Abstract
N-Acetylgalactosamine kinase (GALK2) is a small molecule kinase from the GHMP family which phosphorylates N-acetylgalactosamine at the expense of ATP. Recombinant GALK2 expressed in, and purified from, Escherichia coli was shown to be active with the following kinetic parameters: Michaelis constant for ATP, 14 ± 3 μM; Michaelis constant for N-acetylgalactosamine, 40 ± 14 μM; and turnover number, 1.0 ± 0.1 s−1. The combination of substrate inhibition by N-acetylgalactosamine and α-methylgalactopyranoside acting as an uncompetitive inhibitor with respect to ATP suggested that the enzyme has an ordered ternary complex mechanism in which ATP is the first substrate to bind. The effects of pH on the kinetic parameters provided evidence for ionizable residues playing a role in substrate binding and catalysis. These results are discussed in the context of the mechanisms of the GHMP kinases.
| Abbreviations: | ||
| GALK1, | = | human galactokinase; |
| GALK2, | = | human N-acetylgalactosamine kinase; |
| GalNAc, | = | N-acetylgalactosamine; |
| GHMP, | = | galactokinase, homoserine kinase, mevalonate kinase, phosphomevalonate kinase; |
| kcat, | = | turnover number; |
| Kic, | = | competitive inhibition constant; |
| Kiu, | = | uncompetitive inhibition constant; |
| Kis, | = | substrate inhibition constant; |
| Km,ATP, | = | Michaelis constant for ATP; |
| Km,GalNAc, | = | Michaelis constant for GalNAc; |
| LB, | = | Luria–Bertani. |
Introduction
The GHMP kinases are a group of small molecule kinases which are primarily involved in intermediary metabolismCitation1,Citation2. The name arises from some of the first members of the family identified—galactokinase, homoserine kinase, mevalonate kinase, and phosphomevalonate kinase. These enzymes have sequence and structural similarityCitation1. On the basis of both sequence and structural alignments, the family has been extended to include a number of other enzymes (e.g. arabinose kinase and archael shikimate kinaseCitation3,Citation4) and two non-enzymatically active proteins—the Saccharomyces cerevisiae transcriptional regulator Gal3pCitation5 and the C. elegans sex-fate determining protein XOL-1Citation6.
N-Acetylgalactosamine kinase (GALK2, EC 2.7.1.157) shows high sequence and structural similarity to galactokinase (GALK1, EC 2.7.1.6)Citation7,Citation8. This mammalian enzyme was originally isolated from kidneyCitation9,Citation10 and shows greatest activity toward N-acetylgalactosamine with lower activity with galactosamine and galactoseCitation9,Citation10 (). Human galactokinase (GALK1) has no detectable activity against N-acetylgalactosamineCitation11. The structural basis of this specificity is the substitution of bulky methionine and cysteine residues in the active site of GALK1 with threonine and glycine respectively ()Citation8. While GALK1 is known to function in the Leloir pathway of galactose catabolismCitation12–14, the precise role of GALK2 in vivo is not known, but it has been suggested that it may play a role in glycoprotein metabolismCitation10.
Figure 1. (a) The reaction catalyzed by N-acetylgalactosamine kinase. (b) The active site of GALK2. Only the substrates and key residues are shown. The loop comprising Thr-184 to Gly-186 accommodates the N-acetyl group of N-acetylgalactosamine (GalNAc). In galactokinase, the threonine and glycine residues in this loop are substituted for bulkier methionine and cysteine side chains respectively. Asp-190 is located with the carboxyl group in between the C1-OH of the sugar and the γ-phosphate of the nucleotide. This residue is likely to be important in the catalytic mechanism of the enzyme and its ionization state is probably influenced by Arg-43. The figure was drawn using PyMol (www.pymol.org) and the PDB file 2A2DCitation8. (c) Structures of N-acetylgalactosamine kinase inhibitors used in this study.
