Abstract
Nineteen 5-nitrothiazolylthiosemicarbazones were synthesized from 5-nitrothiazole by three-step synthesis and evaluated for in vitro activities against seven mycobacterial species. Among them, N-(5-nitro-1, 3-thiazol-2-yl)-2-((Z)-4-[(phenylmethyl)oxy]phenylmethylidene)hydrazine-1-carbothioamide (4m) was found to be the most active compound with a minimum inhibitory concentration (MIC) of 0.23 μM against Mycobacterium tuberculosis H37 Rv, and was three times more potent than isoniazid and equally active as rifampicin. Compound 4m also inhibited six non-tubercular mycobacteria with MICs ranging from 1.88 to 30.25 μM.
Introduction
Mycobacterium tuberculosis (MTB), a human pathogen causing tuberculosis (TB), is responsible for the deaths of millions of people every year, and continues to claim more lives than any other single infectious agentCitation1. A chief reason that TB persists as a global killer and is on the rise in parts of the world is that existing antibiotics require up to 6 months of daily use, making it difficult for people to complete the treatment. Those who miss doses, in turn, fuel the emergence of drug-resistant strains of MTB (MDR-TB) that cause the illness. The treatment of MDR-TB is characterized by relatively less effective, poorly tolerated, and expensive drugs that may need to be administered for yearsCitation2. A key element in these events is the explosive combination of TB and the human immune-deficiency virus (HIV) epidemicCitation3. This convergence of HIV and TB poses difficult problems, not only because the viral infection increases mortality from TB, but also because optimization of antiviral and antimycobacterial drugs given together presents further difficulties: for example, rifampicin inhibits RNA polymerase but also induces CYP450 enzymes, which metabolize certain anti-HIV drugs. These problems illustrate the importance but also the difficulties inherent in finding new drugs to treat TB. Even in terms of drug-sensitive disease, the duration of therapy is still very long, and the drugs used have toxic effects. Unfortunately, the last truly novel drug, rifampicin, approved for the treatment of TB was discovered 45 years ago; this partially explains the inadequacy of the present armamentarium of antitubercular drugs. Earlier we reported the antitubercular activities of various oxazolyl and N-hydroxy-thiosemicarbazones which inhibited MTB with a minimum inhibitory concentration (MIC) of 0.05 μg/mLCitation4,Citation5. In continuation we present herein preliminary results concerning the synthesis and in vitro antimycobacterial activities of novel 5-nitrothiazolylthiosemicarbazones.
Materials and methods
Chemistry
Melting points were measured on an electrothermal melting point apparatus (Buchi BM530) in open capillary tubes and are uncorrected. 1H-nuclear magnetic resonance (NMR) spectra were scanned on a Jeol Fx 400 MHz NMR spectrometer using dimethylsulfoxide (DMSO)-d6 as solvent. Chemical shifts are expressed in δ (ppm) relative to tetramethylsilane. Elemental analyses (C, H, and N) were performed on a PerkinElmer model 240C analyzer and the data were within ±0.4% of the theoretical values.
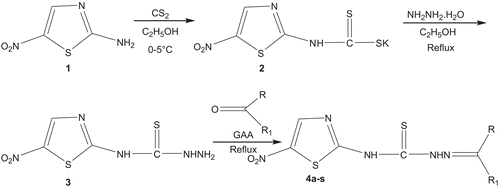
Synthesis of N-(5-nitrothiazol-2-yl)hydrazinecarbothioamide (3)
To a solution of 2-amino-5-nitrothiazole (0.01 mol) in absolute ethanol (20 mL) were added potassium hydroxide (0.01 mol) and carbon disufhide (0.75 mL), and the mixture was stirred at 0–5°C for 1 h to form the potassium salt of dithiocarbamate (2). To the stirred mixture of dithiocarbamate salt was added hydrazine hydrate (0.01 mol) and the mixture was refluxed for 3 h with stirring. Completion of the reaction was monitored using thin layer chromatography (TLC). Ethanol was distilled off and finally crystals of thiosemicarbazide were filtered, with yield: 92%; M.P.: 150–152°C.
