Abstract
Novel 1-[[4-(4-bromophenyl)-5-(2-furyl)-4H-1,2,4-triazole-3-yl]mercaptoacetyl]-4-alkyl/aryl-3-thiosemicarbazides (5–12) were synthesized by the reaction of 4-(4-bromophenyl)-5-(2-furyl)-4H-1,2,4-triazole-3-ylmercaptoacetylhydrazide (4) with substituted isothiocyanates. Cyclodehydration of thiosemicarbazides with concentrated sulfuric acid yielded 2-[4-(4-bromophenyl)-5-(2-furyl)-4H-1,2,4-triazole-3-yl]mercaptomethyl-5-alkyl/arylamino-1,3, 4-thiadiazoles (13–17). The new compounds were evaluated for in vitro antifungal activity using the microdilution method. The tested compounds showed varying degrees of activity against Microsporum gypseum NCPF-580, Microsporum canis, Trichophyton mentagrophytes, Trichophyton rubrum, and Candida albicans ATCC 10231 (MIC 8–4 μg/mL).
Introduction
The increasing clinical importance of drug-resistant bacterial pathogens and opportunistic infections caused by fungi, especially in immunocompromised patients receiving immunosuppressive and cytotoxic therapies, has stimulated the search for new, more effective antibacterial and antifungal agents.
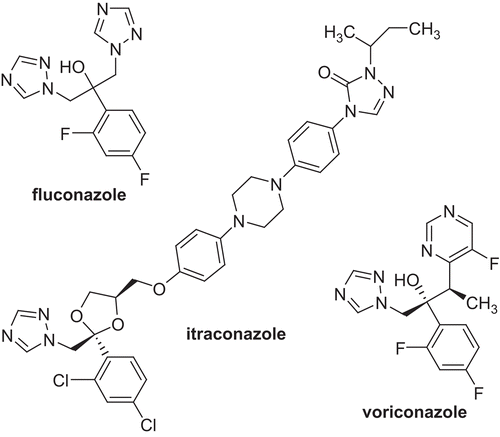
The options for treatment of opportunistic infections are still led by amphotericin B and a few azole (N-substituted imidazole and triazole) antifungal agents. Fluconazole, itraconazole, and the newest triazole antifungal agent voriconazole, with a structure related to that of fluconazole and a spectrum of activity comparable to that of itraconazole, are currently being used for the treatment of systemic mycoses ()Citation1. Due to the adverse effects encountered with currently available drugs, the race to market a new azole continues, and many potent antifungal 1,2,4-triazole compounds have been developed recentlyCitation2–4.
Triazoles cure mycoses by selectively inhibiting fungal cytochrome P-450-mediated lanosterol 14α-demethylase. They prevent demethylation of the natural substrate lanosterol, cause accumulation of 14α-methyl sterols in fungal cells, and decrease ergosterol, which affects membrane structure and functions, resulting in inhibition of the growth of fungi.
Literature surveys reveal that 1,3,4-thiadiazole derivatives as well as 1,2,4-triazoles are associated with antibacterial and antifungal activitiesCitation5–9.
In view of the above considerations and in continuation of our previous work on 1,2,4-triazoles and 1,3,4- thiadiazolesCitation10–15, it was considered worthwhile to design new thiosemicarbazide (5–12) and 1,3,4-thiadiazole (13–17) derivatives featuring a 1,2,4-triazole nucleus, to investigate their antifungal properties. The present article describes the synthesis and antifungal evaluation of 5–17.
Materials and methods
2-Furoic acid hydrazide, ethyl bromoacetate, and the isothiocyanates were commercially available. Melting points were determined on a Büchi (Tottoli) apparatus and are uncorrected. Elemental analyses were performed on PerkinElmer 247 and Carlo Erba 1106 elemental analyzers. Ultraviolet (UV; EtOH) and infrared (IR; KBr, cm−1) spectra were run on Shimadzu 2100 S and PerkinElmer 577 (grating) instruments, respectively. 1H-nuclear magnetic resonance (NMR) spectra were recorded on Bruker DPX-400, Bruker AC 200, and Bruker 80 instruments; C3-H, C4-H, and C5-H represent furan protons. Mass spectra (liquid chromatography-mass spectrometry (LC-MS), atmospheric pressure chemical ionization (APCI)) were recorded on a Finnigan LCQ spectrometer in the positive ionization mode.
