Abstract
A new natural flavonoid patuletin 3′-β-xylofuranoside was isolated from Leuzea carthamoides leaves. The antioxidant activity of this compound was evaluated by the DPPH radical assay and ferric reducing antioxidant power (FRAP) assay, and the results were compared with those for trolox and quercetin. DPPH radical scavenging activity of the tested compounds was expressed by the parameter EC50: patuletin 3′-β-xylofuranoside (56.0 μM), trolox (27.8 μM), and quercetin (25.3 μM). The ferric reducing activity of the compounds was demonstrated as FRAP values at 4 and 60 min: patuletin 3′-β-xylofuranoside (28.4 μM, 35.8 μM), trolox (19.3 μM, 20.2 μM), and quercetin (54.3 μM, 79.9 μM). The structure/activity relationship of the flavonoid is also discussed. The results indicate significant antioxidant potency of patuletin 3′-β-xylofuranoside.
Introduction
Plants and their secondary metabolites exhibit a wide range of biological health benefit effectsCitation1,Citation2. For this reason, new compounds are being investigated worldwideCitation3–5.
Flavonoids belong to the large group of plant secondary metabolites called polyphenols (8000 compounds)Citation6. Polyphenols have been reported to exhibit a wide range of biological effects, including for example antibacterial, antiviral, anticancer, antiinflammatory, antiallergic, and especially cardiovascular-protective actionsCitation2,Citation7. It is presumed that these effects are associated with the antioxidant activity of polyphenolsCitation8,Citation9.
According to their structural features, flavonoids are divided into various subclasses such as flavones, flavonols, flavanones, or flavan-3-ols (catechins). All subtypes can also occur as flavonoid glycosidesCitation9.
L. carthamoides (Rhaponticum carthamoides (Aster- aceae)) is a widely used medicinal plant. The principal bioactive constituents are ecdysteroids, flavonoids, and phenolic acids. L. carthamoides is traditionally used as a tonic, stimulant, and adaptogenCitation10.
Previous studies have demonstrated that the components and prepared extracts from L. carthamoides possess important biological effects such as antioxidant, antiplatelet, or antimicrobial activityCitation10–12. In this study, a new natural compound, patuletin 3′-β-xylofuranoside isolated from L. carthamoides, is described. The antioxidant activities of the isolated compound and antioxidant standards (trolox and quercetin) evaluated by 2,29-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) assays are also discussed.
Materials and methods
Dried powdered leaves of Leuzea carthamoides (Willd.) DC (Asteraceae) were obtained from Radka Simakova (medicinal plants cultivation; Ohnisov, Czech Republic). The sample was identified by L. Opletal.
Patuletin 3′-β-xylofuranoside was obtained from the phenolic fraction of the L. carthamoides leaves according to the isolation procedure of Koleckar et al.Citation10.
Antioxidant activity was determined using the DPPH radical scavenging assay modified to a sequential injection analysis (SIA) methodCitation13,Citation14, and the FRAP assay according to the method of Firuzi et al.Citation15.
Results
Structure and characteristics
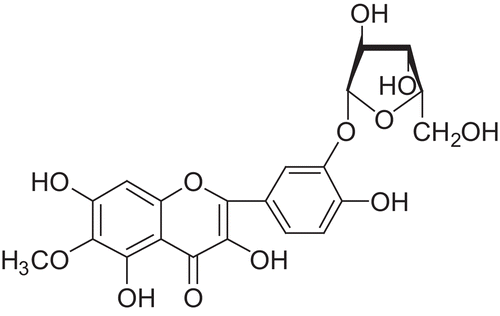
The structure of the isolated compound is shown in .
Patuletin 3′-β-xylofuranoside
Yellow powder; UV max (MeOH): 264; mp 275–278°C; 1H NMR (300 MHz, DMSO) δ 12.50 (1H, bs, OH-5), 10.79 (1H, bs, OH-7), 9.53 (1H, bs, OH-4′), 9.49 (1H, bs, OH-3), 7.91 (1H, d, J = 2.4 Hz, H-2′), 7.77 (1H, dd, J = 8.7 Hz, J = 2.4 Hz, H-6′), 6.97 (1H, d, J= 8.7 Hz, H-5′), 6.53 (1H, s, H-8), 5.59 (1H, bs, OH), 5.15 (2H, bs, OH), 4.77 (1H, d, J =7.5 Hz, CH-1′), 3.86–3.77 (1H, m, CH-5′), 3.74 (3H, s, OCH3), 3.47–3.18 (4H, m, CH-2′, CH-3′, CH-4′, CH-5′); 13C NMR (75 MHz, DMSO) δ 146.4 (C-2), 135.9 (C-3), 176.4 (C-4), 151.5 (C-5), 131.1 (C-6), 151.9 (C-7), 94.0 (C-8), 157.5 (C-9), 103.6 (C-10), 123.7 (C-1′), 116.4 (C-2′), 145.0 (C-3′), 149.4 (C-4′), 116.9 (C-5′), 122.4 (C-6′), 103.1 (C-1′), 73.3 (C-2′), 76.1 (C-3′), 69.6 (C-4′), 66.0 (C-5′), 60.2 (OCH3). ESI-MS (positive mode) m/z [M + H]+ 465, MS/MS (465) m/z [M + H–C5O4H8]+ 333.
