Abstract
The present study describes for the first time the in vitro properties (inhibition of NO production and anticholinesterase) of Phagnalon saxatile (L.) Cass. (Asteraceae). The methanolic extract showed antioxidant activity that was measured by DPPH assay and β-carotene bleaching test. The same extract inhibited NO production in the murine monocytic macrophage cell line RAW 264.7. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibition was assessed by modifications of Ellman’s method. Purification of the MeOH extract of P. saxatile allowed the isolation of phenolic compounds. Among them, the compounds that most effectively inhibited lipopolysaccharide-induced NO production were caffeic acid and methylchlorogenic acid, with IC50 values of 7 μg/mL and 12 μg/mL, respectively. Luteolin and 3,5-dicaffeoylquinic acid exhibited the most promising activity against AChE with an IC50 of 25.2 and 54.5 μg/mL, respectively, while caffeic acid and luteolin exhibited higher activity against BChE with an IC50 of 32.2 and 37.2 μg/mL, respectively.
Introduction
Oxidative damage, caused by the action of free radicals, may initiate and promote the progression of a number of chronic diseases, including cancer, cardiovascular diseases, inflammation, diabetes, and Alzheimer’s diseaseCitation1. Nitric oxide (NO•) is a diatomic free radical produced from l-arginine by constitutive and inducible nitric oxide synthase (cNOS and iNOS) in numerous mammalian cells and tissues. Nitric oxide (NO), superoxide (O2−), and their reaction product peroxynitrite (ONOO−) may be generated in excess during the host response against viral and antibacterial infections and contribute to some pathogenesis by promoting oxidative stress, tissue injury, and even cancerCitation2,Citation3. Recently, the effects of selected polyphenolics on NO production in lipopolysaccharide (LPS)-activated RAW 264.7 macrophage cells have been reported. These studies have shown that some polyphenolics or methanol extracts of plants inhibit NO productionCitation4–7. Most clinically important medicines belong to steroidal or non-steroidal anti-inflammatory chemical therapeutics for treatment of inflammation-related diseases. Though these have potent activity, long-term administration is required for the treatment of chronic disease. Furthermore, these drugs have various and severe adverse effects.
In recent years, oxidative stress has been described in the pathological changes that occur in Alzheimer’s disease (AD)Citation8,Citation9. Acetylcholine (ACh) is found in the synapse of the cerebral cortex, and a deficiency in the cerebral cortex is one of the major features seen in sufferers of ADCitation10. Acetylcholinesterase (AChE) inhibitors such as tacrine, donepezil, and the natural products rivastigmine and galantamine are currently the only effective treatment for AD. These approved drugs are limited in use due to their adverse side-effects, such as gastrointestinal disturbance, and bioavailability problemsCitation11–13. The continuing search for novel anticholinesterases from plants as therapeutic agents for dementia and other central nervous system disorders is motivated by the current need for new substances of natural origin targeted to the brain areas affected, with effectiveness in the described fields of application and low degrees of toxicity and side-effects for man.
The genus Phagnalon (Asteraceae) is represented by about 30 species distributed worldwide, six of which are typical of the Mediterranean regionCitation14. Different Phagnalon sp. are used in popular medicine, and a variety of extracts have been examinedCitation15–21. Among the Bedouins of the Negev desert, the bark of P. rupestre (L.) DC. is widely used to induce deliberate burns for the healing of various ailmentsCitation15, while in the Palestinian area the whole plant is used to treat asthma and headache, and as an analgesic for toothache. The aqueous and ethanolic extracts of this plant showed antimicrobial activity against both positive and negative bacteria and C. albicansCitation16. Phytochemical investigations on this species are mainly devoted to the study of P. rupestre, and report the presence of terpenoidsCitation22, flavonoidsCitation23, hydroquinone glycosides, and caffeoylquinic acid derivativesCitation24. Pharmacological studies have been led only on P. rupestre: prenylhydroquinone glucosides and caffeoylquinic esters from this plant have been studied for a variety of biological activities.