Compounds 1–4 were prepared as described in previous worksCitation11,Citation16.
[4-(4-Bromophenyl)-5-(2-furyl)-4H-1,2,4-triazole-3-yl]mercaptoacetic acid hydrazide (4) Yield: 65%, m.p. 171–2°C. IR: 3627, 3567 (N-H); 1671 (C=O). 1H-NMR (400 MHz, CDCl3 + DMSO-d6): 9.48 (br.s, 1H, NH); 7.66 (d, J = 8.6 Hz, 2H, ar); 7.47 (d, J = 3.7 Hz, 1H, C5-H); 7.29 (d, J = 8.6 Hz, 2H, ar); 6.37 (dd, J = 3.5,1.8 Hz, 1H, C4-H); 6.23 (d, J = 3.5 Hz, 1H, C3-H); 3.81 (s, 2H, SCH2); 3.45 (br.s, 1H, NH2).
General procedure of the synthesis of 1-[[4-(4-bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptoacetyl]-4-alkyl/aryl-3-thiosemicarbazides (5–12)
A solution of 4 (5 mmol) in ethanol (30 mL) and an appropriately substituted isothiocyanate (5 mmol) was heated under reflux for 3–4 h. The prepicitate that formed after cooling was collected by filtration and recrystallized from ethanol (5–9, 11, and 12) or ethanol/chloroform (3:1) (10).
1-[[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptoacetyl]-4-allyl-3-thiosemicarbazide (6) UV: 275.1 (4.31); 245.8 (4.44). IR: 3330, 3150 (N-H); 1705 (C=O); 1620, 1520, 1485 (C=N, C=C). 1H-NMR (200 MHz, DMSO-d6): 10.22 (s, 1H, NH); 9.39 (s, 1H, NH); 8.31 (s, 1H, NH); 7.84 (d, J = 8.6 Hz, 2H, ar); 7.78 (s, 1H, C5-H); 7.49 (d, J = 8.6 Hz, 2H, ar); 6.55 (dd, J = 3.4, 1.7 Hz, 1H, C4-H); 6.34 (d, J = 3.4 Hz, 1H, C3-H); 5.85 (m, 1H, −CH=CH2); 5.12 (d, J = 17.0 Hz, 1H, =CH2); 4.62 (dd, J = 10.0, 1.4 Hz, 1H,=CH2); 4.17 (t, 2H, N-CH2); 3.94 (s, 2H, SCH2).
1-[[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptoacetyl]-4-butyl-3-thiosemicarbazide (7) UV: 274.8 (4.29); 244.5 (4.42). IR: 3290, 3140 (N-H); 1705 (C=O); 1540, 1510, 1485 (C=N, C=C). 1H-NMR (200 MHz, DMSO-d6): 10.12 (s, 1H, NH); 9.21 (s, 1H, NH); 8.06 (t, J = 5.4 Hz, 1H, NH); 7.84 (d, J = 8.6 Hz, 2H, ar); 7.77 (d, J = 1.3 Hz, 1H, C5-H); 7.49 (d, J = 8.6 Hz, 2H, ar); 6.55 (dd, J = 3.4, 1.8 Hz, 1H, C4-H); 6.35 (d, J = 4.3 Hz, 1H, C3-H); 3.89 (s, 2H, SCH2); 3.51(q, J = 6.6 Hz, 2H, N-CH2); 1.52 (qn, J = 7.5 Hz, 2H, CH2); 1.28 (sx, J = 7.3 Hz, 2H, CH2); 0.87 (t, J = 7.2 Hz, 3H, CH3).