General method for the synthesis of thiosemicarbazones (4a–s)
To a solution of 3 (0.003 mol) in dimethylformamide (DMF), an appropriate aldehyde or ketone was added. The pH was maintained at 4–5 by adding glacial acetic acid (GAA). The mixture was refluxed with stirring for 10–15 h and completion of the reaction was monitored using TLC. The mixture was added to crushed ice to obtain final target products 4a–s. Product was recrystallized from 95% ethanol.
N-(5-Nitro-1,3-thiazol-2-yl)-2-[(Z)-phenylmethylidene]hydrazine-1-carbothioamide (4a)
Yield: 40.8%; M.P.: > 250°C; 1H-NMR (DMSO-d6) δ (ppm): 7.12–7.17 (m, 1H, Ar-H), 7.27–7.31 (m, 2H, Ar-H), 7.42–7.45 (m, 2H, Ar-H), 7.80 (s, 1H, benzylidine H), 7.99 (s, 1H, H of thiazole), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C11H9N5O2S2; Calculated for C, 42.99; H, 2.95; N, 22.79; Found: C, 43.04; H, 2.90; N, 22.76%.
2-[(Z)-(2-Hydroxyphenyl)methylidene]-N-(5-nitro-1,3-thiazol-2-yl)hydrazine-1-carbothioamide (4b)
Yield: 50.3%; M.P.: 167°C; 1H-NMR (DMSO-d6) δ (ppm): 6.96–6.99 (m, 1H, Ar-H), 7.14–7.18 (m, 1H, Ar-H), 7.21–7.25 (m, 1H, Ar-H), 7.33 (s, 1H, benzylidine H), 7.36–7.38 (m, 1H, Ar-H), 7.99 (s, 1H, H of thiazole), 11.4 (s, 1H, OH); 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C11H9N5O3S2; Calculated for C 40.86; H, 2.81; N, 21.66; Found: C, 40.88; H, 2.80; N, 21.70%.
2-[(Z)-(2-Methylphenyl)methylidene]-N-(5-nitro-1,3-thiazol-2-yl)hydrazine-1-carbothioamide (4c)
Yield: 41.7%; M.P.: 184°C; 1H-NMR (DMSO-d6) δ (ppm): 2.78 (s, 3H, CH3), 6.98–7.00 (m, 1H, Ar-H), 7.27–7.29 (m, 1H, Ar-H), 7.33–7.34 (m, 1H, Ar-H), 7.64 (s, 1H, benzylidine H), 7.99 (s, 1H, H of thiazole), 8.13–8.14 (m, 1H, Ar-H), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C12H11N5O2S2; Calculated for C, 44.85; H, 3.45; N, 21.79; Found: C, 44.88; H, 3.43; N, 21.76%.
2-(Z)-[4-(Dimethylamino)phenyl]methylidene-N-( 5-nitro-1,3-thiazol-2-yl)hydrazine-1-carbothioamide (4d)
Yield: 80.1%; M.P.: > 250°C; 1H-NMR (DMSO-d6) δ (ppm): 2.44 (s, 6H, CH3), 6.44–6.46 (m, 2H, Ar-H), 7.64–7.66 (m, 2H, Ar-H), 7.80 (s, 1H, benzylidine H), 7.98 (s, 1H, H of thiazole), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C13H14N6O2S2; Calculated for C, 44.56; H, 4.03; N, 23.98; Found: C, 44.52; H, 3.98; N, 23.96%.
2-(Z)-[4-(Methyloxy)phenyl]methylidene-N-(5-nitro- 1,3-thiazol-2-yl)hydrazine-1-carbothioamide (4e)
Yield: 41.7%; M.P.: 246°C; 1H-NMR (DMSO-d6) δ (ppm): 3.84 (s, 3H, -OCH3), 6.73–6.75 (m, 2H, Ar-H), 7.31–7.34 (m, 2H, Ar-H), 7.80 (s, 1H, benzylidine H), 7.98 (s, 1H, H of thiazole), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C12H11N5O3S2; Calculated for C, 42.72; H, 3.29; N, 20.76; Found: C, 42.70; H, 3.33; N, 20.91%.
