Abstract
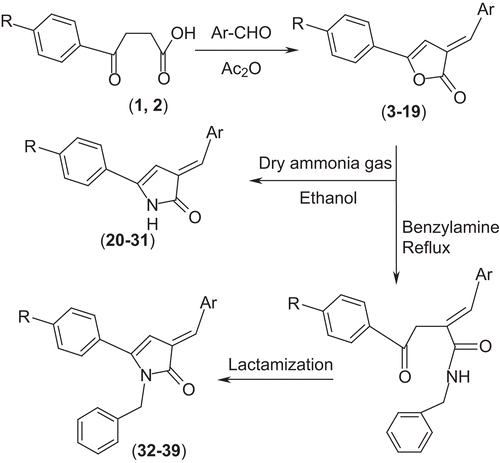
In the present investigation, 17 new synthetic butenolides, i.e. 2-arylidene-4-(4-chloro/ethyl-phenyl)but-3-en-4-olides (3–19) have been synthesized from 3-(4-chloro-benzoyl)propionic acid or 3-(4-ethyl-benzoyl)propionic acid using appropriate reagents. Some of the selected butenolides were reacted with ammonia and benzylamine to give the corresponding pyrrolones (20–31) and N-benzyl-pyrrolones (32–39) respectively. All the compounds were screened for their antibacterial activity against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa and antifungal activity against Candida albicans, Aspergillus niger, and Rhizopus oryza. Minimum inhibitory concentration (MIC) values of the compounds are reported. The pyrrolone derivatives discovered in this study may provide valuable therapeutic intervention for the treatment of microbial diseases, especially against fungal species.
Introduction
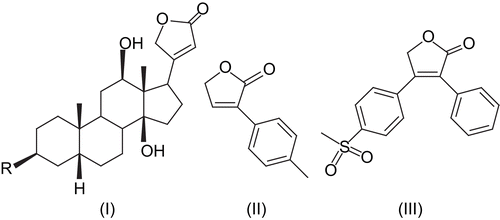
Butenolides, a family of α,β-unsaturated lactones, also known as furanones, are ubiquitous chemical moieties found in many natural productsCitation1,2. They are typical products of a polyketide biochemical synthesis pathwayCitation2. Butenolide ring systems acquire a special place in natural chemistry and in heterocyclic chemistry because this is a frequently encountered structural motif in many pharmacologically relevant compounds. Some common examples of compounds having a butenolide ring are cardiotonic digitoxines (I) from Digitalis speciesCitation3, antifungal incrustoporine (II)Citation4, and COX-2 inhibitor rofecoxib (III) ()Citation5, and many others are encountered among fungi, bacteriaCitation6, and gorgoriansCitation7. Their saturated analogs act as signaling substances in bacteriaCitation8 and enhance spore formation of streptomycetes, or induce metabolite productionCitation9. The γ-lactone ring present in butenolides is significantly reactive, and has been utilized for the synthesis of nitrogen hetrocycles (pyrrolones) of potential biological activityCitation10,Citation11.
Pyrrolones are five-membered heterocyclic lactams, either Δ1 or Δ2 derivatives, and have been found to possess important pharmacological activities, especially antimicrobialCitation12–14. They are also present in many natural productsCitation15: typical examples are the anti-tumor alkaloid jatrophamCitation16 and the platelet aggregation inhibitor PI-091Citation17,18.
Over recent decades, the incidence of systemic microbial infections has been increasing dramatically due to an increase in the number of immunocompromised hosts. Immunosuppression due to malignancy, immunosuppressive therapies, human immunodeficiency virus (HIV) infection, broad-spectrum antimicrobial treatment, and age, as well as invasive procedures and mucosal barriers, places patients at risk for microbial infectionsCitation19. These observations place new emphasis on the need, as well as search, for alternative, new, and more effective antimicrobial agents with a broad-spectrum activityCitation20,21. We previously examined in these laboratories the antimicrobial activity of a number of butenolides, and the results were encouragingCitation11–13. The present work describes the synthesis and characterization of new butenolides and their nitrogen analogs (isosteres), i.e. pyrrolones, with a study of their antibacterial and antifungal activities against some selected microbes.
Materials and methods
Chemistry
Melting points were determined by the open tube capillary method, and are uncorrected. The purity of the compounds was checked by thin layer chromatography (TLC) on silica gel G plates. The infrared (IR) spectra were measured on potassium bromide pellets using a PerkinElmer 1725X spectrophotometer. 1H nuclear magnetic resonance (NMR) spectra were recorded on a Bruker spectrospin DPX-300 MHz, in CDCl3 with tetramethylsilane (TMS) as an internal standard; chemical shifts (δ) are reported in parts per million (ppm) downfield from TMS. The splitting pattern abbreviations are as follows: s, singlet; d, doublet; dd, double doublet; t, triplet; q, quartet; m, multiplet. Mass spectra were recorded on a Jeol JMS-D 300 instrument fitted with a JMS 2000 data system at 70 eV. Spectral data are consistent with assigned structures. Elemental analyses were performed on a PerkinElmer model 240 analyzer (C, H, N) and found to be within a range of ±0.4% of theoretical values. 3-(4-Chloro-benzoyl)propionic acid (1) and 3-(4-ethyl-benzoyl)propionic acid (2) were prepared according to the method reported in the literatureCitation11.
General procedure for the synthesis of 2-arylidene-4-(4-chloro/ethyl-phenyl)but-3-en-4-olides (3–19)
A solution of an aromatic aldehyde (3 mmol) and 3-(4-chloro-benzoyl)propionic acid 1 or 3-(4-ethyl-benzoyl)propionic acid 2 (equimolar; 3 mmol) in acetic anhydride (10 mL) with triethylamine (2–3 drops) was refluxed for 4 h under anhydrous conditions. After completion of reaction, the contents were poured into crushed ice in small portions under stirring. A colored solid mass separated out, which was filtered, washed with water, and crystallized from a mixture of methanol:chloroform (1:1).