1-[[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptoacetyl]-4-phenyl-3-thiosemicarbazide (8) UV: 271.8 (4.37); 225.0 (4.41). IR: 3300, 3160 (N-H); 1710 (C=O); 1590, 1540, 1485 (C=N, C=C). 1H-NMR (200 MHz, DMSO-d6): 10.31 (s, 1H, NH); 9.66 (s, 2H, NH); 7.83 (d, J = 8.6 Hz, 2H, ar); 7.77 (d, J = 1.3 Hz, 1H, C5-H); 7.53 (t, J = 8.3 Hz, 4H, ar); 7.34 (t, J = 7.9 Hz, 2H, ar); 7.16 (t, J = 7.3 Hz, 1H, ar); 6.55 (dd, J = 3.4, 1.8 Hz, 1H, C4-H); 6.35 (d, J = 3.4 Hz, 1H, C3-H); 3.97 (s, 2H, SCH2).
1-[[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptoacetyl]-4-(4-bromophenyl)-3-thiosemicarbazide (9) UV: 273.5 (4.44); 226.0 (4.48). IR: 3360, 3200 (N-H); 1705, 1695 (C=O); 1585, 1540, 1480 (C=N, C=C). 1H-NMR (200 MHz, DMSO-d6): 10.41 (s, 1H, NH); 9.89 (s, 1H, NH); 9.79 (s, 1H, NH); 7.85 (d, J = 8.6 Hz, 2H, ar); 7.80 (d, J = 1.5 Hz, 1H, C5-H); 7.56–7.50 (m, 6H, ar); 6.56 (dd, J = 3.4, 1.8 Hz, 1H, C4-H); 6.32 (d, J = 3.4 Hz, 1H, C3-H); 3.95 (s, 2H, SCH2).
1-[[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptoacetyl]-4-(4-chlorophenyl)-3-thiosemicarbazide (10) UV: 274.0 (4.45); 228.0 (4.52). IR: 3330, 3150 (N-H); 1705 (C=O); 1620, 1520, 1485 (C=N, C=C). 1H-NMR (200 MHz, DMSO-d6): 10.40 (s, 1H, NH); 9.89 (s, 1H, NH); 9.81 (s, 1H, NH); 7.85 (d, J = 8.5 Hz, 2H, ar); 7.80 (d, J = 1.6 Hz, 1H, C5-H); 7.41–7.54 (m, 4H, ar); 7.39 (d, J = 8.6 Hz, 2H, ar); 6.55 (dd, J = 3.5, 1.8 Hz, 1H, C4-H); 6.31 (d, J = 3.5 Hz, 1H, C3-H); 3.94 (s, 2H, SCH2).
1-[[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptoacetyl]-4-(4-fluorophenyl)-3-thiosemicarbazide (11) UV: 268.6 (4.48); 226.8 (4.56). IR: 3340, 3200 (N-H); 1710, 1685 (C=O); 1605, 1520, 1500, 1480 (C=N, C=C). 1H-NMR (200 MHz, DMSO-d6): 10.32 (s, 1H, NH); 9.72 (s, 1H, NH); 9.69 (s, 1H, NH); 7.84 (d, J = 8.6 Hz, 2H, ar); 7.77 (s, 1H, C5-H); 7.55–7.49 (m, 4H, ar); 7.17 (t, J = 5.8 Hz, 2H, ar); 6.55 (dd, J = 3.5, 1.8 Hz, 1H, C4-H); 6.34 (d, J = 3.5 Hz, 1H, C3-H); 3.96 (s, 2H, SCH2).