Phagnalon saxatile (L.) Cass. is one of five suffruticose chamaephyte species growing wild in Italy, mostly in southern ItalyCitation25. A recent ethnobotanical study classifies it as a nutritional plant utilized for medicinal purposesCitation26. A survey of the literature shows the presence in the aerial parts of the plant of an essential oil constituted mainly of sesquiterpenes, fatty acids, and waxesCitation27, 3,3-dimethylallyl-p-benzoquinoneCitation28, and flavonoids such as apigenin, apigenin-7-glucoside, and luteolinCitation22, but phytochemical data are incomplete and pharmacological information on the plant and its metabolites is lacking. The objectives of the present study were (1) to investigate the phytochemical characteristics of P. saxatile flowering aerial parts, which exhibit significant antioxidant, inhibition of NO production, and anticholinesterase activities, and (2) to relate these activities to pure isolated compounds.
Materials and methods
General experimental procedures
Nuclear magnetic resonance (NMR) spectra were acquired on a Bruker DRX-600 (Billerica, MA, USA) (1H at 599.19 MHz, 13C at 150.86 MHz) spectrometer: δ (ppm), J in Hz, spectra referred to CHD2OD as internal standard. Mass spectra were recorded with an API 2000 (Applied Biosystems, Foster City, CA, USA). Ultraviolet (UV) spectra were measured on a UV/VIS Jasco V530 spectrophotometer. Reverse-phase high performance liquid chromatography (HPLC) was performed with a TSP SpectraSeries P 100 equipped with a rheodyne injector and a refractive index detector, using a column C18 β-Bondapack (Waters, Milford, MA, USA; p.s. 10 μm, 300 × 7.8 mm, flow rate 2.5 mL/min). Thin layer chromatography (TLC) was performed on plates coated with silica gel 60 F254 (Merck, Whitehouse Station, NJ, USA), 0.25 mm. Elemental analysis was carried out with a PerkinElmer 240 apparatus. All solvents (analytical and deuterated grade) were purchased from Carlo Erba Reagenti, Milan, Italy.
Plant material
The flowering aerial parts of Phagnalon saxatile (L.) Cass. were collected in June 2005, in Cefalù, capo Playa (Sicily, Southern Italy). A voucher specimen of the plant (SN 49) is kept in the Herbarium Neapolitanum (NAP), Faculty of Pharmacy, University of Naples “Federico II”.
Extraction procedures and isolation of compounds
Plant material (452.5 g) was chopped and then sequentially extracted by cold maceration with 3 × 2.5 L of petroleum ether, CHCl3, and CH3OH. The methanolic solution was concentrated under reduced pressure obtaining a residue (15.8 g) that was chromatographed in 2 g lots on a Sephadex LH-20 (Pharmacia) column, eluting with CH3OH. Fractions of 20 mL were collected and analyzed by TLC using BuOH/CH3COOH/H2O (60:15:25, v/v/v) as eluent and Ce(SO4)2 in H2SO4 as spray reagent. Fractions 12–14 (2.20 g), 15, 16 (1.14 g), 17 (0.178 g), 18 (0.263 g), 19–22 (1.09 g), and 23–27 (0.525 g) were gathered according to TLC analysis and further purified by reverse HPLC: fraction 12–14 (CH3OH/H2O, 40:60, as eluent) yielded compound (1) (6.6 mg; Rt = 9 min) and compound (2) (1.4 mg; Rt = 10.5 min); fraction 15, 16 (CH3OH/H2O, 40:60, as eluent) yielded compounds (3) (55.2 mg; Rt = 6.5 min), (4) (15.0 mg; Rt = 9 min), and (5) (13.4 mg; Rt = 13.5 min); fraction 17 (CH3OH/H2O, 55:45, as eluent) yielded compound (6) (10.6 mg; Rt = 10.5 min); fraction 18 (CH3OH/H2O, 60:40, as eluent) yielded compound (7) (0.8 mg; Rt = 6.5 min); fraction 19–22 (CH3OH/H2O, 60:40, as eluent) yielded compounds (8) (130.5 mg; Rt = 4.5 min) and (9) (22.4 mg; Rt = 12.5 min); fraction 23–27 (CH3OH/H2O 70:30 as eluent) yielded compounds (10) (4.7 mg; Rt = 6.5 min) and (11) (4.3 mg; Rt = 7.5 min). The structures of the compounds were determined by comparison of their NMR and mass spectrometry (MS) data with literature valuesCitation24.