2-[(Z)-(2-Nitrophenyl)methylidene]-N-(5-nitro-1,3-thiazol-2-yl)hydrazine-1-carbothioamide (4f)
Yield: 70.1%; M.P.: 167°C; 1H-NMR (DMSO-d6) δ (ppm): 6.69–6.70 (m, 2H, Ar-H), 7.72–7.73 (m, 1H, Ar-H), 7.78–7.79 (m, 1H, Ar-H), 7.9 (s, 1H, benzylidine H), 8.0 (s, 1H, H of thiazole), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C11H8N6O4S2; Calculated for C, 37.50; H, 2.29; N, 23.85; Found: C, 37.53; H, 2.31; N, 23.90%.
2-[(Z)-(4-Nitrophenyl)methylidene]-N-(5-nitro-1,3- thiazol-2-yl)hydrazine-1-carbothioamide (4g)
Yield: 80.2%; M.P.: > 250°C; 1H-NMR (DMSO-d6) δ (ppm): 7.80 (s, 1H, benzylidine H), 7.96–7.97 (m, 2H, Ar-H), 8.0 (s, 1H, H of thiazole), 8.23–8.24 (m, 2H, Ar-H), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C11H8N6O4S2; Calculated for C, 37.50; H, 2.29; N, 23.85; Found: C, 37.51; H, 2.34; N, 23.86%.
2-[(Z)-(3-Bromophenyl)methylidene]-N-(5-nitro-1,3- thiazol-2-yl)hydrazine-1-carbothioamide (4h)
Yield: 70.6%; M.P.: > 250°C; 1H-NMR (DMSO-d6) δ (ppm): 7.21–7.23 (m, 1H, Ar-H), 7.37–7.38 (m, 1H, Ar-H), 7.46–7.47 (m, 1H, Ar-H),7.75 ( s, 1H, benzylidine H), 7.78–7.79 (m, 1H, Ar-H), 8.0 (s, 1H, H of thiazole), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C11H8BrN5O2S2; Calculated for C, 34.21; H, 2.09; N, 18.13; Found: C, 34.22; H, 2.13; N, 18.12%.
2-[(Z)-(4-Fluorophenyl)methylidene]-N-(5-nitro-1,3-thiazol-2-yl)hydrazine-1-carbothioamide (4i)
Yield: 40.2%; M.P.: 184–185°C; 1H-NMR (DMSO-d6) δ (ppm): 7.30–7.32 (m, 2H, Ar-H), 7.54–7.58 (m, 2H, Ar-H), 7.80 (s, 1H, benzylidine H), 8.0 (s, 1H, H of thiazole), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C11H8FN5O2S2; Calculated for C, 40.61; H, 2.48; N, 21.53; Found: C, 40.64; H, 2.43; N, 21.50%.
2-[(Z)-(4-Chlorophenyl)methylidene]-N-(5-nitro-1,3- thiazol-2-yl)hydrazine-1-carbothioamide (4j)
Yield: 52.8%; M.P.: 178–179°C; 1H-NMR (DMSO-d6) δ (ppm): 7.40–7.42 (m, 2H, Ar-H), 7.49–7.50 (m, 2H, Ar-H), 7.80 (s, 1H, benzylidine H), 8.0 (s, 1H, H of thiazole), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C11H8ClN5O2S2; Calculated for C, 38.65; H, 2.36; N, 20.49; Found: C, 38.64; H, 2.40; N, 20.53%.
N-(5-Nitro-1,3-thiazol-2-yl)-2-(Z)-[2-(trifluoromethyl)phenyl]methylidenehydrazine-1-carbothioamide (4k)
Yield: 84.6%; M.P.: 166–167°C; 1H-NMR (DMSO-d6) δ (ppm): 7.39 (s, 1H, benzylidine H), 7.59–7.60 (m, 1H, Ar-H), 7.66–7.67 (m, 1H, Ar-H), 8.0 (s, 1H, H of thiazole), 8.03–8.06 (m, 1H, Ar-H), 8.22–8.24 (m, 1H, Ar-H), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C12H8F3N5O2S2; Calculated for C, 38.40; H, 2.15; N, 18.66; Found: C, 38.43; H, 2.11; N, 18. 70%.