1-[[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptoacetyl]-4-(4-nitrophenyl)-3-thiosemicarbazide (12) UV: 384.0 (4.01); 278.0 (4.42); 228.0 (4.48). IR: 3310, 3250 (N-H); 1717 (C=O); 1616, 1564, 1512, 1492 (C=N, C=C). 1H-NMR (200 MHz, DMSO-d6): 10.47 (s, 1H, NH); 10.16 (s, 1H, NH); 10.07 (s, 1H, NH); 8.21 (d, J = 9.1 Hz, 2H, ar); 7.98 (d, J = 8.8 Hz, 2H, ar); 7.84 (d, J = 8.5 Hz, 2H, ar);7.78 (s, 1H, C5-H); 7.52 (d, J = 8.8 Hz, 2H, ar); 6.55 (dd, J = 3.3, 1.7 Hz, 1H, C4-H); 6.34 (d, J = 3.4 Hz, 1H, C3-H); 3.97 (s, 2H, SCH2).
General procedure of the synthesis of 2-[4-(4-bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptomethyl-5-alkyl/aryl-amino-1,3,4-thiadiazoles (13–17)
An appropriate thiosemicarbazide (5–12) (2 mmol) was added portionwise to H2SO4 (96%) (5.3 mL) cooled in an ice bath with constant stirring. After dissolution, the reaction mixture was further agitated for 30 min at room temperature, poured over crushed ice, and neutralized by the addition of saturated Na2CO3 solution. The prepicitate thus obtained was collected by filtration, washed with water, and recrystallized from ethanol (13, 14, 16, and 17) or ethanol/chloroform (3:1) (15).
2-[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptomethyl-5-ethyl-amino-1,3,4-thiadiazole (13) UV: 278.2 (4.37); 224.4 (4.26). IR: 3280 (N-H); 1615, 1580, 1510, 1480 (C=N, C=C). 1H-NMR (200 MHz, CDCl3): 7.64 (d, J = 8.7 Hz, 2H, ar); 7.38 (d, J = 1.2 Hz, 1H, C5-H); 7.15 (d, J = 8.7 Hz, 2H, ar); 6.43 (d, J = 3.5 Hz, 1H, C4-H); 6.38 (dd, J = 3.6, 1.9 Hz, 1H, C3-H); 5.70 (s, 1H, NH);4.65 (s, 2H, SCH2); 3.33 (q, J = 6.8 Hz, 2H, CH2); 1.24 (t, J = 6.8 Hz, 3H, CH3). MS-APCI (m/z, %): 463 (MH+, 49), 465 (MH + 2, 100).
2-[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptomethyl-5-butyl-amino-1,3,4-thiadiazole (14) UV: 279.0 (4.46); 225.0 (4.33). IR: 3210 (N-H); 1610, 1580, 1515, 1480 (C=N, C=C). 1H-NMR (200 MHz, CDCl3): 7.65 (d, J = 8.5 Hz, 2H, ar); 7.39 (s, 1H, C5-H); 7.15 (d, J = 8.6 Hz, 2H, ar); 6.43 (d, J = 3.5 Hz, 1H, C4-H); 6.38 (d, J = 3.6 Hz, 1H, C3-H); 5.43 (s, 1H, NH); 4.66 (s, 2H, SCH2); 3.28 (t, J = 6.9 Hz, 2H, CH2); 1.62 (qn, J = 6.9 Hz, 2H, CH2); 1.38 (sx, J = 6.9 Hz, 2H, CH2); 0.92 (t, J = 6.9 Hz, 3H, CH3).
2-[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptomethyl-5-(4-chlorophenyl)-amino-1,3,4-thiadiazole (15) UV: 287.2 (4.08). IR: 3285 (N-H); 1600, 1545, 1490 (C=N, C=C). 1H-NMR (200 MHz, DMSO-d6): 10.37 (s, 1H, NH); 7.78 (d, J = 8.0 Hz, 3H, C5-H, ar); 7.62 (d, J = 8.0 Hz, 2H, ar); 7.40 (t, J = 8.0 Hz, 4H, ar); 6.55 (dd, J = 3.4, 1.7 Hz, 1H, C4-H); 6.37 (d, J = 3.5 Hz, 1H, C3-H); 4.65 (s, 2H, SCH2).
