Abstract
In this investigation we studied the trypanocidal activity of the ethyl esters of N-propyl (Et-NPOX) and N-isopropyl (Et-NIPOX) oxamates on bloodstream trypomastigotes and on the clinically relevant intracellular amastigotes of Trypanosoma cruzi acute infected mice. In the infected and treated mice, the levels of parasitemia were drastically reduced between days 15 and 20 of treatment and almost to zero between days 35 and 40. We also found that Et-NPOX completely eliminated amastigote nests in the myocardium of mice infected with INC-5 or NINOA T. cruzi strain, and in skeletal muscle the reduction in the number of amastigote nests was between 60 and 80% in both strains. Also, Et-NIPOX reduced by 60–80% the number of amastigote nests in the myocardium and skeletal muscle of mice infected with these T. cruzi strains. In contrast, nifurtimox, used for comparison, produced a reduction of amastigote nests of only 20–40% in the studied tissues in both strains.
Introduction
Chagas’ disease is an endemic parasitic disease in Latin America, and it is caused by Trypanosoma cruziCitation1. The vectorial transmission is effected by triatomine insectsCitation2; humans and wild and domestic animals are the natural reservoirs of T. cruzi. It is estimated that 16–18 million people are infected by T. cruzi and that some 100 million are at risk of acquiring Chagas’ diseaseCitation2,Citation3. There are three stages of the human disease: the acute stage which appears shortly after infection, characterized by a large increase of bloodstream trypomastigotes, followed by a silent or asymptomatic stage, and the chronic stage, characterized by a large increase of intracellular amastigotes, which may last several years and irreversibly affects internal organs such as the heart, esophagus, colon, and peripheral nervous systemCitation4.
Chagas’ disease remains practically incurable due to the limited interest in developing new antichagasic drugs and the fact that the clinically available drugs for the treatment of Chagas’ disease, benznidazole and nifurtimox (Nx), markedly reduce the parasitemia in the acute stage but they are ineffective in the chronic stageCitation5. Natural resistance to these drugs has been suggested as an important factor to explain the low rate of cure detected in chagasic patientsCitation6. Thus, a search for new agents that exhibit trypanocidal activity against intracellular T. cruzi amastigotes seems justifiable.
In previous investigations we designed and synthesized N-propyl oxamate (NPOX) and N-isopropyl oxamate (NIPOX) as possible inhibitors of T. cruzi α-hydroxyacid dehydrogenase (HADH)-isozyme II, and we found that these oxamates were indeed competitive and selective inhibitors of this isozyme ()Citation7,Citation8. Since HADH-isozyme II participates in the energetic metabolism of T. cruziCitation9–11, a trypanocidal effect can be expected with these inhibitorsCitation12,Citation13. However, when we tested the trypanocidal activity of NPOX and NIPOX, we were not able to detect any trypanocidal effect with these oxamates. In contrast, the corresponding ethyl esters (Et-NPOX and Et-NIPOX), acting as prodrugs, exhibited trypanocidal activity on cultured epimastigotes (in vitro) and murine trypanosomiasis (in vivo) in all the tested T. cruzi strainsCitation8,Citation14,Citation15. The increased effectiveness of Et-NPOX and Et-NIPOX resulted from their better absorption by this parasite and their efficient hydrolysis inside T. cruzi by its carboxylesterasesCitation16,Citation17, generating the active HADH-isozyme II inhibitors and NIPOX in situCitation14,Citation15.
Figure 1. Structures of α-ketocaproate and α-ketoisocaproate, the best substrates of T. cruzi α-HADH-isozyme II, and the inhibitors N-propyl oxamate and N-isopropyl oxamate.
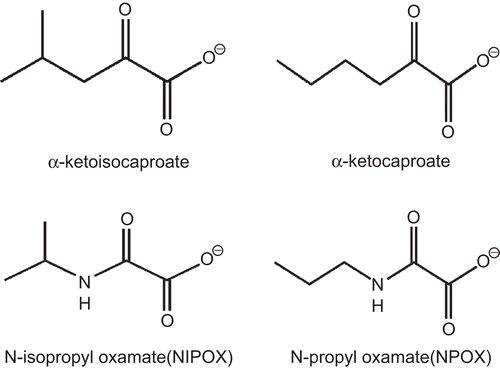
Accordingly, in the present investigation we studied the possible trypanocidal activity of the ethyl esters of N-propyl and N-isopropyl oxamates on bloodstream trypomastigotes and intracellular amastigotes of Trypanosoma cruzi acute infected mice.