2-[(Z)-(2,6-Dichlorophenyl)methylidene]-N-(5-nitro- 1,3-thiazol-2-yl)hydrazine-1-carbothioamide (4l)
Yield: 84.6%; M.P.: 166–167°C; 1H-NMR (DMSO-d6) δ (ppm): 7.08–7.10 (m, 1H, Ar-H), 7.34–7.36 (m, 2H, Ar-H), 7.98 (s, 1H, benzylidine H), 8.5 (s, 1H, H of thiazole), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C11H7Cl2N5O2S2; Calculated for C, 35.12; H, 1.88; N, 18.61; Found: C, 35.13; H, 1.91; N, 18.64%.
N-(5-Nitro-1,3-thiazol-2-yl)-2-((Z)-4-[(phenylmethyl)oxy]phenylmethylidene)hydrazine-1-carbothioamide (4m)
Yield: 40.4%; M.P.: 188–190°C; 1H-NMR ( DMSO-d6) δ (ppm): 4.98 (s, 2H, CH2 of benzyl), 6.68–6.70 (m, 2H, Ar-H), 7.04–7.06 (m, 1H, Ar-H), 7.11–7.12 (m, 2H, Ar-H), 7.13–7.14 (m, 2H, Ar-H), 7.27–7.28 (m, 2H, Ar-H), 7.80 s, 1H, benzylidine H), 8.0 (s, 1H, H of thiazole), 11.9 (s, 2H, D2O exchangeable NH); Chemical Formula: C18H15N5O3S2; Calculated for C, 52.29; H, 3.66; N, 16.94; Found: C, 52.26; H, 3.70; N, 16.98%.
N-(5-Nitro-1,3-thiazol-2-yl)-2-[(Z)-1-phenylethylidene]hydrazine-1-carbothioamide (4n)
Yield: 54.4%; M.P.: 188–189°C; 1H-NMR (DMSO-d6) δ (ppm): 2.32 (s, 3H, CH3), 7.25–7.27 (m, 3H, Ar-H), 7.39–7.42 (m, 2H, Ar-H), 7.95 (s, 1H, H of thiazole), 9.77 (s, 2H, D2O exchangeable NH); Chemical Formula: C12H11N5O2S2; Calculated for C, 44.85; H, 3.45; N, 21.79; Found: C, 44.90; H, 3.49; N, 21.76%.
2-[(Z)-1-(2-Hydroxyphenyl)ethylidene]-N-(5-nitro-1,3-thiazol-2-yl)hydrazine-1-carbothioamide (4o)
Yield: 50.1%; M.P.: 146–147°C; 1H-NMR (DMSO-d6) δ (ppm): 2.21 (s, 3H, CH3), 6.95–6.97 (m, 1H, Ar-H), 7.14–7.16 (m, 1H, Ar-H), 7.18–7.19 (m, 1H, Ar-H), 7.34–7.35 (m, 1H, Ar-H), 7.95 (s, 1H, H of thiazole), 10.40 (s, 2H, D2O exchangeable NH); Chemical Formula: C12H11N5O3S2; Calculated for C, 42.72; H, 3.29; N, 20.76; Found: C, 42.76; H, 3.26; N, 20.79%.
2-[(Z)-1-(4-Methylphenyl)ethylidene]-N-(5-nitro-1,3- thiazol-2-yl)hydrazine-1-carbothioamide (4p)
Yield: 43.1%; M.P.: 149–150°C; 1H-NMR (DMSO-d6) δ (ppm): 2.32 (s, 3H, CH3), 2.34 (s, 3H, 4-CH3), 7.20–7.22 (m, 2H, Ar-H), 7.88–7.90 (m, 2H, Ar-H), 7.95 (s, 1H, H of thiazole), 9.77 (s, 2H, D2O exchangeable NH); Chemical Formula: C13H13N5O2S2; Calculated for C, 46.55; H, 3.91; N, 20.88; Found: C, 46.60; H, 3.94; N, 20.89%.