2-[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptomethyl-5-(4-fluorophenyl)-amino-1,3,4-thiadiazole (16) UV: 283.7 (4.39). IR: 3280 (N-H); 1620, 1550, 1510, 1490 (C=N, C=C). 1H-NMR (80 MHz, DMSO-d6): 7.84–7.05 (m, 9H, C5-H, ar); 6.55 (dd, J = 3.5, 1.8 Hz, 1H, C4-H); 6.34 (d, J = 2.8 Hz, 1H, C3-H); 4.64 (s, 2H, SCH2).
2-[4-(4-Bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptomethyl-5-(4-nitrophenyl)-amino-1,3,4-thiadiazole (17) UV: 355.0 (4.32); 278.0 (4.23); 228.0 (4.31). IR: 3261 (N-H); 1615, 1568, 1508, 1487 (C=N, C=C). 1H-NMR (200 MHz, DMSO-d6): 10.99 (s, 1H, NH); 8.23 (d, J = 9.2 Hz, 2H, ar); 7.80–7.76 (m, 5H, C5-H, ar); 7.42 (d, J = 8.4 Hz, 2H, ar); 6.54 (dd, J = 3.4, 1.8 Hz, 1H, C4-H); 6.35 (d, J = 3.4 Hz, 1H, C3-H); 4.69 (s, 2H, SCH2). MS-APCI (m/z, %): 556 (MH+, 91), 558 (MH + 2, 100).
Antifungal activity
The microdilution method was used according to a standard protocol of the National Committee for Clinical Laboratory Standards (NCCLS)Citation17,Citation18. Five strains were tested from each of the following species: Microsporum gypseum NCPF-580, Microsporum canis, Trichophyton mentagrophytes, Trichophyton rubrum, and Candida albicans ATCC 10231.
The medium used was RPMI 1640 broth with l-glutamine and 0.165 M MOPS buffer (3-(N-morpholino)propanesulfonic acid; 34.54 g/L) and without sodium bicarbonate. The medium was adjusted to pH 7.0 at 25°C. Sterility control of each bottle was performed before it was used.
Itraconazole (ITC) was provided by the manufacturer as a standard powder. All compounds were dissolved in 100% dimethyl sulfoxide (DMSO) according to NCCLS methodsCitation17,Citation18. The final compound concentrations were 32–0.01 μg/mL.
Preparation of inoculum suspensions was based mainly on NCCLS guidelinesCitation19 and was described previouslyCitation20. The isolates were subcultured onto potato dextrose agar (PDA) plates at 28°C, during 7–14 days. The fungal colonies were covered with 1 mL of sterile 0.85% saline, and suspensions were made by gently probing the surface with the tip of a Pasteur pipette. The resulting mixture of conidial and hyphal fragments was withdrawn and transferred to a sterile tube. Heavy particles were allowed to settle for 15–20 min at room temperature; the upper suspension was mixed with a vortex for 15 s. The turbidity of supernatants was measured spectrophotometrically at a wavelength of 530 nm, and transmission was adjusted to 65–75%. These stock suspensions were diluted 1:50 in RPMI medium to obtain the final inoculum sizes ranging from 0.4 × 104 to 5 × 104 CFU/mL.
Preparation of inoculum suspensions of C. albicans was based mainly on NCCLS guidelines and was described previouslyCitation19. C. albicans colonies grown on Sabouraud dextrose agar for 24 h at 35°C were suspended in 5 mL of sterile 0.85% saline. The turbidity of the mixed suspension was measured at 530 nm to obtain 75–77% transmission and adjusted to 1 × 106–5 × 106 CFU/mL by a spectrophotometric method. These stock suspensions were diluted 1:50 in RPMI medium, and further diluted 1:20 with medium to obtain the two-fold test inoculum (1 × 103–5 × 103 CFU/mL). The (two-fold) inoculum was diluted 1:1 when wells were inoculated, and the desired final inoculum size was achieved (0.5 × 103–2.5 × 103 CFU/mL).