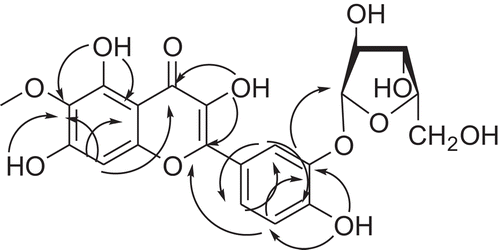
The structure of the compound was unequivocally corroborated by 2D nuclear magnetic resonance (NMR). All gHMQC (gradient-selected heteronuclear multiple quantum coherence) correlations are shown in . Xylose is bonded via a hydroxy group at position 3′ of the benzene ring; the chemical shift of the carbon atom is 145.0 ppm. A cross-peak to the carbon atom at 145.0 ppm and the hemiacetal hydroxyl of the sugar is displayed. The structure of the glycoside was confirmed by comparison of the spectra of aglycone and xylose obtained upon hydrolysis with those of the authentic samples.
Antioxidant activity
The results are shown in .
Table 1. DPPH radical scavenging activity (EC50) and FRAP value (at 4 and 60 min) of patuletin 3′-β-xylofuranoside, trolox, and quercetin.
Discussion
Patuletin 3′-β-xylofuranoside showed significant antioxidant activity in both assays. The DPPH radical scavenging activity of patuletin 3′-β-xylofuranoside was relatively close to those of trolox and quercetin, which were used as antioxidant standards. The ferric reducing activity of the tested compounds followed the order: quercetin > patuletin 3′-β-xylofuranoside > trolox.
The results are in agreement with the generally known structure/antioxidant activity relationship data for flavonoidsCitation8,Citation10. Important structural criteria for high DPPH radical scavenging and ferric reducing activity of flavonoids are (1) ortho-dihydroxy groups in the B-ring or in the A-ring, (2) 3-hydroxyl group in the C-ring, and (3) 2,3-double bond in conjugation with 4-oxo function in the C-ringCitation10,Citation14. The structure of the highly antioxidant-active compound quercetin satisfies all mentioned structural requirements, while in the structure of the less active patuletin 3′-β-xylofuranoside the ortho-dihydroxy groups are missing.
The study has presented a new flavonol glycoside, patuletin 3′-β-xylofuranoside. The compound was isolated from L. carthamoides, a well known medicinal plant growing in Central and Eastern Europe. The previously performed studies of the authors demonstrated significant antioxidant and antiplatelet action of compounds and extracts of L. carthamoidesCitation10,Citation11. This study isolates a highly antioxidant-active natural compound, patuletin 3′-β-xylofuranoside. In conclusion, these results suggest L. carthamoides as a promising cardiovascular-protective plant.
Acknowledgements
This work was supported by a grant from the Charles University (Czech Republic), GAUK 124/2005/B-BIO/FaF, and a research project of the Ministry of Education of the Czech Republic (MSM 0021620822).
Declaration of interest: The authors report no conflicts of interest.
References
- Kondratyuk TP, Pezzuto JM. Natural product polyphenols of relevance to human health. Pharm Biol 2004;42:46–63.
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 2006;99:191–203.
- Yang L, Gong J, Wang F, Zhang Y, Wang Y, Hao X, et al. Synthesis and antioxidant evaluation of novel silybin analogues. J Enzyme Inhib Med Chem 2006;21:399–404.
- Cervenka F, Koleckar V, Rehakova Z, Jahodar L, Kunes J, Opletal L, et al. Evaluation of natural substances from Evolvulus alsinoides L. with the purpose of determining their antioxidant potency. J Enzyme Inhib Med Chem 2008;23:574–8.
- Kontogiorgis CA, Savvoglou K, Hadjipavlou-Litina DJ. Antiinflammatory and antioxidant evaluation of novel coumarin derivatives. J Enzyme Inhib Med Chem 2006;21:21–9.
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 1998;56:317–33.
- Koleckar V, Kubikova K, Rehakova Z, Kuca K, Jun D, Jahodar L, et al. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev Med Chem 2008;8:436–47.
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res Fund Mol M 2005;579:200–13.
- Cook NC, Samman S. Flavonoids—chemistry, metabolism, cardio- protective effects, and dietary sources. J Nutr Biochem 1996;7:66–76.
- Koleckar V, Opletal L, Brojerova E, Rehakova Z, Cervenka F, Kubikova K, et al. Evaluation of natural antioxidants of Leuzea carthamoides as a result of a screening study of 88 plant extracts from the European Asteraceae and Cichoriaceae. J Enzyme Inhib Med Chem 2008;23:218–24.
- Koleckar V, Brojerova E, Rehakova Z, Kubikova K, Cervenka F, Kuca K, et al. In vitro antiplatelet activity of flavonoids from Leuzea carthamoides. Drug Chem Toxicol 2008;31:27–35.
- Chobot V, Buchta V, Jahodarova H, Pour M, Opletal L, Jahodar, L, et al. Antifungal activity of a thiophene polyine from Leuzea carthamoides. Fitoterapia 2003;74:288–90.
- Polasek M, Skala P, Opletal L, Jahodar L. Rapid automated assay of anti-oxidation/radical-scavenging activity of natural substances by sequential injection technique (SIA) using spectrophotometric detection. Anal Bioanal Chem 2004;379:754–8.
- Koleckar V, Jun D, Opletal L, Jahodar L, Kuca K. Assay of radical scavenging activity of antidotes against chemical warfare agents by DPPH test using sequential injection technique. J Appl Biomed 2007;5:81–4.
- Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta 2005;1721:174–84.