2-[(Z)-1-(3-Aminophenyl)ethylidene]-N-(5-nitro-1,3-thiazol-2-yl)hydrazine-1-carbothioamide (4q)
Yield: 43.1%; M.P.: 149–150°C; 1H-NMR (DMSO-d6) δ (ppm): 2.32 (s, 3H, CH3), 6.61–6.63 (m, 1H, Ar-H), 7.02 (s, 2H, NH2 D2O exchangeable), 7.14–7.16 (m, 1H, Ar-H), 7.18–7.19 (m, 1H, Ar-H), 7.26–7.28 (m, 1H, Ar-H), 7.95 (s, 1H, H of thiazole), 9.77 (s, 2H, D2O exchangeable NH); Chemical Formula: C12H12N6O2S2; Calculated for C, 42.85; H, 3.60; N, 24.98; Found: C, 42.87; H, 3.64; N, 24.90%.
2-[(Z)-1-(4-Bromophenyl)ethylidene]-N-(5-nitro-1,3- thiazol-2-yl)hydrazine-1-carbothioamide (4r)
Yield: 38.4%; M.P.: 156–158°C; 1H-NMR (DMSO-d6) δ (ppm): 2.32 (s, 3H, CH3), 7.51–7.53 (m, 2H, Ar-H), 7.91–7.93 (m, 2H, Ar-H), 7.95 (s, 1H, H of thiazole), 9.77 (s, 2H, D2O exchangeable NH); Chemical Formula: C12H10BrN5O2S2; Calculated for C, 36.01; H, 2.52; N, 17.50; Found: C, 36.10; H, 2.54; N, 17.49%.
2-[(Z)-1-(4-Nitrophenyl)ethylidene]-N-(5-nitro-1,3-thiazol-2-yl)hydrazine-1-carbothioamide (4s)
Yield: 40.1%; M.P.: 188–189°C; 1H-NMR (DMSO-d6) δ (ppm): 2.32 (s, 3H, CH3), 7.51–7.90–7.91 (m, 2H, Ar-H), 7.95 (s, 1H, H of thiazole), 8.18–8.20 (m, 2H, Ar-H), 9.77 (s, 2H, D2O exchangeable NH); Chemical Formula: C12H10N6O4S2; Calculated for C, 39.34; H, 2.75; N, 22.94; Found: C, 39.36; H, 2.69; N, 23.1%.
Antimycobacterial activity in log-phase cultures
All compounds were screened in triplicate for their in vitro antimycobacterial activity against log-phase cultures of MTB, and non-tubercular mycobacterial (NTM) species such as M. smegmatis ATCC 14468, M. microti MTCC 1727, M. vaccae MTCC 997, M. phlei MTCC 1724, M. fortuitum MTCC 951, and M. kansasii MTCC 3058 in Middlebrook 7H11agar medium supplemented with OADC (albumin–dextrose–sodium chloride) by the agar dilution method, similar to that recommended by the National Committee for Clinical Laboratory Standards, for the determination of MICCitation6. The MDR-TB clinical isolate was obtained from the Tuberculosis Research Center, Chennai, India, and was resistant to isoniazid, rifampicin, and ciprofloxacin. The MIC is defined as the minimum concentration of compound required to give complete inhibition of bacterial growth.
Cytotoxicity
All the compounds were further examined for toxicity (IC50) in a mammalian Vero cell line at a concentration of 62.5 μg/mL by the serial dilution methodCitation7. After 72 h of exposure, viability was assessed on the basis of cellular conversion of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) into a formazan product using the Promega CellTiter 96 non-radioactive cell proliferation assay.
Results and discussion
Synthesis
The synthesis of N-(5-nitrothiazol-2-yl)hydrazinecarbothioamide (3) was carried out in two steps with 92% yield, as shown in . First, to a solution of 2-amino-5-nitrothiazole (1) in ethanol were added potassium hydroxide and carbon disulfide, and the mixture was stirred to form the corresponding potassium salt of dithiocarbamate (2). To the stirred mixture was added hydrazine hydrate, and stirring was continued with reflux for 3 h followed by distillation to give compound 3. Thiosemicarbazide on condensation with various carbonyl compounds in the presence of glacial acetic acid afforded various novel 5-nitrothiazolyl-2-thiosemicarbazones (4a–s) (). In the 1H-NMR spectra, the signals of the respective protons of the prepared derivatives were verified on the basis of their chemical shifts, multiplicities, and coupling constants. The spectra of all the compounds showed a D2O exchangeable singlet at δ 11.9 and 9.77 ppm, corresponding to NH protons of benzaldehyde-derived thiosemicarbazones (4a–m) and acetophenone-derived thiosemicarbazones (4n–s), respectively. Compounds 4a–m showed a singlet at δ ranging 7.4–7.8 ppm, corresponding to the carbimino H of benzaldehyde, and compounds 4n–s showed a singlet at δ 2.32 ppm, corresponding to the carbimino CH3 of acetophenone. All the compounds showed a singlet in the range of δ 7.8–8.0 ppm, which corresponds to the C-4 hydrogen of the thiazole ring. The elemental analysis results were within ±0.4% of the theoretical values.