Microdilution plates (96 U-shaped) were prepared and frozen at −70°C until needed. Rows from 2 to 12 contained the series of drug dilutions in 100 μL volumes and the first row contained 100 μL of drug-free medium, which served as the growth control. Each well was inoculated on the day of the test with 100 μl of the corresponding inoculum. Microplates of dermatophytes were incubated at 28°C for 7 days and microplates of C. albicans were incubated at 35°C, 24 and 48 h. The microplates were read visually with the aid of an inverted reading mirror after 7 days for dermatophytes and after 24 and 48 h for C. albicans. For all drugs, except itraconazole, the minimum inhibitory concentration (MIC) was the lowest concentration showing 100% growth inhibition, and the MIC value of itraconazole was determined according to the NCCLS M27-A2 as 80% inhibition.
Results and discussion
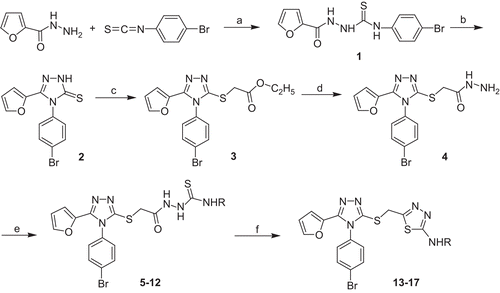
The synthetic approach utilized for the required compounds 1–17 is outlined in . The preparation of the key intermediate [4-(4-bromophenyl)-5-(2-furyl)-4H-1,2,4- triazole-3-yl]mercaptoacetic acid hydrazide (4) was achieved in four steps starting from furoic acid hydrazide, employing a previously described methodologyCitation11,Citation16. Reaction of 4 with appropriately substituted isothiocyanates afforded corresponding 1-[[4-(4-bromophenyl)-5-(2-furyl)-1,2,4-triazole-3-yl]mercaptoacetyl]-4-alkyl/aryl-3-thiosemicarbazides (5–12), which in turn were treated with concentrated H2SO4 at 0–4°C to yield 2-[4-(4-bromophenyl)-5-(2-furyl)-1,2,4-triazole- 3-yl]mercaptomethyl-5-alkyl/arylamino-1,3,4-thiadiazoles (13–17) (). The structures of 5–17 were established by elemental analysis () and spectral data (UV, IR, 1H-NMR, LC-MS).
Table 1. Physicochemical data of compounds 5–17.
Scheme 1. Reagents and conditions: (a) EtOH, reflux, 2 h; (b) 1M NaOH, reflux, 3 h, 1 M HCl; (c) BrCH2COOEt, KOH, EtOH, reflux, 2h; (d) H2NNH2.H2O, EtOH, reflux, 3 h; (e) RNCS, EtOH, reflux, 3–4 h; (f) (i) conc. H2SO4, 0–4°C, (ii) Na2CO3.
The IR spectra of the thiosemicarbazide derivatives 5–12 showed two N-H bands in the 3360–3290 and 3250– 3140 cm−1 regions. The C=O stretchings were observed at about 1717– 1685 cm−1. Supportive data were obtained from the 1H-NMR spectra of 5–12 as they showed N1-H, N2-H, and N4-H resonances in the δ 10.41–8.06 ppm regionCitation21. Singlets observed at about δ 3.97–3.87 ppm were attributed to the SCH2 protonsCitation12.
Cyclodehydration of 1-acyl or 1-aroyl-3-thiosemicarbazides in the presence of a strong base or a strong mineral acid leads to 2,4-dihydro-3H-1,3,4-triazole-3-thiones or 5-amino-1,3,4-thiadiazoles. An explanation for the different modes of cyclization is provided by consideration of the relative nucleophilicities of the terminal thioamide function. In a base the sulfur function is ionized, which increases the nucleophilicity of the 4-amino group leading to 1,2,4-triazole formation, whereas in a strong acid the 4-amino group is protonated and cannot participate in the condensation and this leads to 1,3,4-thiadiazole formationCitation22.