1,1-Diphenyl-2-picrylhydrazyl (DPPH) assay
This experimental procedure was adapted from Wang et al.Citation29, modified by our workgroup as reported previouslyCitation30. The absorbance was measured using a PerkinElmer Lambda 40 UV/VIS spectrophotometer at 517 nm against a blank, which was without DPPH. All tests were run in triplicate. Ascorbic acid was used as a positive control. A decrease of DPPH solution absorbance indicates an increase of DPPH radical scavenging activity. This activity is given as % DPPH radical-scavenging, calculated by the equation:
β-Carotene bleaching test
Antioxidant activity was determined using a modified β-carotene bleaching testCitation31. Briefly, 1 mL of β-carotene solution (0.2 mg/mL in chloroform) was added to 0.02 mL of linoleic acid and 0.2 mL of 100% Tween 20. After evaporation of chloroform and dilution with water, 5 mL of the emulsion was transferred into different test tubes containing 0.2 mL of samples in 70% ethanol at different concentrations. Standard (propyl gallate) at the same concentration as samples was used for comparison. The tubes were then gently shaken and placed at 45°C in a water bath for 60 min. The absorbance of the samples, standard, and control was measured at 470 nm using a PerkinElmer Lambda 40 UV/VIS spectrophotometer against a blank, consisting of an emulsion without β-carotene. The measurement was carried out at initial time (t = 0) and successively at 30 and 60 min. All samples were assayed in triplicate and results averaged. The antioxidant activity (AA) was measured in terms of successful bleaching of β-carotene by using the following equation:
where A0 and A°0 are the absorbance values measured at the initial incubation time for samples/standard and control, respectively, while At and A°t are the absorbance values measure in the samples/standard and control, respectively, at t = 30 min and t = 60 min.
Cell culture
The murine monocytic macrophage cell line RAW 264.7 (European Collection of Cell Cultures, London, UK) was grown in a plastic culture flask in Dulbecco’s modified Eagle’s medium (DMEM) with l-glutamine supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic/antimitotic solution (penicillin/streptomycin) under 5% CO2 at 37°C. After 4–5 days cells were removed from the culture flask by scraping and were centrifuged for 10 min at 1500 rpm. The medium was then removed and the cells were resuspended with fresh DMEM. Cell counts and viability were assessed using a standard trypan blue cell counting technique. The cell concentration was adjusted to 1 × 106 cells/mL in the same medium, and 100 μL of the above concentration was cultured in a 96-well plate for 1 day to become nearly confluent. Concentrations ranging from 10 to 100 μg/mL of the samples were prepared from stock solutions by serial dilution in DMEM to give a volume of 100 μL in each well of a microtiter plate (96-well). Then cells were cultured with vehicle, P. saxatile MeOH extract, and isolated compounds in the presence of 1 μg/mL LPS for 24 h.
Assay for cytotoxic activity
Cytotoxicity was determined using the 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma) assay reported by Tubaro et al.Citation32, with some modification. The assay for each concentration of samples was performed in triplicate and the culture plates were kept at 37°C with 5% (v/v) CO2 for 1 day. After 24 h of incubation, 100 μL of medium was removed from each well. Subsequently, 100 μl of 0.5% (w/v) MTT (Sigma, Italy), dissolved in phosphate buffered saline, was added to each well and allowed to incubate for a further 4 h. After that, 100 μL of dimethylsulfoxide (DMSO) was added to each well to dissolve the formazan crystals. Absorbance values at 550 nm were measured with a microplate reader (GDV DV 990 B/V; Roma, Italy). Cytotoxicity was expressed as CD50, which is the concentration to reduce the absorbance of treated cells by 50% compared with the control (untreated cells).