Materials and methods
Chemicals
Trypan blue (tetrasodium salt), hematoxylin, eosin, and crystal violet were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Other chemicals used were of the highest purity available. Nifurtimox (tetrahydro-3-methyl-4-[5-nitro-furfurilidene)amine]-2-methyl-tetrahydro-1,4-thiazine-4,4-dioxide was from Bayer, Mexico. NPOX, NIPOX, and the corresponding esters, Et-NPOX and Et-NIPOX, were synthesized according to methods reported elsewhereCitation7,Citation14.
T. cruzi strains
Two T. cruzi strains, NINOA and INC-5, isolated from chronic chagasic patients of two different endemic areas of Mexico, were used in this investigation. The T. cruzi stock strains were isolated by xenoculture according to Bronfen et al.Citation18. Following the method described by Chiari et al.Citation19, feces of infected triatomine insects were inoculated intraperitoneally into laboratory mice and cardiac blood was cultured subsequently at 28°C on either enriched biphasic blood agar medium or the monophasic liquid medium, liver infusion tryptone broth (LIT), supplemented with 10% heat-inactivated fetal calf serum.
Trypanocidal assay
The level of parasitemia was determined by counting in a Neubauer chamber the number of parasites in 5 μL of blood collected from the mouse tail and diluted 1:10 in ammonium chloride, according to Filardi and BrenerCitation20 and Barr et al.Citation21. The reduction in parasitemia was evaluated by comparing the number of parasites obtained at each time point after drug administration with the number of parasites obtained at the same time points in infected, non-treated mice.
Evaluation of drug activity on intracellular amastigotes of T. cruzi acute infected mice
Eighty male National Insitutes of Health (NIH) albino mice (18–20 g per mouse, 10 mice per group) were inoculated intraperitoneally with 1 × 10Citation3 bloodstream trypomastigotes. Four groups were infected with T. cruzi NINOA strain and the other four groups were infected with T. cruzi INC-5 strain. The trypanocidal prodrugs Et-NIPOX and Et-NPOX, and the drug Nx, were administered orally, dissolved in a 5% gum Arabic solution, at a dose of 10 mg/kg per day during 60 days. The first dose was given 24 h after the infection.
Mice were divided into the following groups: (1) infected with T. cruzi (NINOA) as a control; (2) infected with T. cruzi (NINOA) and treated with Et-NPOX; (3) infected with T. cruzi (NINOA) and treated with Et-NIPOX; (4) infected with T. cruzi (NINOA) and treated with Nx; (5) infected with T. cruzi (INC-5) as a control; (6) infected with T. cruzi (INC-5) and treated with Et-NPOX; (7) infected with T. cruzi (INC-5) and treated with Et-NIPOX; (8) infected with T. cruzi (INC-5) and treated with Nx. The experimental procedure was carried out in accordance with the “Guide for the care and use of laboratory animals” published by the US NIH, publication numberCitation22. Levels of parasitemia were determined in a Neubauer hemocytometer beginning 24 h after infection.
Histopathological studies of mouse hearts and left legs were done 60 days post-infection. Thin (3 μm) sections of heart tissue from T. cruzi infected mice, treated or not treated with NPOX, NIPOX, or Nx (10 mg/kg per day), were formaldehyde-fixed, dehydrated, and embedded in paraffin. Sections were stained by hematoxylin–eosin (H&E) and analyzed by light microscopy (×40). Fifty randomly selected microscopic fields were examined to quantify the number of amastigote nests. Histopathological studies of the hearts and legs of each group were done in triplicate. The mean number of amastigote nests in the tissue slices of the infected group not submitted to drug treatment was taken as 100%. The results were evaluated statistically, using Student’s t test for parasitemia and the χCitation2 test for histopathology; the significance level was set at p < 0.05.