2-Benzylidene-4-(4-chloro-phenyl)but-3-en-4-olide (3)
Yellow crystals, yield 68%, mp 194°C; IR (cm−1, KBr): 1763, 1602, 836; 1H-NMR (CDCl3) δ 6.93 (s, 1H, butenolide ring), 7.41 (s, olefinic H, merged), 7.62 and 7.68 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.41 (m, 6H, 5H arylidene ring + olefinic proton); MS: m/z 282 (M+), 139, 111, 105, 77. Anal. Calcd. for C17H11ClO2: C, 72.22; H, 3.92; found: C, 72.43; H, 3.90%.
2-(2-Chloro-benzylidene)-4-(4-chloro-phenyl)but-3-en-4-olide (4)
Yellowish orange crystals, yield 72%, mp 220°C; IR (cm−1, KBr): 1768, 1605, 831; 1H-NMR (CDCl3) δ 6.84 (s, 1H, butenolide ring), 7.46 (s, 1H, olefinic H), 7.44 and 7.67 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.64 (m, 4H, H-3, 4, 5, 6, arylidene ring); MS: m/z 317 (M+), 139, 105, 77. Anal. Calcd. for C17H10Cl2O2: C, 64.38; H, 3.18; found: found: C, 64.20; H, 3.19%.
2-(3-Chloro-benzylidene)-4-(4-chloro-phenyl)but-3-en-4-olide (5)
Yellow crystals, yield 70%, mp 240°C; IR (cm−1, KBr): 1773, 1605, 832; 1H-NMR (CDCl3) δ 6.78 (s, 1H, butenolide ring), 7.42 (s, 1H, olefinic H), 7.52 and 7.69 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.36 (m, 3H, H-4, 5, 6, arylidene), 7.62 (s, 1H, H-2, arylidene ring). MS: m/z 317 (M+), 139, 111, 105. Anal. Calcd. for C17H10Cl2O2: C, 64.38; H, 3.18; found: C, 64.19; H, 3.16%.
2-(4-Bromo-benzylidene)-4-(4-chloro-phenyl)but-3-en- 4-olide (6)
Dark yellow crystals, yield 75%, mp 226°C; IR (cm−1, KBr): 1771, 1605, 835; 1H-NMR (CDCl3) δ 6.81 (s, 1H, butenolide ring), 7.47 (s, 1H, olefinic H), 7.43 and 7.59 (d, each, J = 8.1 Hz, A2B2, arylidene ring), 7.56 and 7.78 (d, each, J = 8.4 Hz, A2B2, phenyl). MS: m/z 361(M+), 139, 77. Anal. Calcd. for C17H10BrClO2: C, 56.46; H, 2.79; found: C, 56.58; H, 2.66%.
2-(2-Nitro-benzylidene)-4-(4-chloro-phenyl)but-3-en-4-olide (7)
Light brown crystals, yield 76%, mp 240°C; IR (cm−1, KBr): 1769, 1608, 828; 1H-NMR (CDCl3) δ 6.60 (s, 1H, butenolide ring), 7.66 (s, 1H, olefinic H), 7.54 and 7.68 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.84 (m, 1H, H-5, arylidene ring), 8.20 (d, 1H, J = 7.8 Hz, H-3, arylidene ring), 8.43 (m, 2H, H-4,6, arylidene ring). MS: m/z 327 (M+) not observed, 139, 111, 105, 77. Anal. Calcd. for C17H10ClNO4: C, 62.30; H, 3.08; N, 4.27; found: C, 62.48; H, 3.09; N, 4.26%.
2-(3-Nitro-benzylidene)-4-(4-chloro-phenyl)but-3-en-4-olide (8)
Reddish orange crystals, yield 78%, mp 226°C; IR (cm−1, KBr): 1771, 1606, 826; 1H-NMR (CDCl3) δ 6.67 (s, 1H, butenolide ring), 7.62 (s, 1H, olefinic H), 7.52 and 7.69 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.64 (m, 1H, H-5, arylidene ring), 7.81 (dd, J = 2, 7.8 Hz, 1H, H-6, arylidene), 8.16 (m, 1H, H-4, arylidene ring), 8.23 (d, J = 2 Hz, 1H, H-2, arylidene ring). MS: m/z 327 (M+), 139, 105, 77. Anal. Calcd. for C17H10ClNO4: C, 62.30; H, 3.08; N, 4.27; found: C, 62.12; H, 3.05; N, 4.29%.
2-(4-Nitro-benzylidene)-4-(4-chloro-phenyl)but-3-en-4- olide (9)
Yellowish orange crystals, yield 82%, mp 264°C; IR (cm−1, KBr): 1765, 1608, 835; 1H-NMR (CDCl3) δ 6.82 (s, 1H, butenolide ring), 7.50 and 7.63 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.56 (s, 1H, olefinic H), 7.59 and 8.12 (d, each, J = 8.1 Hz, A2B2, arylidene ring). MS: m/z 327 (M+), 111, 105, 77. Anal. Calcd. for C17H10ClNO4: C, 62.30; H, 3.08; N, 4.27; found: C, 62.47; H, 3.10; N, 4.28%.
2-(4-Fluoro-benzylidene)-4-(4-chloro-phenyl)but- 3-en-4-olide (10)
Yellow crystals, yield 64%, mp 222°C; IR (cm−1, KBr): 1761, 1601, 833; 1H-NMR (CDCl3) δ 6.87 (s, 1H, butenolide ring), 7.42 (m, olefinic H, merged), 7.63 and 7.68 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.14 (m, 2H, H-3, 5, arylidene ring), 7.42 (m, 3H, olefinic proton + H-2, 6 arylidene ring). MS: m/z 300 (M+), 139, 105, 77. Anal. Calcd. for C17H10ClFO2: C, 67.90; H, 3.35; found: C, 67.81; H, 3.36%.
2-(4-Methoxy-benzylidene)-4-(4-chloro-phenyl)but-3-en-4-olide (11)
Red crystals, yield 74%, mp 214°C; IR (cm−1, KBr): 1755, 1597, 844; 1H-NMR (CDCl3) δ 3.82 (s, 3H, OCH3), 6.90 (s, 1H, butenolide ring), 6.93 and 7.45 (d, each, J = 8.4 Hz, 2 × A2B2, arylidene ring), 7.42 (s, 1H, olefinic H), 7.50 and 7.67 (d, each, JI = 7.8 Hz, A2B2, phenyl). MS: m/z 312 (M+), 107, 105, 77. Anal. Calcd. for C18H13ClO3: C, 69.13; H, 4.19; found: C, 68.93; H, 4.17%.