Table 1. Antimycobacterial activities of 5-nitrothiazolylthiosemicarbazones.
Biological investigation
In the first phase, the compounds were screened for their in vitro antimycobacterial activity against log-phase cultures of MTB, and NTM species such as M. smegmatis ATCC 14468, M. microti MTCC 1727, M. vaccae MTCC 997, M. phlei MTCC 1724, M. fortuitum MTCC 951, and M. kansasii MTCC 3058 by the agar dilution method for determination of the MIC. The minimum inhibitory concentration (MIC) is defined as the minimum concentration of compound required to give complete inhibition of bacterial growth. MICs of the synthesized compounds along with those of the standard drugs for comparison are reported ().
In the initial screening against MTB, the newer compounds showed activity with MICs ranging from 0.23 to 77.31 μM. Two compounds (4k and 4m) showed excellent activity, with MIC < 1 μM. When compared to isoniazid (INH) (MIC: 0.66 μM), both compounds (4k and 4m) were found to be more active, with MICs of 0.52 and 0.23 μM, respectively. Compound 4m was also found to be equally active as rifampicin (RIF) (MIC: 0.23 μM). Eight compounds (4f–h, 4j, 4k, 4m, 4r, 4s) were more potent than ciprofloxacin (CIP) (MIC: 4.71 μM). Compound N-(5-nitro-1,3-thiazol-2-yl)-2-((Z)-4-[(phenylmethyl)oxy]phenylmethylidene)hydrazine-1-carbothioamide (4m) was found to be the most active compound in vitro with an MIC of 0.23 μM against MTB, and was 2 and 19 times more potent than INH and CIP, respectively. With respect to the structure–MTB activity relationship, in the carbimino terminal, benzaldehyde-derived thiosemicarbazones (4a–m) were more potent than acetophenone-derived thiosemicabazones (4n–s). The antimycobacterial activity was enhanced by the introduction of strongly deactivating electron-withdrawing groups such as nitro and trifluoromethyl groups in the phenyl moiety, whereas the introduction of electron-donating groups such as hydroxyl, methyl, and dimethylamino groups decreased the activity. The introduction of the bulky benzyloxy group in the phenyl ring enhanced the activity.
All the compounds were also screened for atypical mycobacteria (AM) infectionCitation8, an illness caused by a type of mycobacterium other than TB which causes a wide variety of infections such as abscesses, septic arthritis, and osteomyelitis. It can also infect the lungs, lymph nodes, gastrointestinal tract, skin, and soft tissues. The incidence of AM infections is rare, but it is increasing as the AIDS population grows. Populations at risk include individuals who have lung disease and those with weakened immune systems. The synthesized compounds inhibited M. smegmatis (MS) with MICs ranging from 1.88 to 16.61 μM, and all the compounds were more potent than INH (MIC: 45.57 μM); MS infects the lungsCitation9. With regard to activity against M. microti (MM; which causes sepsis tuberculosa acutissima in immunocompetent personsCitation10), the compounds showed activity with MICs ranging from 13.32 to 148.20 μM, and only one compound (4k) was more potent than INH (MIC: 22.82 μM). M. vaccae, which causes cutaneous and pulmonary infectionsCitation11, was inhibited by the synthesized compounds with MICs ranging from 2.21 to 148.63 μM, and all the compounds were more potent than INH (MIC: 182.3 μM). All the compounds also inhibited M. phlei (MP), which causes abscessesCitation12, with MICs ranging from 1.95 to 71.34 μM, and were more potent than INH (MIC: 91.15 μM). Against M. fortuitum (which causes infection in immunosuppressed personsCitation13), the compounds showed excellent activity, with MICs ranging from 2.01 to 297.27 μM, and nine compounds were more potent than INH (MIC: 22.82 μM). The compounds were also screened against M. kansasii, which causes central nervous system infection and cutaneous lymphadenitisCitation14, and was inhibited with MICs ranging from 2.21 to 77.79 μM; all compounds were more potent than INH (MIC: 182.3 μM). Compound 4m inhibited all seven mycobacterium species with MICs ranging from 0.23 to 30.25 μM, and was more potent than INH except against MM.