Results
Effect of Et-NIPOX, Et-NPOX, and Nx on acute parasitemia of mice infected with INC-5 T. cruzi strain
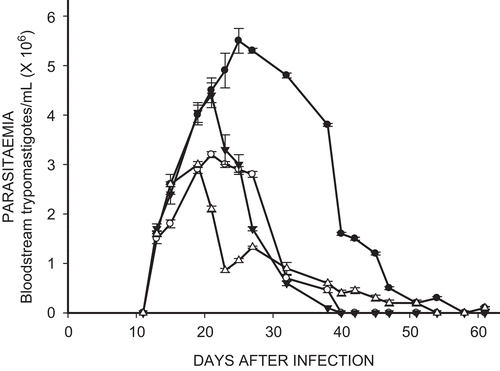
shows that treatment of the infected mice with Et-NIPOX, Et-NPOX, and Nx, at a dose of 10 mg/kg during 60 days, markedly decreased the parasitemia induced by INC-5 T. cruzi strain. This figure also shows that after 20 days of treatment, parasitemia sharply decreased, and after 40 days of treatment the parasitemia was reduced to zero in the groups treated with Et-NPOX and Nx. In the group treated with Et-NIPOX, the parasitema was no longer evident after 55 days of treatment. In contrast, in the infected and non-treated group the parasitemia remained very high between days 20 and 40 after infection.
Figure 2. Effect of ethyl ester of N-propyl oxamate (Et-NPOX) (▵), ethyl ester of N-isopropyl oxamate (Et-NIPOX) (▾), and nifurtimox (○) on acute parasitemia of mice infected with INC-5 T. cruzi strain, using as a control (•) infected and non-treated mice. The drugs were administered orally 10 mg/kg per day during 60 days.
Effect of Et-NIPOX, Et-NPOX, and Nx on acute parasitemia of mice infected with NINOA T. cruzi strain
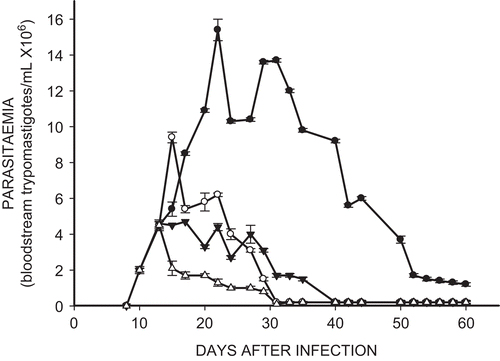
shows that treatment of the infected mice with Et-NIPOX, Et-NPOX, and Nx, at a dose of 10 mg/kg during 60 days, markedly decreased the parasitemia induced by NINOA T. cruzi strain. This figure also shows that after 15 days of treatment, the parasitemia sharply decreased, and after 35 days of treatment the parasitemia was reduced almost to zero in all the treated groups. In contrast, in the infected and non-treated group the parasitemia remained very high between days 15 and 40 after infection.
Figure 3. Effect of ethyl ester of N-propyl oxamate (Et-NPOX) (▵), ethyl ester of N-isopropyl oxamate (Et-NIPOX) (▾), and nifurtimox (○) on acute parasitemia of mice infected with NINOA T. cruzi strain, using as a control (•) infected and non-treated mice. The drugs were administered orally 10 mg/kg per day during 60 days.
Effect of Et-NIPOX, Et-NOPX, and Nx on amastigote nests in myocardium of mice infected with INC-5 or NINOA T. cruzi strain
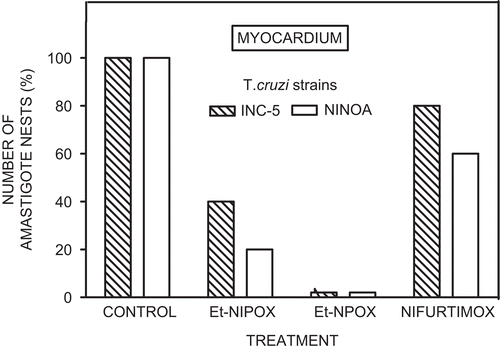
shows that Et-NIPOX, administered at a dose of 10 mg/kg during 60 days, reduced by 60–80% the number of amastigote nests in cardiac muscle of mice infected with INC-5 or NINOA T. cruzi strain. Also, Et-NPOX completely eliminated amastigote nests in cardiac muscle of mice infected with INC-5 or NINOA T. cruzi strain. In contrast, nifurtimox, the drug used for comparison, produced a reduction of amastigote nests of only 20–40%. These experiments are in agreement with previous reports describing the low trypanocidal effect of nifurtimox in the chronic stage of Chagas’ diseaseCitation7.
Figure 4. Effect of ethyl ester of N-isopropyl oxamate (Et-NIPOX), ethyl ester of N-propyl oxamate (Et-NPOX), and nifurtimox on T. cruzi amastigote nests in myocardium of mice infected with INC-5 (hatched columns) or NINOA (open columns) T. cruzi strain. The drugs were administered orally 10 mg/kg per day during 60 days.