The cyclization reaction proceeds via enol and enethiol intermediates formed by participation of the N1-H, N2-H, C=O, and C=S functions of the thiosemicarbazides. Thus, the absence of C=O stretchings in the IR spectra, observation of a single NH resonance (δ 10.99–5.43 ppm)Citation11 assigned to the proton of the 5-alkylamino group, and downfield shift of the SCH2 resonances (δ 4.69–4.64 ppm) when compared to those of the thiosemicarbazides (δ 3.97–3.89 ppm) 5–12 in the 1H-NMR spectra of 13–17 provided confirmatory evidence of the formation of expected 1,3,4-thiadiazoles. Further spectral details supporting the structure of the cyclic products are presented in “Materials and methods.”
Abundant (MH)+ and (MH + 2)+ ions observed in the APCI (+) mass spectra of 13 and 17 (13: (MH)+ m/z 463, (MH + 2)+ m/z 465; 17: (MH)+ m/z 556, (MH + 2)+ m/z 558) confirmed their molecular weights.
In line with literature findings, selected compounds (4, 6, 7, 9, 11–17) were tested for antifungal activity against Microsporum gypseum NCPF-580, Microsporum canis, Tricophyton mentagrophytes, Tricophyton rubrum, and Candida albicans ATCC 10231 by microdilution assay using itraconazole as the standard. As can be seen in , the tested compounds inhibited the growth of fungal strains responsible for mycoses at varying concentrations (MIC 8–4 μg/mL). Both aliphatic and aromatic substituents were tolerated, and potency was not altered except in the case of compounds 15 and 17. The most susceptible microorganism was T. mentagrophytes, and two thiosemicarbazide derivatives 9 and 12 demonstrated a wider spectrum of activity with lower MIC values (). Although no apparent structure–activity relationship can be derived from the above considerations, it may be speculated that the compounds have some potential which deserves further attention for the development of new antifungal candidates.
Table 2. Antifungal activity of compounds 4, 6, 7, 9, 11–17 (MIC: μg/mL)a.
Declaration of interest: This work was supported in part by the Research Fund of Istanbul University (Project Numbers: BYPS-1-19 / 22012007 and UDP-77 / 26082002.
References
- Greer ND. Voriconazole: the newest triazole antifungal agent. BUMC Proc 2003;16:241–8.
- Bartroli J, Turmo E, Alguero M, Boncompte E, Vericat ML, Conte L, et al. New azole antifungals. 2. Synthesis and antifungal activity of heterocycle carboxamide derivatives of 3-amino-2-aryl-1-azolyl-2-butanol. J Med Chem 1998;41:1855–68.
- Bartroli J, Turmo E, Alguero M, Boncompte E, Vericat ML, Conte L, et al. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J Med Chem 1998;41:1869–82.
- Upadhayaya RS, Sinha N, Jain S, Kishore N, Chandra R, Arora SK. Optically active antifungal azoles: synthesis and antifungal activity of (2R,3S)-2-(2,4-difluorophenyl)-3-(5-(2-(4-aryl-piperazin-1-yl)ethyl)tetrazol-2-yl/1-yl)-butan-2-ol. Bioorg Med Chem 2004;12:2225–38.
- Foroumadi A, Mirzaei M, Shafie A. Antituberculosis agents II. Evaluation of in vitro antituberculosis activity and cytotoxicity of some 2-(1-methyl-5-nitro-2-imidazolyl)-1,3,4-thiadiazole derivatives. Farmaco 2001;56:621–3.
- Doğan HN, Duran A, Rollas S, Sener G, Uysal MK, Gülen D. Synthesis of new 2,5-disubstituted-1,3,4-thiadiazoles and preliminary evaluation of anticonvulsant and antimicrobial activities. Bioorg Med Chem 2002;10:2893–8.