2-(3,4-Dimethoxy-benzylidene)-4-(4-chloro-phenyl)but-3-en-4-olide (12)
Yellowish orange crystals, yield 72%, mp 218°C; IR (cm−1, KBr): 1771, 1598, 837; 1H-NMR (CDCl3) δ 3.92 (s, 6H, 2 × OCH3), 6.85 (s, 1H, butenolide ring), 6.98 (d, J = 7.8 Hz, 1H, H-5, arylidene ring), 7.17 (d, J = 2 Hz, 1H, H-2, arylidene ring), 7.39 (dd, J = 2, 7.8 Hz, 1H, H-6, arylidene ring), 7.59 (s, 1H, olefinic H), 7.45 and 7.74 (d, each, J = 8.1 Hz, A2B2, phenyl). MS: m/z 342 (M+), 139, 111, 77. Anal. Calcd. for C19H15ClO4: C, 66.58; H, 4.41; found: C, 66.80; H, 4.43%.
2-(2,4-Dichloro-benzylidene)-4-(4-chloro-phenyl)but-3-en-4-olide (13)
Dark orange crystals, yield 64%, mp 172°C; IR (cm−1, KBr): 1776, 1611, 833; 1H-NMR (CDCl3) δ 6.41 (s, 1H, butenolide ring), 7.3 and 7.48 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.37 (m, 1H, H-6, arylidene ring), 7.42 (s, 1H, olefinic H), 7.54 (m, 2H, H-3, 5, arylidene ring). MS: m/z 351 (M+), 139, 105, 111, 77. Anal. Calcd. for C17H9Cl3O2: C, 58.07; H, 2.58; found: C, 57.26; H, 2.57%.
2-(4-Acetoxy-3-methoxy-benzylidene)-4-(4-chloro-phenyl)but-3-en-4-olide (14)
Yellowish red crystals, yield 62%, mp 148°C; IR (cm−1, KBr): 1767, 1597, 829; 1H-NMR (CDCl3) δ 2.34 (s, 3H, OCOCH3), 3.90 (s, 3H, OCH3), 6.67 (s, 1H, butenolide ring), 7.32 and 7.64 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.36 (m, 1H, H-5, arylidene ring), 7.42 (s, 1H, olefinic H), 7.54 (m, 2H, H-2, 6, arylidene ring). MS: m/z 370 (M+), 139, 105, 77. Anal. Calcd. for C20H15ClO5: C, 64.79; H, 4.08; found: C, 64.58; H, 4.09%.
2-Benzylidene-4-(4-ethyl-phenyl)but-3-en-4-olide (15)
Yellow needles, yield 71%, mp 134°C; IR (cm−1, KBr): 1761, 1611, 833; 1H-NMR (CDCl3) δ 1.25 (t, 3H, CH3), 2.69 (q, 2H, CH2), 6.83 (s, 1H, butenolide ring), 7.34 (s, olefinic H), 7.12 and 7.68 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.37 (m, 5H, 5H arylidene ring); MS: m/z 276 (M+), 133, 105, 77. Anal. Calcd. for C19H16O2: C, 82.58; H, 5.84; found: C, 83.01; H, 5.49%.
2-(4-Nitro-benzylidene)-4-(4-ethyl-phenyl)but-3-en-4-olide (16)
Brown crystals, yield 78%, mp 184°C; IR (cm−1, KBr): 1785, 1609, 824; 1H-NMR (CDCl3) δ 1.26 (t, 3H, CH3), 2.71 (q, 2H, CH2), 6.85 (s, 1H, butenolide ring), 7.26 and 7.68 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.42 (s, 1H, olefinic H), 7.49 and 8.04 (d, each, J = 8.1 Hz, A2B2, arylidene ring). MS: m/z 321 (M+), 133. Anal. Calcd. for C19H15NO4: C, 71.02; H, 4.70; N, 4.36; found: C, 71.32; H, 4.95; N, 4.54%.
2-(4-Methoxy-benzylidene)-4-(4-ethyl-phenyl)but-3-en-4-olide (17)
Dark yellow needles, yield 72%, mp 116°C; IR (cm−1, KBr): 1770, 1605, 836; 1H-NMR (CDCl3) δ 1.26 (t, 3H, CH3), 2.70 (q, 2H, CH2), 3.86 (s, 3H, OCH3), 6.82 (s, 1H, butenolide ring), 7.26 and 7.66 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.37 and 7.59 (d, each, J = 8.1 Hz, A2B2, arylidene ring), 7.55 (s, 1H, olefinic H). MS: m/z 306(M+), 133, 105. Anal. Calcd. for C20H18O3: C, 78.41; H, 5.92; found: C, 78.58; H, 5.66%.
2-(3,4-Dimethoxy-benzylidene)-4-(4-ethyl-phenyl)but-3-en-4-olide (18)
Yellowish orange crystals, yield 70%, mp 122°C; IR (cm−1, KBr): 1768, 1599, 833; 1H-NMR (CDCl3) δ 1.26 (t, 3H, CH3), 2.70 (q, 2H, CH2), 3.89 (s, 6H, 2 × OCH3), 6.77 (s, 1H, butenolide ring), 6.96 (d, J = 7.8 Hz, 1H, H-5, arylidene ring), 7.15 (d, J = 2 Hz, 1H, H-2, arylidene ring), 7.38 (dd, J = 2, 7.8 Hz, 1H, H-6, arylidene ring), 7.48 (s, 1H, olefinic H), 7.28 and 7.71 (d, each, J = 8.1 Hz, A2B2, phenyl). MS: m/z 336 (M+), 133, 105. Anal. Calcd. for C21H20O4: C, 74.98; H, 5.99; found: C, 74.65; H, 6.28%.