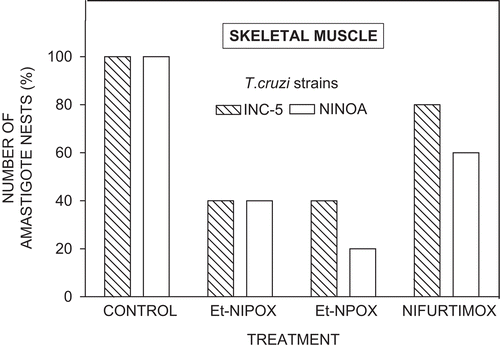
Effect of Et-NPOX, Et-NIPOX, and Nx on amastigote nests in skeletal muscle of mice infected with INC-5 or NINOA T. cruzi strain
shows that Et-NPOX and Et-NIPOX, administered at a dose of 10 mg/kg during 60 days, produced in skeletal muscle a reduction in the number of amastigote nests of 60–80%. In contrast, nifurtimox, the drug used for comparison, produced a reduction of amastigote nests of only 20–40% in skeletal muscle of mice infected with INC-5 or NINOA T. cruzi strain. These experiments are in agreement with previous reports describing the low trypanocidal effect of this compound in the chronic stage of Chagas’ disease7.
Figure 5. Effect of ethyl ester of N-isopropyl oxamate (Et-NIPOX), ethyl ester of N-propyl oxamate (Et-NPOX), and nifurtimox on T. cruzi amastigote nests in skeletal muscle of mice infected with INC-5 (hatched columns) or NINOA (open columns) T. cruzi strain. The drugs were administered orally 10 mg/kg per day during 60 days.
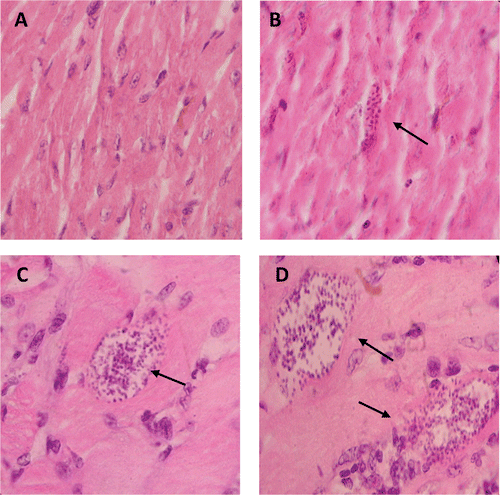
Histopathological studies of myocardium and skeletal muscle of mice infected with NINOA or INC-5 T. cruzi strain and treated with Et-NPOX
shows some of the histopathological studies of myocardium and skeletal muscle of mice infected with NINOA or INC-5 T. cruzi strain and treated with Et-NPOX, 10 mg/kg per day during 60 days. This figure also shows that Et-NPOX completely eliminated amastigote nests in the myocardium of mice infected with NINOA or INC-5 T. cruzi strain, whereas in skeletal muscle the reduction of amastigote nests by Et-NPOX was around 60–80%.
Figure 6. Histopathological studies of myocardium and skeletal muscle of mice infected with NINOA or INC-5 T. cruzi strain. (A) Myocardium from (NINOA) T. cruzi infected mice treated with the ethyl ester of N-propyl oxamate (Et-NPOX). (B) Myocardium from (NINOA) T. cruzi infected, non-treated mice. (C) Skeletal muscle from (NINOA) T. cruzi infected mice treated with Et-NPOX. (D) Skeletal muscle from (NINOA) T. cruzi infected, non-treated mice. Similar results were obtained in myocardium and skeletal muscle from (INC-5) T. cruzi infected mice, non-treated and treated with the ethyl ester of N-isopropyl oxamate (Et-NIPOX). The drugs were administered orally 10 mg/kg per day during 60 days. Tissue slices were stained with hematoxylin–eosin and were analyzed with a ×40 objective. The arrows indicate the location of amastigote nests.
Discussion
Since the clinically available drugs for the treatment of Chagas’ disease reduce only the severity of the acute disease, but they are ineffective in chronic stages of the infectionCitation5, in this investigation we studied the possible trypanocidal activity of the ethyl esters of N-propyl and N-isopropyl oxamates on bloodstream trypomastigotes and on the intracellular amastigotes of Trypanosoma cruzi acute infected mice.
