule TRX were studied with regard to their antioxidant ability as well as their inhibition of soybean lipoxygenase.
Results and discussion
In this work, we evaluated in vitro and in vivo a series of novel TRX derivatives that were expected to offer protection against inflammation and radical attack.
Antioxidants are defined as substances that, even at low concentration, significantly delay or prevent oxidation of easily oxidizable substrates. There has been increased interest in using antioxidants for medical purposes in recent years. It is well known that free radicals play an important role in inflammatory processesCitation18. Many non-steroidal anti-inflammatory drugs have been reported to act either as inhibitors of free radical production or as radical scavengersCitation19. Consequently, compounds with antioxidant properties could be expected to offer protection in rheumatoid arthritis and inflammation and lead to potentially effective drugs. Thus, we tested the TRX derivatives with regard to their antioxidant ability and in comparison to well known antioxidant agents, e.g. butylated hydroxyl toluene and trolox.
For estimating the antioxidative potential of chemical components, different experimental approaches have been usedCitation20. Most of them require spectrophotometric measurement and a certain reaction time in order to obtain reproducible resultsCitation21. The DPPH assay is used as a radical scavenging measuring method. DPPH is a stable free radical in methanolic solution. In its oxidized form, the DPPH radical has an absorbance maximum centered at about 517 nmCitation22. The DPPH assay is described as a simple, rapid, and convenient method independent of sample polarity for the screening of many samples for radical scavenging activityCitation23. These advantages make the DPPH method interesting for testing our analogs.
The interaction of the examined compounds with the stable free radical DPPH is shown in . This interaction indicates their radical scavenging ability in an iron-free system. Only TRX derivative 6 (37.5%) showed an almost equipotent interaction to the reference compound BHT (31%). Compounds 2–4 presented very low interaction to DPPH at 0.1 mM (11–14%), whereas compound TRX and derivatives 1, 5, and 7–11 did not show any interaction at 0.1 mM. The interaction remained in most cases highly constant. With the exception of TRX derivative 6 and the TRX derivativatives 1 and 5, which showed 100% interaction after 60 min, no changes were observed after 60 min for the rest of the derivatives. It is evident that the presence of the hydroxamate function correlated with high interaction values, whereas blocking the free hydroxyl group in ester 5 completely suppressed this interaction. No changes were observed between the acid 4 and its tert-butyl ester 3. The insertion of a double bond in the side-chain as well as a phenyl group did not influence notably the antioxidant activity.
Leukotrienes play an important role as mediators of a variety of inflammatory and allergic reactions and are derived from the biotransformation of arachidonic acid catalyzed by lipoxygenase (LOX). Lipoxygenases play a role in membrane lipid peroxidation by forming hydroperoxides in the lipid bilayerCitation24. Inhibitors of LOX have attracted attention initially as potential agents for the treatment of inflammatory and allergic diseases, but their therapeutic potential has now been expanded to certain types of cancer and cardiovascular diseases.
In this context, we decided to further evaluate the synthesized TRX derivatives for inhibition of soybean lipoxygenase (LOX) by the UV absorbance based enzyme assayCitation25 (). Most of the LOX inhibitors are antioxidants or free radical scavengersCitation26, since lipoxygenation occurs via a carbon-centered radical. Some studies suggest a relationship between LOX inhibition and the ability of the inhibitors to reduce Fe3+ at the active site to the catalytically inactive Fe2+. LOXs contain a “non-heme” iron per molecule in the enzyme active site as high-spin Fe2+ in the native state and high-spin Fe3+ in the activated state. Several LOX inhibitors are excellent ligands for Fe3+. This inhibition is related to their ability to reduce the iron species in the active site to the catalytically inactive ferrous form.
Perusal of the IC50 or percent inhibition values () shows that TRX derivative 3 is the most active (9.4 μM) within the set followed by compound 2 (79 μM). It is therefore apparent that the highest inhibitory activity is secured by the trioxsalen derivative 3, bearing a highly lipophilic α,β-unsaturated tert-butyl ester side-chain, followed by compound 2, bearing also an unsaturated and lipophilic group, namely the acetyl group. It is also apparent that all esters in this series of compounds presented higher interaction values with the enzyme compared to their corresponding acids. It is also interesting to note that insertion of a second double bond in the side-chain diminished this interaction, whereas a phenyl group had a much less deleterious effect (compounds 3, 7, and 10). Although lipophilicity is referred toCitation27 as an important physicochemical property for LOX inhibitors, all the above tested derivatives do not follow this concept.