2-(2,4-Chloro-benzylidene)-4-(4-ethyl-phenyl)but-3-en-4-olide (19)
Orange crystals, yield 68%, mp 168°C; IR (cm−1, KBr): 1777, 1619, 819; 1H-NMR (CDCl3) δ 1.27 (t, 3H, CH3), 2.73 (q, 2H, CH2), 6.84 (s, 1H, butenolide ring), 7.31 and 7.58 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.35 (m, 1H, H-6, arylidene ring), 7.41 (s, 1H, olefinic H), 7.55 (m, 2H, H-3, 5, arylidene ring). MS: m/z 345 (M+), 133, 77. Anal. Calcd. for C19H14Cl2O2: C, 66.10; H, 4.09; found: C, 66.34; H, 4.30%.
General procedure for the synthesis of 3-arylidene-5-(substituted-phenyl)-2(3H)-pyrrolone (20–31)
Dry ammonia gas was passed into anhydrous ethanolic solution of 2-arylidene-4-(4-chloro-phenyl)but-3-en-4-olide (1.0 gm) for 1 h at room temperature, ethanol was distilled off under reduced pressure, and the solid mass so obtained was crystallized from methanol/acetone to give 20–31.
3-Benzylidene-5-(4-chloro-phenyl)-2(3H)-pyrrolone (20)
Light brown crystals, yield 74%, mp 224°C; IR (cm−1, KBr): 3381, 1687, 1608, 802; 1H-NMR (CDCl3) δ 6.58 (s, 1H, pyrrolone ring), 7.41(m, 5H, arylidene ring), 7.43 (s, 1H, olefinic H), 7.47 and 7.55 (d, each, J = 8.1 Hz, A2B2, phenyl), 8.42 (s, 1H, NH). MS: m/z 281 (M+), 138, 111, 77. Anal. Calcd. for C17H12ClNO: C, 72.47; H, 4.29; N, 4.97; found: C, 72.56; H, 4.21; N, 5.04%.
3-(2-Chloro-benzylidene)-5-(4-chloro-phenyl)-2(3H)-pyrrolone (21)
Dark brown crystals, yield 72%, mp 246°C; IR (cm−1, KBr): 3454, 1707, 1613, 821; 1H-NMR (CDCl3) δ 6.55 (s, 1H, pyrrolone ring), 7.41 (s, 1H, olefinic H), 7.45 and 7.55 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.62 (m, 4H, H-3, 4, 5, 6, arylidene ring), 8.57 (s, 1H, NH). MS: m/z 316(M+), 138, 77. Anal. Calcd. for C17H11Cl2NO: C, 64.58; H, 3.51; N, 4.43; found: C, 64.45; H, 3.58; N, 4.51%.
3-(4-Bromo-benzylidene)-5-(4-chloro-phenyl)-2(3H)-pyrrolone (22)
Brown crystals, yield 68%, mp 212°C; IR (cm−1, KBr): 3464, 1712, 1616, 818; 1H-NMR (CDCl3) δ 6.67 (s, 1H, pyrrolone ring), 7.43 (s, 1H, olefinic H), 7.39 and 7.48 (d, each, J = 8.1 Hz, A2B2, arylidene ring), 7.51 and 7.67 (d, each, J = 8.4 Hz, A2B2, phenyl), 8.61 (s, 1H, NH). MS: m/z 360 (M+), 138, 111, 77. Anal. Calcd. for C17H11BrClNO: C, 56.62; H, 3.07; N, 3.88; found: C, 56.45; H, 3.16; N, 3.80%.
3-(3-Nitro-benzylidene)-5-(4-chloro-phenyl)-2(3H)-pyrrolone (23)
Yellowish orange needles, yield 82%, mp 238°C; IR (cm−1, KBr): 3427, 1685, 1593, 813; 1H-NMR (CDCl3) δ 6.59 (s, 1H, pyrrolone ring), 7.55 (s, 1H, olefinic H), 7.51 and 7.66 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.62 (m, 1H, H-5, arylidene ring), 7.79 (dd, J = 2, 7.8 Hz, 1H, H-6, arylidene ring), 8.15 (m, 1H, H-4, arylidene ring), 8.21 (d, J = 2 Hz, 1H, H-2, arylidene ring), 8.85 (s, 1H, NH). MS: m/z 326 (M+), 138, 77. Anal. Calcd. for C17H11ClN2O3: C, 62.49; H, 3.39; N, 8.57; found: C, 62.65; H, 3.30; N, 8.51%.
3-(4-Nitro-benzylidene)-5-(4-chloro-phenyl)-2(3H)-pyrrolone (24)
Orange crystals, yield 86%, mp 252°C; IR (cm−1, KBr): 3435, 1708, 1610, 821; 1H-NMR (CDCl3) δ 6.57 (s, 1H, pyrrolone ring), 7.48 and 7.60 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.51 (s, 1H, olefinic H), 7.57 and 8.02 (d, each, J = 8.4 Hz, A2B2, arylidene ring), 8.57 (s, 1H, NH). MS: m/z 326 (M+), 138, 111, 77. Anal. Calcd. for C17H11ClN2O3: C, 62.49; H, 3.39; N, 8.57; found: C, 62.58; H, 3.32; N, 8.50%.
3-(4-Fluoro-benzylidene)-5-(4-chloro-phenyl)-2(3H)-pyrrolone (25)
Light brown crystals, yield 76%, mp 186°C; IR (cm−1, KBr): 3471, 1696, 1603, 826; 1H-NMR (CDCl3) δ 6.63 (s, 1H, pyrrolone ring), 7.15 and 7.43 (d, each, J = 8.4 Hz, A2B2, arylidene ring), 7.47 (s, 1H, olefinic H), 7.46 and 7.62 (d, each, J = 8.1 Hz, A2B2, phenyl), 8.61 (s, 1H, NH). MS: m/z 299 (M+), 138, 111, 77. Anal. Calcd. for C17H11ClFNO: C, 68.12; H, 3.70; N, 4.67; found: C, 68.20; H, 3.55; N, 4.58%.
3-(4-Methoxy-benzylidene)-5-(4-chloro-phenyl)-2(3H)-pyrrolone (26)
Dark red needles, yield 80%, mp 208°C; IR (cm−1, KBr): 3408, 1693, 1611, 824; 1H-NMR (CDCl3) δ 3.78 (s, 3H, OCH3), 6.49 (s, 1H, pyrrolone ring), 6.96 and 7.44 (d, each, J = 8.4 Hz, A2B2, arylidene ring), 7.38 (s, 1H, olefinic H), 7.55 and 7.71 (d, each, J = 8.1 Hz, A2B2, phenyl), 8.33 (s, 1H, NH). MS: m/z 311 (M+), 138, 107, 77. Anal. Calcd. for C18H14ClNO2: C, 69.35; H, 4.53; N, 4.49; found: C, 69.18; H, 4.60; N, 4.54%.