Some compounds were further examined for toxicity (IC50) in a mammalian Vero cell line at a concentration of 62.5 μg/mL (). After 72 h of exposure, viability was assessed on the basis of cellular conversion of MTT into a formazan product using the Promega CellTiter 96 non-radioactive cell proliferation assay. The compounds with nitro group substitution were found to be toxic (4f, 4g, and 4s). Other compounds were not found to be toxic; these results are important, as these compounds with their decreased cytoliability are much more attractive in the development of a compound for the treatment of TB. This is primarily due to the fact that the eradication of TB requires a lengthy course of treatment, and the need for an agent with a high margin of safety becomes a primary concern. Compound 4m showed a selectivity index (IC50/MIC) of more than > 657 against log-phase MTB.
Acknowledgment
The authors are thankful to the Department of Biotechnology (BT/01/COE/05/06/01), Government of India, for their financial assistance.
Declaration of interest: The authors report no conflicts of interest.
References
- Snider DEJ, Raviglione M, Kochi A. Tuberculosis: pathogenesis, protection, and control. In: Bloom BR, ed. Tuberculosis: Pathogenesis, Protection, and Control. Washington, DC: American Society of Microbiology, 1994:2–11.
- Zignol M, Hosseini MS, Wright A, Weezenbeek CL, Nunn P, Watt CJ, et al. Global incidence of multidrug-resistant tuberculosis. J Infect Dis 2006;194:479–85.
- Friedland G. Tuberculosis, drug resistance, and HIV/AIDS: a triple threat. Curr Infect Dis Rep 2007;9:252–61.
- Sriram D, Yogeeswari P, Thirumurugan R, Pavana RK. Discovery of newer antitubercular oxazolyl thiosemicarbazones. J Med Chem 2006;49:3448–50.
- Sriram D, Yogeeswari P, Prathiba D, Senthilkumar P, Debjani B. N-Hydroxythiosemicarbazones: synthesis and in-vitro antitubercular activity. Bioorg Med Chem Lett 2007;17:1888–91.
- National Committee for Clinical Laboratory Standards. Antimycobacterial susceptibility testing for Mycobacterium tuberculosis. Proposed standard M24-T. Villanova, PA: National Committee for Clinical Laboratory Standards, 1995.
- Gundersen LL, Nissen-Meyer J, Spilsberg B. Synthesis and antimycobacterial activity of 6-arylpurines: the requirements for the N-9 substituent in active antimycobacterial purines. J Med Chem 2002;45:1383–6.
- Katoch VM. Infections due to non-tuberculous mycobacteria (NTM). Indian J Med Res 2004;120:290–304.
- Schreiber J, Burkhardt U, Rusch-Gerdes S. Non-tubercular mycobacterial infection of the lungs due to Mycobacterium smegmatis. Pneumologie 2001;55:238–43.
- Geiss HK, Feldhues R, Niemann S. Landouzy septicemia (sepsis tuberculosa acutissima) due to Mycobacterium microti in an immunocompetent man. Infection 2005;33:393–6.
- Hachem R, Raad I, Rolston KV. Cutaneous and pulmonary infections caused by Mycobacterium vaccae. Clin Infect Dis 1996;23:173–5.
- Spiegl PV, Feiner CM. Mycobacterium phlei infection of the foot: a case report. Foot Ankle Int 1994;15:680–3.
- O’Brien RJ, Geiter LJ, Snider DE. The epidemiology of non-tuberculous mycobacteria disease in the United States; results from a national survey. Am Rev Respir Dis 1987;135:1007–14.
- Tabatabaei N, Stout J. Goldschmidt-Clermont P. Central nervous system infection and cutaneous lymphadenitis due to Mycobacterium kansasii in an immunocompetent patient. Infection 2007;35:291–4.