Inhibition of nitric oxide (NO) production in LPS-stimulated RAW 264.7 cells
The presence of nitrite, a stable oxidized product of NO, was determined in cell culture media using Griess reagent (1% sulfanamide and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in 2.5% H3PO4)Citation33; 100 μL of cell culture supernatant was removed and combined with 100 μL of Griess reagent in a 96-well plate, followed by spectrophotometric measurement at 550 nm using a microplate reader (GDV DV 990 B/V). The nitrite concentration in the supernatant was determined by comparison with a sodium nitrite standard curve.
Microtiter cholinesterase inhibition assay
Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibition was assessed by modification of Ellman’s methodCitation34, which is based on the reaction of released acetyl- or butyrylthiocholine to give a colored product with a chromogenic reagent. AChE or BChE 20 μL (0.20 U/mL in buffer pH 8) and P. saxatile isolated compounds (25 μL) were added to 50 μL of buffer, pH 8, and pre-incubated in an ice bath at 4°C for 30 min. Duplicate wells were also treated this way with 20 μL of physostigmine (0.1 mM) to allow interference of the test substances in the assay to be assessed, and to control for any hydrolysis of acetylcholine not due to enzyme activity. The reaction was started by adding dithionitrobenzene (DTNB) solution (125 μL of 0.05 mM in buffer, pH 7) and acetylthiocholine (ATCI) or butyrylthiocholine BTCI (25 μl, 0.018 mM in buffer, pH 7), and tubes were incubated in a water bath for 20 min at 37°C. The reaction was stopped by placing the assay solution tubes in an ice bath and adding physostigmine (20 μL, 0.018 mM in buffer, pH 7). Blanks were used of reagents without compounds, and a positive control was set up which was the same as the blank except that physostigmine (20 μL, 0.018 mM in buffer, pH 7) was added. The production of yellow anion was immediately recorded on a spectrophotometer at 405 nm. The inhibition rate (%) was calculated by the equation:
Results and discussion
Compound characteristics in P. saxatile MeOH extract
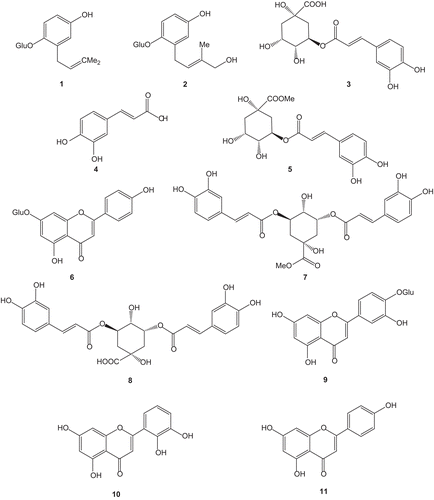
The major constituents in the MeOH extract were isolated using several repeated procedures of RP-18 silica gel HPLC. The structures of the known compounds (), 1-O-β-glucopyranosyl-2(3′,3′-dimethylallyl) hydroquinone (1), 1-O-β-glucopyranosyl-2(3′-hydroxymethyl-3′-methylallyl) hydroquinone (2), chlorogenic acid (3), caffeic acid (4), methylchlorogenic acid (5), apigenin 7-O-β-glucopyranoside (6), 3,5-di-O-caffeoylquinic acid methyl ester (7), 3,5-di-O-caffeoylquinic acid (8), luteolin-4′-O-glucopyranoside (9), luteolin (10), and apigenin (11), were determined by comparison of their NMR and MS data with literature valuesCitation22. All these compounds have been previously investigated for their antioxidant activity. In fact, Chiang et al.Citation35 have accredited to chlorogenic acid, caffeic acid, and 3,5-di-O-caffeoylquinic acid a very high antioxidant activity. The antioxidant activity of flavonoids was confirmed by Bae et al.Citation36. The compounds present in a sufficient amount (3–6, 8–11) were investigated for their anti-inflammatory and anticholinesterase activities in order to relate biological activities of the extract to pure isolated compounds.