3-(2,4-Chloro-benzylidene)-5-(4-chloro-phenyl)-2(3H)-pyrrolone (27)
Reddish orange crystals, yield 75%, mp 184°C; IR (cm−1, KBr): 3471, 1686, 1606, 821; 1H-NMR (CDCl3) δ 6.45 (s, 1H, pyrrolone ring), 7.41 and 7.58 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.43 (m, 1H, H-6, arylidene ring), 7.49 (s, 1H, olefinic H), 7.63 (m, 2H, H-3, 5, arylidene ring), 8.31 (s, 1H, NH). MS: m/z 350 (M+), 138, 111. Anal. Calcd. for C17H10Cl3NO: C, 58.23; H, 2.87; N, 3.99; found: C, 58.35; H, 2.80; N, 4.06%.
3-Benzylidene-5-(4-ethyl-phenyl)-2(3H)-pyrrolone (28)
Light brown crystals, yield 74%, mp 224°C; IR (cm−1, KBr): 3381, 1687, 1608, 802; 1H-NMR (CDCl3) δ 1.26 (t, 3H, CH3), 2.71 (q, 2H, CH2), 6.43 (s, 1H, pyrrolone ring), 7.04 (m, 2H, H-2, 6, arylidene ring), 7.31 and 7.57 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.38 (m, 3H, H-3, 4, 5, arylidene ring), 7.43 (s, 1H, olefinic H), 8.42 (s, 1H, NH). MS: m/z 281 (M+), 132, 105, 77. Anal. Calcd. for C17H12ClNO: C, 72.47; H, 4.29; N, 4.97; found: C, 72.56; H, 4.21; N, 5.04%.
3-(4-Nitro-benzylidene)-5-(4-ethyl-phenyl)-2(3H)-pyrrolone (29)
Orange crystals, yield 86%, mp 252°C; IR (cm−1, KBr): 3435, 1708, 1610, 821; 1H-NMR (CDCl3) δ 1.26 (t, 3H, CH3), 2.70 (q, 2H, CH2), 6.52 (s, 1H, pyrrolone ring), 7.29 and 7.61 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.42 (s, 1H, olefinic H), 7.47 and 8.03 (d, each, J = 8.1 Hz, A2B2, arylidene ring), 8.56 (s, 1H, NH). MS: m/z 320 (M+), 132, 105, 77. Anal. Calcd. for C19H16N2O3: C, 71.24; H, 5.03; N, 8.74; found: C, 71.58; H, 5.32; N, 8.50%.
3-(4-Methoxy-benzylidene)-5-(4-ethyl-phenyl)-2(3H)-pyrrolone (30)
Red fine needles, yield 78%, mp 216°C; IR (cm−1, KBr): 3443, 1696, 1610, 822; 1H-NMR (CDCl3) δ 1.25 (t, 3H, CH3), 2.73 (q, 2H, CH2), 3.81 (s, 3H, OCH3), 6.55 (s, 1H, pyrrolone ring), 7.28 and 7.64 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.39 and 7.57 (d, each, J = 8.4 Hz, A2B2, arylidene ring), 7.48 (s, 1H, olefinic H), 8.19 (s, 1H, NH). MS: m/z 305 (M+), 132, 77. Anal. Calcd. for C20H19NO2: C, 78.66; H, 6.27; N, 4.59; found: C, 78.38; H, 6.60; N, 4.54%.
3-(2,4-Chloro-benzylidene)-5-(4-ethyl-phenyl)-2(3H)-pyrrolone (31)
Dark orange crystals, yield 76%, mp 234°C; IR (cm−1, KBr): 3451, 1689, 1603, 815; 1H-NMR (CDCl3) δ 1.27 (t, 3H, CH3), 2.70 (q, 2H, CH2), 6.53 (s, 1H, pyrrolone ring), 7.30 and 7.58 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.36 (m, 1H, H-6, arylidene ring), 7.51 (s, 1H, olefinic H), 7.64 (m, 2H, H-3, 5, arylidene ring), 8.51 (s, 1H, NH). MS: m/z 344 (M+), 132, 105, 77. Anal. Calcd. for C19H15Cl2NO: C, 66.29; H, 4.39; N, 4.07; found: C, 66.35; H, 4.80; N, 4.00%.
General procedure for the synthesis of 3-arylidene-5-(4-chloro-phenyl)-1-benzyl-2(3H)-pyrrolone (32–39)
Synthesis of these compounds involved the following two steps.
(i) Synthesis of γ-ketobenzylamide Butenolide (3 mmol) and benzylamine (4 mmol) were refluxed in dry benzene (CARE—CARCINOGEN) for 2 h. The color of the butenolide slowly discharged. On completion of reaction, excess benzene was distilled off and the solid mass so obtained was washed with petroleum ether and dried. The compound obtained was used without crystallization.
(ii) Lactamization of γ-ketobenzylamide γ-Ketobenzylamide (3 mmol) was refluxed in hydrochloric acid (6 N; 20 mL) for 1 h. Upon refluxing, the solid gained color. The contents were then cooled and the solid mass so obtained was filtered, washed with water, and recrystallized from methanol to give 32–39.
3-Benzylidene-5-(4-chloro-phenyl)-1-benzyl-2(3H)-pyrrolone (32)
Brown flakes, yield 72%, mp 136°C; IR (cm−1, KBr): 1752, 1613, 805; 1H-NMR (CDCl3) δ 4.82 (s, 2H, CH2), 6.25 (s, 1H, pyrrolone ring), 7.05 and 7.64 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.37 (m, 5H, benzyl), 7.46 (m, 3H, H-3, 4, 5, arylidene ring), 7.55 (s, 1H, olefinic H), 7.62 (m, 2H, H-2, 6, arylidene ring). MS: m/z 371 (M+), 280, 138, 91, 77. Anal. Calcd. for C24H18ClNO: C, 77.52; H, 4.88; N, 3.77; found: C, 77.24; H, 4.87, N, 3.78%.