In this investigation, all compounds were studied in order to identify their possible inhibitory activity on lipid peroxidation. Azo compounds generating free radicals through spontaneous thermal decomposition are useful for free radical production studies in vitro. The water-soluble azo compound AAPH has been extensively used as a clean and controllable source of thermally produced alkylperoxyl free radicals. In our studies, AAPH was used as a free radical initiator to follow oxidative changes of linoleic acid to conjugated diene hydroperoxide. Compounds 3, 5, 9, and 11 showed higher inhibition (80–91%) of lipid peroxidation than the reference compound trolox (63%). It is interesting to note that among the analogs with a double bond in the side-chain, the tert-butyl esters are more potent inhibitors than the corresponding acids, but this is reversed in the analogs with a phenyl ring in the side-chain, wherein the acids present higher inhibitory activity.
Regression analyses of the values of percent inhibition of lipid peroxidation (AAPH %) for the subgroup of compounds 2–4, 7, and 9–11 revealed that low lipophilicity is the main physicochemical parameter influencing their interaction, followed by an indicator variable taking the value 1 for the presence of a free COOH group. A free COOH group is more significant for the inhibition of lipid peroxidation than an ester group:
The most potent compound in this set of TRX derivatives, namely ester 3, was selected to be examined in vivo using the functional model of carrageenan-induced rat paw edema, on the basis that it both highly inhibited LOX and presented high in vitro antioxidant activities. Carrageenan-induced edema is a non-specific inflammation resulting from a complex of diverse mediatorsCitation28. As shown in , this compound induced equipotent inhibition, essentially identical to the effect produced by the commonly used standard, namely indomethacin.
Conclusion
A series of TRX derivatives, bearing a variety of substituents at position 4′ of the psoralen ring-system, were synthesized using literature procedures or typical peptide synthetic methods, and their reducing abilities were determined using the stable radical DPPH at 0.1 mM after 20–60 min. The reducing abilities ranged from 0 to 37.5% (20 min) and 0 to 100% (60 min). This interaction expresses the reducing activity of compounds and indicates their ability to scavenge free radicals. The highest activity was shown by TRX derivative 6, bearing an α,β-unsaturated free hydroxamic acid function as side-chain. Inhibitory activities were measured against soybean lipoxygenase, in vitro. The compounds were tested in several concentrations and IC50 or percent inhibition values were determined. TRX derivative 3 showed the best inhibitory activity followed by TRX derivative 2, bearing an α,β-unsaturated tert-butyl ester and an acetyl side-chain, respectively. TRX derivative 3 showed much higher inhibition on lipoxygenase compared to caffeic acid and better inhibitory activity of lipid peroxidation compared to trolox. The highest inhibition of lipid peroxidation was shown by the tert-butyl ester 5 of the hydroxamic acid 6, followed by the TRX derivatives 11 and 9, bearing a benzoic or cinnamic acid side-chain, and compound 3. Finally, compound 3 with the most promising biological profile also showed equipotent anti-inflammatory activity in vivo to indomethacin.
In general, the presence of an α,β-unsaturated hydroxamic acid side-chain at position C-4′ of the psoralen ring-system seems to increase substantially the antioxidant properties of trioxsalen. On the other hand, the highest inhibitory ability of LOX is secured by the presence of tert-butyl acrylate or acetyl side-chains, whereas the highest inhibition of lipid peroxidation is shown by derivatives with tert-butyl acrylate or α,β-unsaturated hydroxamate side-chains and benzoic or cinnamic acid side-chains. Finally, incorporation of the tert-butyl acrylate side-chain at position 4′ of the psoralen ring-system provides not only the strongest inhibitor of LOX and strong inhibition of lipid peroxidation within this family of compounds but also significant anti-inflammatory activity.
Acknowledgements
We thank the European Social Fund (ESF) Operational Programme for Educational and Vocational Training II (EPEAEK II) and particularly the Program PYTHAGORAS II for funding the synthetic part of this work. We would also like to thank Dr. C. Hansch and Biobyte Corp., Claremont, CA, USA for free access to the C-QSAR program.
A part of this research has been presented at the 13th Panhellenic Symposium of Pharmacochemistry, 2008.
Declaration of interest: The authors report no conflicts of interest.
References
- Bonish B, Jullien D, Dutronc Y, Bei Huang B, Modlin R, Spada FM, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-γ production by NK-T cells. J Immunol 2000;165:4076–85.
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science 1983;220:568–72.