Free radical scavenging and antioxidant activity
The DPPH radical-scavenging activity of all samples was measured. The effect of antioxidants on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was thought to be due to their hydrogen-donating ability. The scavenging effects of the methanolic extract and fractions on DPPH were examined at different concentrations (10, 25, 50, 100, 250, 500, and 1000 μg/mL). As shown in , the methanolic extract showed significant activity (IC50 25 μg/mL). Antioxidant activity, which was demonstrated by the ability of the samples to inhibit the bleaching of β-carotene, was measured and compared to that of the control, which contained no antioxidant component. The methanolic extract of P. saxatile inhibited oxidation of linoleic acid. As shown in , the methanolic extract showed antioxidative activity after 30 min of incubation as well as after 60 min of incubation (IC50 14 μg/mL and 41 μg/mL, respectively), indicating that its activity was not correlated with time of heating.
Table 1. Free radical scavenging and lipid peroxidation inhibition of methanolic extract from P. saxatile.
Inhibition of NO production
The anti-inflammatory activity of P. saxatile MeOH extract and isolated compounds was studied in vitro, analyzing their inhibitory effects on chemical mediators released from macrophages. Once activated by inflammatory stimulation, macrophages produce a large number of cytotoxic molecules. Treatment of RAW 264.7 macrophages with LPS (1 μg/mL) for 24 h induces NO production, as assessed by measuring the accumulation of nitrite, a stable metabolite of NO, in the medium, based on the Griess reaction. NO, a macrophage-derived mediator, is considered to play a key role in the inflammatory response, based on its occurrence at inflammatory sites and its ability to induce many of the hallmarks of the inflammatory response. The beneficial effect of P. saxatile extract on inhibition of the production of inflammatory mediators in macrophages can be mediated through oxidative degradation of the products of phagocytes, such as O2− and HOCl. As shown in , incubation of RAW 264.7 cells with the methanol extract and isolated compounds of P. saxatile induced a significant inhibitory effect on LPS-induced nitrite production. The MeOH extract of P. saxatile showed significant inhibition of LPS-induced NO production in RAW 264.7 cells in a dose-dependent manner, with an IC50 value of 82 μg/mL. The pure compounds that most effectively inhibited LPS-induced NO production were caffeic acid (4) and methylchlorogenic acid (5), with IC50 values of 7 μg/mL and 12 μg/mL, respectively (). Although the anti-inflammatory activity of caffeic acid has already been studiedCitation37, methylchlorogenic acid was investigated for the first time. In contrast with Wang and MazzaCitation38, who showed that chlorogenic acid has no inhibitory effect, but on the contrary may induce the production of NO, we found that both chlorogenic acid and its methyl ester showed anti-inflammatory activity. As regards flavonoids, several articlesCitation39,Citation40 have discussed their anti-inflammatory activity, particularly of apigenin and luteolin. No data are available for the glucopyranosides of these compounds, which in our study showed significant activity on NO production. In fact luteolin-4′-glucopyranoside (9) showed an IC50 value of 30 μg/mL, while apigenin-7-glucopyranoside (6) showed an IC50 value of 25 μg/mL. Phenolic compounds are one of the most important groups of secondary metabolites in edible plants. Epidemiological studies have indicated that naturally occurring phenolic compounds may play an important role in the maintenance of human health and prevention of several diseasesCitation41. This is also believed to be correlated to the anti-oxidative properties of phenolics. In particular, flavonoids have a broad spectrum of biological properties, e.g. antinflammatory. It has been previously reported that the presence of free hydroxyls on a flavonoid nucleus enhances the inhibition of inflammation, whereas substitution by methoxy groups or sugars diminishes the activityCitation42. Based on structure and activity relationship analysis, we observed that substitution of the C4′ hydroxyl group with glucose, as in the case of luteolin-4′-O-glucopyranoside (9), and substitution of the C7 in the case of apigenin 7-O-β-glucopyranoside (6), resulted in an approximately two-fold increase in IC50 values for inhibition of NO production relative to that of luteolin (10) or apigenin (11), containing free hydroxyl groups in the C4′ and C7 position, respectively.
Table 2. Inhibition of NO production in LPS-induced RAW 264.7 macrophages by methanolic extract and compounds isolated from P. saxatile.