T. cruzi presents a complex life cycle involving several morphological and functionally different stages that adapt to a variety of conditions imposed by the insect vector and mammalian host environments. The trypomastigotes, the infective form of T. cruzi, live in the blood, and they are dependent upon their own vigorous cell motility for extravasation and dissemination within the hostCitation23. It is evident that cell motility plays an important role in the pathogenesis of Chagas’ disease. Taking advantage of this cell motility, the bloodstream trypomastigotes invade mammalian cells where they undergo differentiation into amastigotes, the replicative form of T. cruzi. The amastigotes then undergo many cycles of multiplication by binary fission, and transform again into mobile bloodstream trypomastigotes, leading to the rupture of colonized cells during the chronic stage of the infection. These tissue lesions can be detected as amastigote nests in histopathological studies. So, to evaluate the possible trypanocidal effect of Et-NPOX and Et-NIPOX on intracellular amastigotes, we used a murine model of acute Chagas’ disease. Male NIH albino mice were inoculated with bloodstream trypomastigotes of INC-5 or NINOA T. cruzi strain. The prodrugs Et-NPOX and Et-NIPOX and the drug Nx used for comparison were administered at a dose of 10 mg/kg per day during 60 days, and at the end of the treatment, the trypanocidal effect of these drugs on intracellular amasatigotes was evaluated by detecting and counting the amastigote nests in myocardium and skeletal muscle of infected, treated and non-treated mice. Additionally, the level of parasitemia was followed over time in the treated and non-treated mice.
In the infected and non-treated mice we obtained classic bell shaped curves of parasitemia, with a maximum peak of 5.5 × 10Citation6 bloodstream trypomastigotes/mL with the INC-5 T. cruzi strain and of 15 × 10Citation6 trypomastigotes/mL with the NINOA T. cruzi strain. In the infected and treated mice the parasitemia was drastically reduced between days 15 and 20, and was reduced almost to zero between days 35 and 40. In contrast, in the infected and non-treated group the parasitemia remained very high between days 15 and 40 after infection.
The trypanocidal experiments on intracellular T. cruzi amastigotes showed that Et-NPOX completely eliminated amastigote nests in the cardiac muscle of mice infected with INC-5 or NINOA T. cruzi strain, and in skeletal muscle the reduction in the number of amastogote nests was between 60 and 80% in both strains. Also, Et-NIPOX reduced by 60–80% the number of amastigote nests in both cardiac and skeletal muscles of mice infected with these T. cruzi strains. In contrast, Nx, the drug used for comparison, produced a reduction of amastigote nests of only 20–40% in cardiac and skeletal muscles of mice infected with these T. cruzi strains. These findings are in agreement with previous reports describing the low trypanocidal effect of this drug in the chronic stage of Chagas’ diseaseCitation7.
These pharmacological studies show that the prodrugs Et-NPOX and Et-NIPOX have an in vivo trypanocidal effect not only on bloodstream trypomastigotes, but also on the clinically relevant intracellular proliferative form of the parasite. The reduction in the number of both circulating and intracellular parasites produced by Et-NPOX and Et-NIPOX was clearly demonstrated. In contrast, Nx showed a poor trypanocidal effect on intracellular amastigotes in this experimental model of the acute stage of Chagas’ disease.
In conclusion, the results we have presented above show that Et-NPOX and Et-NIPOX have trypanocidal activity against bloodstream trypomastigotes and intracellular amastigotes of INC-5 and NINOA T. cruzi strains in a murine model of acute Chagas’ disease.
At the dose and the treatment duration used in this work, Et-NPOX and Et-NIPOX given by the oral route were very well tolerated by mice. No deleterious effects on weight gain and general physical condition of the treated animals, or in the histopathological studies of tissues such as the myocardium and the skeletal muscle, were observed.
Acknowledgements
This work was partially supported by research grants from the Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-IPN), México. Four of the authors (C.W., L.R.-P., B.N., I.B.) are fellows of SNI-CONACYT and COFAA-IPN, and three of the authors (C.A.-A., F.Z.-M., J.L.T.-R.) are fellows of CONACYT and PIFI-IPN.
Declaration of interest: The authors report no conflicts of interest.
References
- Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA. Chagas’ disease. Postgrad Med J 2006;82:788–98.
- World Health Organization. Chagas’ disease special program for research and training in tropical diseases. Geneva: WHO, 1993:67–75.
- World Health Organization. Control of Chagas’ disease. Tech Rep Ser 2002;905:1–109.
- Moncayo A. Progress towards interruption of transmission of Chagas’ disease. Mem Inst Oswaldo Cruz 1999;94:401–4.