- Nakano H, Inoue T, Kawasaki N, Miyataka H, Matsumoto H, Taguchi T, et al. Synthesis and biological activities of novel antiallergic agents with 5-lipoxygenase inhibiting action. Bioorg Med Chem 2000;8:373–80.
- Hussain H, Shornick LP, Shannon VR, Wilson JD, Funk CD, Pentland AP, et al. Epidermis contains platelet-type 12-lipoxygenase that is overexpressed in germinal layer keratinocytes in psoriasis. J Am J Physiol 1994;266:C243–53.
- Harats D, Shaish A, George J, Mulkins M, Kurihara H, Levkovitz H, et al. Overexpression of 15-lipoxygenase in vascular endothelium accelerates early atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 2000;20:2100–5.
- Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, et al. Lipoxygenase inhibitors as potential cancer chemopreventives. J Cancer Epidemiol Biomark Prev 1999;8:467–83.
- Avis I, Hong SH, Martinez A, Moody T, Choi YH, Trepel J, et al. Five-lipoxygenase inhibitors can mediate apoptosis in human breast cancer cell lines through complex eicosanoid interactions. FASEB J 2001;15:2007–9.
- Gia O, Magno SM, Gonzalez-Diaz H, Quezada E, Santana L, Uriarte E, et al. Design, synthesis and photobiological properties of 3,4-cyclopentenepsoralens. Bioorg Med Chem 2005;13:809–17 and references cited therein.
- Leite VC, Santos RF, Chen LC, Guillo LA. Psoralen derivatives and longwave ultraviolet irradiation are active in vitro against human melanoma cell line. J Photochem Photobiol 2004;76:49–53 and references cited therein.
- van der Kerkhof PCM. The management of psoriasis. Neth J Med 1998;52:40–5 and references cited therein.
- Militsopoulou M, Bariamis SE, Athanassopoulos CM, Papaioannou D. Synthetic studies towards the development of psoralen-acidic retinoid conjugates and hybrids. Synthesis 2008;(21):3433–42.
- Pilkington T, Brogden RN. Acitretin. A review of its pharmacology and therapeutic use. Drugs 1992;43:597–627.
- Kim JS, Kim JC, Shim SH, Lee EJ, Jin W, Bae K, et al. Chemical constituents of the root of Dystaenia takeshimana and their anti-inflammatory activity. Arch Pharmacol Res 2006;29:617–23.
- Kontogiorgis C, Hadjipavlou-Litina D. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidants agents. J Enzyme Inhib Med Chem 2003;18:63–9.
- Biobyte Corp., C-QSAR Database, 201 West 4th Str., Suite 204, Claremont CA, California 91711, USA.
- Liegois C, Lermusieau G, Colin S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J Agric Food Chem 2000;48:1129–34.
- Gavalas A, Kourounakis A, Litina D, Kourounakis P. Anti-inflammatory and Immunomodulating effects of the novel agent γ-(2-aminoethylamino)-2-butyrothienone. Arzneimittelforschung 1991;41:423–6.
- Flohe L, Beckman R, Giertz H, Loschen G. Oxygen-centered free radicals as mediators of inflammation. In: Sies H, ed. Oxidative Stress. London: Academic Press, 1985:403–35.
- Saldanha LA, Elias G, Gao MNA. Oxygen radical scavenging activity of phenylbutenones and their correlation with antiinflammatory activity. Arzneimittelforschung 1990;40:89–91.
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 2005;53:4290–303.
- Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 2004;85:633–40.
- Molyneux P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 2004;26:211–19.
- Koleva II, van Beek TA, Linssen JPH, de Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 2001;13:8–17.
- Kuhn H, Belkner J, Wiesner R, Brash AR. Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. J Biol Chem 1990;265:18351–61.
- Taraporewala RB, Kauffman JM. Synthesis and structure activity relationships of anti-inflammatory 9,10-dihydro-9-oxo-2-acridine-alkanoic acids and 4-(2-carboxyphenyl)aminobenzenealkanoic acids. J Pharm Sci 1990;79:173–8.
- Muller K. 5-Lipoxygenase and 12-lipoxygenase: attractive targets for the development of novel antipsoriatic drugs. Arch Pharm (Weinheim) 1994;327:3–19.
- Pontiki E, Hadjipavlou-Litina D. Lipoxygenase inhibitors: a comparative QSAR study review and evaluation of new QSARs. Med Res Rev 2008;28:39–117.
- Shen TY. Non steroidal anti-inflammatory agents. In: Wolf ME, ed. Burger’s Medicinal Chemistry. New York: John Wiley and Sons, 1980:1217–19.