- Nasr MN, Gineinah MM, El-Bendary ER. Synthesis and in vitro antibacterial evaluation of novel imidazo[2’,1’:5,1]-1,2,4-triazolo[4, 3-c]-quinazoline derivatives of 5-thioxo-1, 2, 4-triazole, 4-oxothiazolidine, and their open-chain counterparts. Arch Pharm Pharm Med Chem 2003;336:560–6.
- Doğan HN, Rollas S, Erdeniz H. Synthesis, structure elucidation and antimicrobial activity of some 3-hydroxy-2-naphthoic acid hydrazide derivatives. Farmaco 1998;53:462–7.
- Modzelewska-Banachiewicz B, Matysiak J, Niewiadomy A. Synthesis and mycological activity of the compounds obtained in the reaction of N3-substituted amidrazones with sulphinyl-bis-2,4-dihydroxybenzenethioyl. Eur J Med Chem 2001;36:75–80.
- Ergenç N, Ulusoy N, Çapan G, Sanış GÖ, Kiraz M. Synthesis and antimicrobial properties of new 4-(alkylidene/arylidene)- amino-5-(2-furanyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones and 6-aryl-3-(2-furanyl)-7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazines. Arch Pharm Pharm Med Chem 1996;329:427–30.
- Karalı N, Çapan G, Ergenç N, Gürsoy A. Synthesis, characterization and preliminary anticonvulsant evaluation of new indoline-2,3-dione derivatives. Sci Pharm 1997;65:277–87.
- Ulusoy N, Çapan G, Ergenç N, Ekinci AC, Vidin A. Synthesis, characterization and anticonvulsant activity of new 4-thiazolidinone and 1,2,4-triazole-3-thione derivatives. Acta Pharm Turc 1998;40:5–8.
- Terzioğlu N, Karalı N, Gürsoy A, ötük G, Kiraz M, Erturan Z. Synthesis and antimicrobial activity of new triazole and thiadiazole derivatives of 4(3H)-quinazolinones. Acta Pharm Turc 1998;40:77–82.
- Ulusoy N, Gürsoy A, ötük G. Synthesis and antimicrobial activity of some 1,2,4-triazole-3-mercaptoacetic acid derivatives. Farmaco 2001;56:947–52.
- Terzioğlu N, Gürsoy A. Synthesis and anticancer evaluation of some new hydrazone derivatives of 2,6-dimethylimidazo[2,1-b][1,3,4]thiadiazole-5-carbohydrazide. Eur J Med Chem 2003;38:781–6.
- Çapan G, Ergenç N, Ötük G. Synthesis and biological evaluation of 4-alkyl/aryl-1-(2-furoil)-3-thiosemicarbazides and 4-alkyl/aryl-2,4-dihydro-5-(2-furyl)-3H-1,2,4-triazole-3-thiones. J Fac Pharm İstanbul 1990–92;26–8:23–9.
- National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. M27-A. Villanova, PA: NCCLS, 1997.
- National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentousa fungi; proposed standard. M38-P. Villanova, PA: NCCLS, 1998.
- Rodríguez-Tudela JL, Berenguer J, Martínez-Suárez JV, Sanchez R. Comparison of a spectrophotometric microdilution method with RPMI-2% glucose with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro susceptibility testing of amphotericin B, flucytosine, and fluconazole against Candida albicans. Antimicrob Agents Chemother 1996;40:1998–2003.
- Fernandez-Torres B, Cabanes FJ, Carillo-Munoz A, Esteban A, Inza I, Abarca L, et al. Collaborative evaluation of optimal antifungal susceptibility testing conditions for dermatophytes. J Clin Microbiol 2002;40:3999–4003.
- Gürsoy A, Karalı N. Synthesis and anticonvulsant activity of new acylthiosemicarbazides and thiazolidones. Farmaco 1995;50:857–66.
- Temple C. Alkyl or Aryl 1,2,4-Triazoline-5-thiones. In: Weisberger A, Taylor EC, eds. The Chemistry of Heterocyclic Compounds, Vol.37, John Wiley & Sons Inc; New York; 1981:402–4.