3-(4-Bromo-benzylidene)-5-(4-chloro-phenyl)-1-benzyl-2(3H)-pyrrolone (33)
Brownish yellow flakes, yield 68%, mp 142°C; IR (cm−1, KBr): 1729, 1610, 811; 1H-NMR (CDCl3) δ 4.82 (s, 2H, CH2), 6.19 (s, 1H, pyrrolone ring), 7.11 and 7.35 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.24 (m, 5H, benzyl), 7.37 and 7.55 (d, each, J = 8.1 Hz, A2B2, arylidene ring), 7.46 (s, 1H, olefinic H). MS: m/z 450 (M+), 156, 91, 77. Anal. Calcd. for C24H17BrClNO: C, 63.95; H, 3.80; N, 3.11; found: C, 63.78; H, 3.55; N, 3.04%.
3-(4-Nitro-benzylidene)-5-(4-chloro-phenyl)-1-benzyl-2(3H)-pyrrolone (34)
Reddish brown crystals, yield 70%, mp 202°C; IR (cm−1, KBr): 1739, 1613, 795; 1H-NMR (CDCl3) δ 4.83 (s, 2H, CH2), 6.21 (s, 1H, pyrrolone ring), 7.24 (m, 5H, benzyl), 7.42 and 7.65 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.51 (s, 1H, olefinic H), 7.55 and 8.08 (d, each, J = 8.1 Hz, A2B2, arylidene ring). MS: m/z 416 (M+) not observed, 325, 138, 91, 77. Anal. Calcd. for C24H17ClN2O3: C, 69.15; H, 4.11; N, 6.72; found: C, 68.97; H, 4.09; N, 6.70%.
3-(4-Fluoro-benzylidene)-5-(4-chloro-phenyl)-1-benzyl-2(3H)-pyrrolone (35)
Dark red needles, yield 72%, mp 142°C; IR (cm−1, KBr): 1736, 1606, 813; 1H-NMR (CDCl3) δ 4.85 (s, 2H, CH2), 6.48 (s, 1H, pyrrolone ring), 7.18 and 7.39 (d, each, J = 8.4 Hz, A2B2, arylidene ring), 7.28 (m, 5H, benzyl), 7.43 and 7.61 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.45 (s, 1H, olefinic H). MS: m/z 389 (M+), 298, 138, 91, 77. Anal. Calcd. for C24H17ClFNO: C, 73.94; H, 4.40; N, 3.59; found: C, 73.68; H, 4.38; N, 3.40%.
3-(4-Hydroxy-3-methoxy-benzylidene)-5-(4-chloro-phenyl)-1-benzyl-2(3H)-pyrrolone (36)
Brown flakes, yield 74%, mp 154°C; IR (cm−1, KBr): 1748, 1606, 815; 1H-NMR (CDCl3) δ 3.92 (s, 3H, OCH3), 5.8 (s, 1H, OH), 4.84 (s, 2H, CH2), 6.26 (s, 1H, pyrrolone ring), 7.25 (m, 5H, benzyl), 7.36 and 7.65 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.34 (m, 1H, H-5, arylidene ring), 7.43 (s, 1H, olefinic H), 7.57 (m, 2H, H-2, 6, arylidene ring). MS: m/z 417 (M+), 326, 138, 91, 77. Anal. Calcd. for C25H20ClNO3: C, 71.86; H, 4.82; N, 3.35; found: C, 71.67; H, 4.80; N, 3.33%.
3-Benzylidene-5-(4-ethyl-phenyl)-1-benzyl-2(3H)-pyrrolone (37)
Light brown flakes, yield 72%, mp 146°C; IR (cm−1, KBr): 1749, 1611, 807; 1H-NMR (CDCl3) δ 1.28 (t, 3H, CH3), 2.75 (q, 2H, CH2), 4.84 (s, 2H, CH2), 6.21 (s, 1H, pyrrolone ring), 7.07 and 7.65 (d, each, J = 8.1 Hz, A2B2, phenyl), 7.24 (m, 5H, benzyl), 7.46 (m, 3H, H-3, 4, 5, arylidene ring), 7.55 (s, 1H, olefinic H), 7.62 (m, 2H, H-2, 6, arylidene ring). MS: m/z 365 (M+), 105, 91, 77. Anal. Calcd. for C26H23NO: C, 85.45; H, 6.34; N, 3.83; found: C, 85.38; H, 6.63; N, 4.05%.
3-(4-Nitro-benzylidene)-5-(4-ethyl-phenyl)-1-benzyl-2(3H)-pyrrolone (38)
Reddish brown crystals, yield 70%, mp 132°C; IR (cm−1, KBr): 1731, 1608, 796; 1H-NMR (CDCl3) δ 1.27 (t, 3H, CH3), 2.71 (q, 2H, CH2), 4.83 (s, 2H, CH2), 6.21 (s, 1H, pyrrolone ring), 7.26 (m, 5H, benzyl), 7.22 and 7.58 (d, each, J = 8.4 Hz, A2B2, phenyl), 7.57 (s, 1H, olefinic H), 7.51 and 8.11 (d, each, J = 8.1 Hz, A2B2, arylidene ring). MS: m/z 410 (M+) not observed, 105, 91, 77. Anal. Calcd. for C26H22N2O3: C, 76.08; H, 5.40; N, 6.82; found: C, 76.22; H, 5.15; N, 6.61%.
3-(4-Methoxy-benzylidene)-5-(4-ethyl-phenyl)-1-benzyl-2(3H)-pyrrolone (39)
Light red crystals, yield 78%, mp 178°C; IR (cm−1, KBr): 1698, 1601, 821; 1H-NMR (CDCl3) δ 1.26 (t, 3H, CH3), 2.74 (q, 2H, CH2), 3.82 (s, 3H, OCH3), 4.85 (s, 2H, CH2), 6.23 (s, 1H, pyrrolone ring), 6.96 and 7.44 (d, each, J = 8.4 Hz, A2B2, arylidene ring), 7.21 (m, 5H, benzyl), 7.48 (s, 1H, olefinic H), 7.55 and 7.83 (d, each, J = 8.1 Hz, A2B2, phenyl). MS: m/z 395 (M+), 91, 77. Anal. Calcd. for C27H25NO2: C, 82.00; H, 6.37; N, 3.54; found: C, 82.22; H, 6.60; N, 3.22%.