- Urbina JA. Parasitological cure of Chagas’ disease: is it possible? Is it relevant? Mem Inst Oswaldo Cruz 1999;94:349–55.
- Rodrigues-Coura J, de Castro SL. A critical review on Chagas’ disease chemotherapy. Mem Inst Oswaldo Cruz 2002;97:3–24.
- Chena MA, Elizondo-Jimenez S, Rodríguez-Páez L, Nogueda-Torres B, Baeza-Ramírez I, Wong-Ramírez C. Trypanosoma cruzi: inhibition of α-hydroxyacid dehydrogenase isozyme II by N-allyl and N-propyl oxamates and their effects on intact epimastigotes. Mem Inst Oswaldo Cruz 2004;99:831–7.
- Elizondo S, Chena MA, Rodríguez-Páez L, Nogueda B, Baeza I, Wong C. Inhibition of Trypanosoma cruzi alpha-hydroxyacid dehydrogenase-isozyme II by N-isopropyl oxamate and its effect on intact epimastigotes. J Enzyme Inhib Med Chem 2003;18:265–71.
- Opperdoes FR. Biochemical peculiarities of trypanosomes African and South American. Br Med Bull 1985;41:130–6.
- Coronel CE, Rovai LE, Gerez de Burgos NM, Burgos C, Blanco A. Properties of α-hydroxyacid dehydrogenase isozymes from Trypanosoma cruzi. Mol Biochem Parasitol 1981;4:29–38.
- Coronel CE, Gerez de Burgos NM, Burgos C, Blanco A. Separación y propiedades catalíticas de la isoenzima de la α-hidroxiácido deshidrogenasa de Trypanosoma cruzi. Medicina (Buenos Aires) 1980;40:159–64.
- Bakker BM, Westerhoff HV, Opperdoes FR, Michels PA. Metabolic control analysis of glycolysis in trypanosomes as an approach to improve selectivity and effectiveness of drugs. Mol Biochem Parasitol 2000;106:1–10.
- Verlinde CL, Hannaert V, Blonski C, Willson M, Périé JJ, Fothergill-Gilmore LA, et al. Glycolysis as a target for the design of new anti-trypanosome drugs. Drug Resist Updat 2001;4:50–65.
- Chena MA, Elizondo S, Rodríguez-Páez L, Nogueda B, Baeza I, Wong C. Trypanocidal activity of N-isopropyl oxamate on cultured epimastigotes and murine trypanosomiasis using different Trypanosoma cruzi strains. J Enzyme Inhib Med Chem 2005;20:189–97.
- Aguirre-Alvarado C, Zaragoza-Martínez F, Rodríguez-Páez L, Nogueda B, Baeza I, Wong C. In vitro and in vivo trypanocidal activity of the ethyl esters of N-allyl and N-propyl oxamates using different Trypanosoma cruzi strains. J Enzyme Inhib Med Chem 2007;22:227–33.
- Aldunate J, Repetto Y, Letelier ME, Morello A. The carboxyl esterases of Trypanosoma cruzi epimastigotes. Comp Biochem Physiol 1987;86:67–71.
- Repetto Y, Aldunate J, Morello A. Trypanosoma cruzi: carboxylesterase activity in intact epimastigotes. Comp Biochem Physiol B 1983;76:61–4.
- Bronfen E, Rocha FSA, Machado GBN. Isolamento de amostras do Trypanosoma cruzi por genodiagnostico e hemoculture de pacientes na fase cronica da doenca de Chagas. Mem Inst Oswaldo Cruz 1989;84:234–40.
- Chiari E, Diaz JCP, Lana M, Chiari CA. Haemocultures for the parasitological diagnosis of human chronic Chagas’ disease. Rev Soc Bras Med Trop 1989;22:19–23.
- Filardi LS, Brener Z. A rapid method for testing in vivo the susceptibility of different strains of Trypanosoma cruzi to active chemotherapeutic agents. Mem Inst Oswaldo Cruz 1984;79:221–5.
- Barr JC, Rose D, Jaines JM. Activity of lytic peptides against intracellular Trypanosoma cruzi amastigotes in vitro and parasitaemia in mice. J Parasitol 1995;81:974–8.
- National Institutes of Health. Guide for the care and use of laboratory animals. DHEW Publication 85–123. Bethesda, MD: Office of Science and Health Reports, DRR/NIH, 1985.
- Hill KL. Biology and mechanism of trypanosome cell motility. Eukaryotic Cell 2003;2:200–8.