Cytotoxicity
The cytotoxic effect of all samples (P. saxatile MeOH extract and isolated compounds) in the presence of LPS (10 μg/mL) was evaluated. The P. saxatile MeOH extract and isolated compounds did not show any cytotoxicity at concentrations of 10–100 μg/mL.
Anticholinesterase activity
This article reports for the first time the cholinesterase inhibitory properties of P. saxatile and derived compounds (). Results evidenced that the MeOH extract was able to inhibit BChE (IC50 523.75 μg/mL) but was unable to affect AChE activity (). As regards the isolated compounds, aglycons were more potent than glycosides in inhibition of the enzyme; in fact IC50 against AChE was 25.2 μg/mL for luteolin and 66.1 μg/mL for luteolin-4′-O-glucopyranoside, and 27.5 and 102.8 μg/mL for apigenin and apigenin-4-glycoside, respectively. Luteolin exhibited the same pattern also against BChE (IC50 of 37.2 and 99.5 μg/mL for aglycon and glycoside, respectively). Previously, our research group reported the ability of linariin and isolinariin A and B, three flavones isolated from Linaria reflexa, to inhibit AChE in a dose-dependent mannerCitation43. Moreover, recent studies have examined the permeability of flavonoids and their known circulating metabolites across the blood–brain barrier (BBB). Flavonoids are hydrolyzed in the gut and then glucuronized, so it is quite possible that a glycosidal form reaches the central nervous system (CNS)Citation44. In particular, Youdim et al.Citation45 reported that flavonoids from particular families are able to permeate the BBB, whereas the entry of others is limited by the actions of efflux transporters expressed at the endothelium surface. However, it should be noted that penetration of the BBB does not necessarily equate to entry into neurons, where flavonoids are believed to elicit their neuroprotective effects. The inhibitory activity of caffeic acid against AChE and BChE is also reported (IC50 of 32.4 and 32.2 μg/mL, respectively). Previously, Borrelli et al.Citation46 showed the ability of caffeic acid phenethyl ester (CAPE) to inhibit AChE. Luteolin and 3,5-dicaffeoylquinic acid exhibited the most promising activity against AChE with IC50 of 25.2 and 54.5 μg/mL, respectively.
Table 3. Cholinesterase inhibitory activity of methanolic extract and compounds isolated from P. saxatile.
Conclusions
Oxidative damage may initiate and promote the progression of a number of chronic diseases, including inflammation and Alzheimer’s disease. The present work showed for the first time the in vitro activity of P. saxatile methanolic extract in the treatment of inflammation and Alzheimer’s disease and its metabolite profile. The beneficial effects may be attributable to the ability of P. saxatile extract to act in different ways. In this study we have demonstrated that the MeOH extract of P. saxatile exhibited significant antioxidant activity and an inhibitory effect on NO production (an inflammatory mediator) in macrophages. Furthermore, anticholinesterase assays on the methanolic extract and isolated phenolic compounds showed that luteolin was the most potent inhibitor of both AChE and BChE. We also observed that the extract of P. saxatile could be a good source of phenolic compounds, which are one of the most bioactive groups of secondary metabolites in plants. The metabolism and bioavailability of these phenolic compounds in vivo are suggested to correlate well to their observed antioxidant properties in vitro.
Therefore, we propose here the potential benefits of P. saxatile extract on the basis of the phytochemical characteristics and the observed bioactive properties. Naturally occurring phytocompounds possess antioxidative, anti-inflammatory, and anticholinesterase properties, which appear to contribute to their chemopreventive or chemoprotective activity. Further studies of the plant extracts and/or the identified compounds from P. saxatile on the pharmacokinetics or mode of action on mechanisms of chemopreventive properties are warranted.
Acknowledgement
The authors thank the CSIAS of University of Naples “Federico II” for technical assistance.
Declaration of interest: The authors report no conflicts of interest.
References
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, 2nd ed. Oxford: Oxford University Press, 1989.
- Maeda H, Akaike T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry 1998;63:854–65.