Microbiology
Antibacterial activity
The newly prepared compounds were screened for their antibacterial activity against Escherichia coli (ATCC-8739), Staphylococcus aureus (ATCC-29737), and Pseudomonas aeruginosa (NCLM-2035) bacterial strains at a concentration of 100 μg mL−1 by the cup plate method. Compounds inhibiting growth of one or more of the above microorganisms were further tested for minimum inhibitory concentration (MIC). The test was carried out according to the turbidity methodCitation22. Ciprofloxacin was used as the standard drug for comparison. Minimum inhibitory concentrations (MICs) were determined by the broth dilution technique. A solution of the compounds was prepared in dimethylformamide (DMF) and a series of doubling dilutions prepared with sterile pipettes. To each of a series of sterile stoppered test tubes, a standard volume of nutrient broth medium was added. A control tube containing no antimicrobial agent was included. The inoculum consisting of an overnight broth culture of microorganisms was added to separate tubes. The tubes were incubated at 37°C for 24 h and examined for turbidity. The lowest concentration (highest dilution) required to arrest the growth of bacteria was regarded as the MIC.
Antifungal activity
The antifungal activity of the compounds was determined against Candida albicans, Aspergillus niger, and Rhizopus oryza by the agar diffusion methodCitation23,24. Sabouraud’s agar medium was prepared by dissolving peptone (1 g), d-glucose (4 g), and agar (2 g) in distilled water (100 mL) and adjusting the pH to 5.7. Normal saline was used to make a suspension of spore of the fungal strain for lawning. A loopful of a particular fungal strain was transferred to 3 mL saline to get a suspension of the corresponding species. Agar medium (20 mL) was poured into each Petri dish. The excess of suspension was decanted and the plates were dried by placing in an incubator at 37°C for 1 h. Wells were made using an agar punch, and each well was labeled accordingly. A control was also prepared in triplicate and maintained at 37°C for 3–4 days. The fungal activity of each compound was compared with griseofulvin as the standard drug. The nutrient broth, which contained a logarithmic serially two-fold diluted amount of test compound and control, was inoculated with approximately 1.6 × 104–6 × 104 cfu mL−1. The cultures were incubated for 48 h at 37°C and the growth was monitored. The lowest concentration (highest dilution) required to arrest the growth of fungus was regarded as the minimum inhibitory concentration (MIC).
Results and discussion
Chemistry
Synthesis of the butenolides 3–19 was brought about by a single step (one pot) reaction using modified Perkin reaction conditions. Overall, 37 new compounds were prepared as outlined in . 2-Arylidene-4-(4-chloro/ethyl-phenyl)but-3-en-4-olides 3–19 were synthesized from 3-(4-chloro-benzoyl)propionic acid 1 or 3-(4-chloro-benzoyl)propionic acid 2 by reacting with aromatic aldehydes in the presence of triethylamine in acetic anhydrideCitation13. The 3-arylidene-5-(4-chloro/ethyl-phenyl)-2(3H)-pyrrolones 20–31 were prepared by reacting the appropriate butenolide with dry ammonia gas in absolute ethanol. The 3-arylidene-5-(chloro/ethyl-phenyl)-1-benzyl-2(3H)-pyrrolones 32–39 were synthesized by reacting the appropriate butenolide with benzylamine in dry benzene to give γ-ketobenzylamides, which were then lactamized in 6 N HCl to give the corresponding benzylpyrrolonesCitation13. Calculations of δ values using incremental parameters for the hydrogen (semicyclic double bond) seem to suggest (E)-configuration. The structures assigned to the compounds were supported by the results of elemental analyses as well as IR, 1H-NMR, and mass spectral data.
In the 1H-NMR spectral data all the compounds showed two singlets of one proton each around δ 6.5 and δ 7.4, which could be assigned to the ring βH and the olefinic hydrogen of the arylidene substituent. The mass spectra of 2-arylidene-4-(4-chloro/ethyl-phenyl)but-3-en-4-olides (3–19) showed an M+ peak at reasonable intensity. The major fragment appears to be R-C6H4-C≡O+ (R = Cl/C2H5) arising from the heterocyclic oxygen and γ-carbon with its substituent. Subsequently it loses CO to give R-C5H4+. There appeared a peak at m/z 77 that corresponds to C6H5+. Occasionally the aryl ring of the arylidene moiety also appeared as Ar+. In the case of pyrrolones (20–31), the major fragmentation is through R-C6H4-C≡N+H (R = Cl/C2H5), which is followed by loss of HCN to give R-C6H4+. In the case of benzylpyrrolones (32–39), loss of 91 mass units corresponding to the benzyl moiety from the molecular ion is observed alongwith peaks at m/z 91, 77. The other pathway is via R-C6H4-C≡N+H (R = Cl/C2H5) arising from C-2 and its substituent, which appears to be novel. This also loses HCN to give R-C6H4+ and then C6H5+. The molecular ion peak, or of its fragments having chloro-substituent(s), appeared as a cluster of peaks.
Microbiology
The newly prepared compounds were screened for their antibacterial activity against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa bacterial species, and antifungal activity against Candida albicans, Rhizopus oryza, and Aspergillus niger. The antibacterial and antifungal screening data showed that compound 22 exhibited very good activity against E. coli, P. aeruginosa, and R. oryza, with MIC 6.25 μg mL−1. A similar type of activity was shown by compound 27 against S. aureus, C. albicans, and A. niger, with MIC 6.25 μg mL−1. Another compound, 23, was active against C. albicans and A. niger, with MIC 6.25 μg mL−1. Compound 24 was also very good in its action against P. aeruginosa and R. oryza at 6.25 μg mL−1 concentration. The results are presented in .
Table 1. Antibacterial and antifungal activity (MIC, μg mL−1).