- Akaike T, Fujii S, Kato A, Yoshitake J, Miyamoto Y, Sawa T, et al. Viral mutation accelerated by nitric oxide production during infection in vivo. FASEB J 2000;14:1447–54.
- Kobuchi H, Droy-Lefaix MT, Christen Y, Packer L. Ginkgo biloba extract (EGb 761): inhibitory effect on nitric oxide production in the macrophage cell line RAW 264.7. Biochem Pharmacol 1997;53:897–903.
- Kim OK, Murakami A, Nakamura Y, Ohigashi H. Screening of edible Japanese plants for nitric oxide generation inhibitory activities in RAW 264.7 cells. Cancer Lett 1998;125:199–207.
- Lin YL, Tsai SH, Lin-Shiau SY, Ho CT, Lin JK. Theaflavin-3,3′-digallate from black tea blocks the nitric oxide synthase by down-regulating the activation of NF-B in macrophages. Eur J Pharmacol 1999;367:379–88.
- Wadsworth T, Koop DR. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem Pharmacol 1999;57:941–9.
- Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus. J Biol Chem 1998;273:1216–18.
- Pratico D, Delanty N. Oxidative injury in diseases of the central nervous system: focus on Alzheimer’s disease. Am J Med 2000;109:577–85.
- Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, et al. Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem 1995;64:749–60.
- Schulz V. Ginkgo extract or cholinesterase inhibitors in patients with dementia: what clinical trials and guidelines fail to consider. Phytomedicine 2003;4:74–9.
- Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA 1997;278:1363–71.
- Melzer D. New drug treatment for Alzheimer’s disease: lessons for healthcare policy. BMJ 1998;316:762–4.
- Tutin TG, Heywoo VH, Burgess NA, More DM, Valentine DH, Walters SM, et al. Flora Europaea, Vol. 4. Cambridge: Cambridge University Press, 1976:133.
- Friedman J, Yaniv Z, Dafni A, Palewitch D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev desert, Israel. J Ethnopharmacol 1986;16:275–87.
- Ali-Shtayeh MS, Yaghmour MR, Faidi YR, Salem K, Al-Nuri MA. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J Ethnopharmacol 1998;60:265–71.
- Góngora L, Giner RM, Máñez S, Recio MdC, Ríos JL. Phagnalon rupestre as a source of compounds active on contact hypersensitivity. Planta Med 2002;68:561–4.
- Góngora L, Giner RM, Máñez S, Recio MdC, Schinella G, Ríos JL. Effects of caffeoyl conjugates of isoprenyl-hydroquinone glucoside and quinic acid on leukocyte function. Life Sci 2002;71:2995–3004.
- Góngora L, Máñez S, Giner RM, Recio MdC, Gray AI, Ríos JL. Phenolic glycosides from Phagnalon rupestre. Phytochemistry 2002;59:857–60.
- Olmos A, Manez S, Giner RM, Recio MdC, Rios JL. Isoprenylhydroquinone glucoside: a new non-antioxidant inhibitor of peroxynitrite-mediated tyrosine nitration. Nitric Oxide 2005;12:54–60.
- Olmos A, Manez S, Giner RM, Recio MdC, Rios JL. Protein tyrosine nitration induced by heme/hydrogen peroxide: inhibitory effect of hydroxycinnamoyl conjugates. Planta Med 2007;73:20–6.
- Bicchi C, Frattini C, Nano GM, Tira S. Hexahydrofarnesylacetone from Phagnalon rupestre. Relata Technica 1979;11:64.
- Dolci M, Tira S. Flavonoids of Gnaphalieae: Phagnalon species. Atti dell’Accademia delle Scienze di Torino, Classe Sci Fis Mater Nat 1982;116:315–18.
- Góngora L, Giner RM, Máñez S, Recio MdC, Ríos JL. New prenylhydroquinone glycosides from Phagnalon rupestre. J Nat Prod 2001;64:1111–13.
- Pignatti S. Flora d’Italia, Vol. 3. Bologna: Edagricole, 1982:40–1.
- Lentini F, Venza F. Wild food plants of popular use in Sicily. J Ethnobiol Ethnomed 2007;3:15–27.
- Senatore F, Formisano C, Grassia A, Rigano D, Bellone G, Bruno M. Chemical composition and antibacterial activity of the essential oil of Phagnalon saxatile (L.) Cass. (Asteraceae) growing wild in Southern Italy. J Essent Oil Bearing Plants 2005;8:258–63.
- Bohlmann F, Kleine KM. über ein neues chinon aus höheren pflanzen. Chem Ber 1966;99:885–8.
- Wang M, Li J, Rangarajan M, Shao Y, La Voie EJ, Huang CT. Antioxidative phenolic compounds from sage (Salvia officinalis). J Agric Food Chem 1998;46:4869–73.
- Conforti F, Loizzo MR, Statti GA, Menichini F. Comparative radical scavenging and antidiabetic activities of methanolic extract and fractions from Achillea ligustica ALL. Biol Pharm Bull 2005;28:1791–4.
- Amin I, Zamaliah MM, Chin WF. Total antioxidant activity and phenolic content in selected vegetables. Food Chem 2004;87:581–6.
- Tubaro A, Florio C, Luxich E, Vertua R, Della Loggia R, Yasumoto T. Suitability of the MTT-based cytotoxicity assay to detect okadaic acid contamination of mussels. Toxicon 1996;34:965–74.
- Prakash H, Ali A, Bala M, Goel HC. Anti-inflammatory effects of Podophyllum hexandrum (RP-1) against lipopolysaccharides induced inflammation in mice. J Pharm Pharm Sci 2005;8:107–14.
- Conforti F, Statti GA, Tundis R, Loizzo MR, Menichini F. In vitro activities of Citrus medica L. cv. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer’s disease. Phytother Res 2007;21:427–33.
- Chiang YM, Chuang DY, Wang SY, Kuo YH, Tsai PW, Shyur LF. Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J Ethnopharmacol 2004;95:409–19.
- Bae K, Jin WY, Thuong TP, Min BS, Na MK, Lee MY, et al. A new flavonoid glycoside from the leaf of Cephalotaxus koreana. Fitoterapia 2007;78:409–13.
- Shin KM, Kim IT, Park YM, Ha J, Choi JW, Park HJ, et al. Anti-inflammatory effect of caffeic acid methyl ester and its mode of action through the inhibition of prostaglandin E2, nitric oxide and tumor necrosis factor-alpha production. Biochem Pharmacol 2004;68:2327–36.
- Wang J, Mazza G. Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor alpha in LPS/IFN-gamma-activated RAW 264.7 macrophages. J Agric Food Chem 2002;50:4183–9.
- Shanmugam K, Holmquist L, Steele M, Stuchbury G, Berbaum K, Schulz O, et al. Plant-derived polyphenols attenuate lipopolysaccharide-induced nitric oxide and tumour necrosis factor production in murine microglia and macrophages. Mol Nutr Food Res 2008;52:427–38.
- Ko H, Weng JR, Tsao LT, Yen MH, Wang JP, Lin CN. Anti-inflammatory flavonoids and pterocarpanoid from Crotalaria pallida and C. assamica. Bioorg Med Chem Lett 2004;14:1011–14.
- Rose JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 2002;22:19–34.
- Arora A, Nair MG, Strasburg GM. Structure-activity relationships for antioxidant activities of series of flavonoids in a liposomal system. Free Radic Biol Med 1998;24:1355–63.
- Loizzo MR, Tundis R, Menichini F, Bonesi M, Statti GA, Deguin B, et al. Acetyl-cholinesterase inhibition by extracts and isolated flavones from Linaria reflexa Desf. (Scrophulariaceae). Nat Prod Com 2007;2:759–63.
- Rice-Evans C. Flavonoids and isoflavones: absorption, metabolism, and bioactivity. Free Radic Biol Med 2004;36:827–8.
- Youdim KA. Flavonoids and the brain: interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med 2004;37:1683–93.
- Borrelli F, Posadas I, Capasso R, Aviello G, Ascione V, Capasso F. Effect of caffeic acid phenethyl ester on gastric acid secretion in vitro. Eur J Pharmacol 2005;521:139–43.