Analysis of the results showed that the synthesized compounds had better activity against fungal strains in comparison to the bacterial strains. The introduction of nitrogen in place of the oxygen atom (pyrrolones) in the butenolide ring significantly enhanced the antimicrobial action. This change in activity may be due to the proton donor capacity of pyrrolones. The introduction of the benzylamine moiety in place of the oxygen atom (benzylpyrrolones) in the butenolide ring decreased the antimicrobial action. Compounds derived from 3-(4-chlorobenzoyl)propionic acid 1 were found to have better antimicrobial activity than those derived from 3-(4-ethylbenzoyl)propionic acid 2.
After analyzing the results, it is conceivable that the derivatives showing significant antimicrobial activity can be further modified to exhibit better potency than the standard drugs. The butenolide and pyrrolone derivatives discovered in this study may provide valuable therapeutic intervention for the treatment of microbial diseases.
Acknowledgements
This article was partially presented at the 56th Indian Pharmaceutical Congress (56-IPC), Kolkata, India.
Financial support provided by the AICTE, New Delhi, under the RPS scheme, is gratefully acknowledged. We are also thankful to Prof. (Mrs.) P. K. Pillai, Head, Department of Microbiology, Majeedia Hospital, New Delhi, for help in performing antimicrobial studies of the compounds.
Declaration of interest: The authors report no conflicts of interest.
References
- Mukku VJ, Speitling M, Laatsch H, Helmke E. New butenolides from two marine Streptomycetes. J Nat Prod 2000;63:1570–2.
- Beck B, Magnin-Lachaux M, Herdtweck E, Domling A. A novel three-component butenolide synthesis. Org Lett 2001;3:2875–8.
- Repke KRH. Toward the discovery of digitalis derivatives with inotropic selectivity. Drug Discov Today 1997;2:110–16.
- Zapf S, Anke T, Sterner O. Incrustoporin, a new antibiotic from Incrustoporia carneola (Bres. ) Ryv. (Basidiomycetes). Acta Chem Scand 1995;49:223–34.
- Rajadhyaksha VD, Dahanukar SA. Rofecoxib: a new selective COX-2 inhibitor. Drug Rev 2001;47:77–8.
- Braun D, Pauli N, Sequin U, Zahner H. New butenolides from the photoconductivity screening of Streptomyces antibioticus (Waksman and Woodruff) Waksman and Henrici 1948 FEMS. Microbiol Lett 1995;126:37–42.
- Guo YW, Gavagnin M, Mollo E, Trivellone E, Cimino G. Three new butenolide lipids from the Caribbean Gorgonian Pterogorgia anceps. J Nat Prod 1999;62:1194–6.
- Yamada Y, Nihira T. In: Barton D, Nakanishi K, eds. Microbial hormones and microbial chemical ecology. Comprehensive Natural Products Chemistry, Vol. 8. Oxford: Elsevier, 1998:377–413.
- Sakuda S, Tanaka S, Mizuno K, Nihira T, Yamada Y. Biosynthetic studies on virginiae butanolide A, a butyrolactone autoregulator from Streptomyces. Part 2. Preparation of possible biosynthetic intermediates and conversion experiments in a cell-free system. J Chem Soc, Perkin Trans 1993;1:2309–15.
- Khattab SA, Honsy M. Conversion of α-arylidene-γ-phenyl-δβ,γ-butenolides into nitrogen heterocycles. Indian J Chem 1980;19B:1038–43.
- Khan MSY, Husain A, Sharma S. Studies on butenolides: 2-arylidene-4-(substituted aryl)but-3-en-4-olides—synthesis, reactions and biological activity. Indian J Chem 2002;41B:2160–71.
- Husain A, Hasan SM, Lal S, Alam MM. Antibacterial and antifungal activities of 2-arylidene-4-(4-methylphenyl)but-3-en-4-olides and their pyrrolone derivatives. Indian J Pharm Sci 2006;68:536–538.
- Husain A, Khan MSY, Hasan SM, Alam MM. 2-Arylidene-4-(4-phenoxy-phenyl)but-3-en-4-olides: synthesis, reactions and biological activity. Eur J Med Chem 2005;40:1394–404.
- Bordner J, Rapoport H. Synthesis of 2,2′-bipyrroles from 2-pyrrolinones. J Org Chem 1965;30:3824–8.
- Birchall GR, Hughes CG, Rees AH. Newer syntheses of the pyoluteorin antibiotics. Tetrahedron Lett 1970;(56):4879–82.
- Dittami JP, Xu F, Qi H, Martin MW. Photocyclization of α,β-unsaturated amide aldehydes: synthesis of jatropham. Tetrahedron Lett 1995;(36):4201–4.
- Shiraki R, Sumino A, Tadano K, Ogawa S. Total synthesis of natural PI-091, a new platelet aggregation inhibitor of microbial origin. J Org Chem 1996;61:2845–52.
- Iwasawa N, Maeyama K. A highly efficient synthesis of (−)-PI-091 construction of the 4-alkoxy-2-butene-4-lactam skeleton from Fischer-type carbene complexes, alkynyllithiums, and tosyl isocyanate. J Org Chem 1997;62:1918–19.
- Davies J. Bacteria on the rampage. Nature 1996;383:219–20.
- Zareef M, Iqbal R, Arfan M. A novel synthesis and antimicrobial activity of 1-[(substituted-phenyl) sulfonyl]pyrrolidin-2-ones. J Enzyme Inhib Med Chem 2007;23:82–6.
- Jat JL, Ojha S, Bhambi D, Dhakar N, Talesara GL. Synthesis and characterization of biologically significant 5,5′-(1,4-phenylene)bis(1-N-alkoxyphthalimido-3-aryl-2-pyrazoline) derivatives. J Enzyme Inhib Med Chem 2008;23:882–7.
- Colle JG, Duguid JP, Fraser AG, Marmion BP. Laboratory strategies in diagnosis. In: Mackie TJ, MacCartney JE, eds. Practical Medical Microbiology, 13th ed. London: Churchill Livingstone, 1989;601–649.
- Khan ZK. In vitro and vivo screening techniques for bioactivity screening and evaluation. In: Proc. Int. Workshop UNIDO-CDRI, 1997, 210–11.
- Varma RS, ed. Antifungal Agents: Past, Present and Future Prospects. Lucknow, India: National Academy of Chemistry & Biology, 1998.