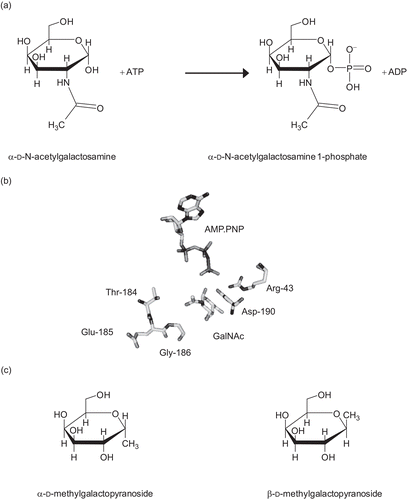
There is interest in developing inhibitors toward some members of the GHMP kinase familyCitation1. For example, inhibition of mevalonate kinase may provide an alternative means to statin drugs for the regulation of cholesterol synthesisCitation1,Citation15–17 and as insecticides which target juvenile hormone biosynthesisCitation18. There is also interest in inhibiting bacterial GHMP kinases for the development of novel antibioticsCitation19, and galactokinase inhibition has been proposed as a therapy for type I galactosemiaCitation20. This severe, and currently untreatable, genetic disease results in irreversible mental and physical impairment during childhoodCitation21,Citation22. These symptoms are believed to be caused by the build-up of the toxic metabolite galactose 1-phosphateCitation23–25. Consequently it was proposed that inhibition of galactokinase would reduce this build-upCitation20. Although the build-up of galactose (and its reduced counterpart, galactitol) can also cause problems, the main symptom is early onset cataracts, which can be addressed by surgery. Recently a number of human galactokinase inhibitors were identified by high throughput screeningCitation26. One issue in the development of specific galactokinase inhibitors is that the high structural similarity between GALK1 and GALK2 means that there is a need to ensure that compounds can discriminate between the two enzymes. Currently, the enzymology of GALK2 is relatively poorly characterized. Here, we describe the inhibition of GALK2 by galactose analogs and the effects of pH on the enzyme’s kinetic parameters. From these data we make inferences about the enzyme’s mechanism.
Materials and methods
Expression and purification of human GALK2
A GALK2 expression clone (generously provided by Professor Hazel Holden, University of Wisconsin, USA) was used to direct the expression of recombinant protein in Escherichia coliCitation8. The plasmid was used to transform competent E. coli HMS174(DE3). A single colony was picked and grown in 5 mL Luria–Bertani (LB) medium supplemented with 100 μg mL−1 kanamycin, shaking overnight at 37°C. This culture was transferred into 1 L of LB (supplemented with 100 μg mL−1 kanamycin) which was then shaken at 37°C for 4 h. The cells were grown for a further 2 h at 20°C, by which time A600nm was typically between 1.5 and 1.8. The expression of GALK2 was then induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) to a final concentration of 2 mM. Induction was allowed to proceed for 18 h at 20°C and then the cells were collected by centrifugation (4200 g for 10 min), resuspended in cell resuspension buffer (50 mM HEPES-OH, pH 7.5, 150 mM NaCl, 10% (v/v) glycerol), and frozen at −80°C.
The cells were thawed and disrupted by sonication (three 30 s pulses at 100 W). Insoluble matter was removed by centrifugation (25,000 g for 15 min) and the supernatant applied to a 1 mL nickel agarose affinity column (His-Select, Sigma) which had been pre-equilibrated in washing buffer (50 mM HEPES-OH, pH 7.5, 500 mM NaCl, 10% (v/v) glycerol). The supernatant was permitted to flow through by gravity and the column was then washed with 20 mL of washing buffer. Protein was eluted in three times 2 mL aliquots of elution buffer (washing buffer supplemented with 250 mM imidazole). Aliquots containing purified GALK2 were identified by 10% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and dialyzed against cell resuspension buffer supplemented with 2 mM dithiothreitol (DTT). Following dialysis, protein concentrations were estimated using the method of BradfordCitation27. The purified protein was stored frozen at −80°C until required.
Assay for N-acetylgalactosamine kinase activity
The assay for N-acetylgalactosamine kinase activity was based on the coupled enzyme assay used for galactokinaseCitation28,Citation29 except that N-acetylgalactosamine was substituted for galactose. In this system, the production of adenosine diphosphate (ADP) is coupled to the oxidation of nicotinamide adenine dinucleotide (NADH) through the actions of pyruvate kinase and lactate dehydrogenase. Reactions were measured in a total volume of 900 μL over a 10 min period at a temperature of 37°C. Each reaction contained 20 mM HEPES-OH, pH 8.0, 150 mM NaCl, 5 mM MgCl2, 10% (v/v) glycerol, 1 mM NADH, 1 mM DTT, 400 μM phsophoenolpyruvate, 7.5 U pyruvate kinase (Sigma), and 10 U lactate dehydrogenase (Sigma). Either GalNAc or ATP was maintained at a saturating concentration (5 mM or 0.5 mM, respectively) and the concentration of the other substrate was varied. Reactions were initiated by the addition of GALK2 (50–230 nM) and monitored by measuring the absorbance at 340 nm. Initial rates (v) were calculated, converted to molar units by use of the extinction coefficient for NADHCitation30 (6220 L mol−1 cm−1) and divided by the enzyme concentration. These values were plotted against the concentration of the variable substrate and the data fitted to the equation v/[GALK2] = kcat.[S]/(Km + [S]) using non-linear curve fittingCitation31 as implemented in the program GraphPad Prism, version 3.02 (GraphPad Software, San Diego, USA). Where substrate inhibition was suspected on the basis of visual inspection of the data, a modified form of this equation was used: v/[GALK2] = kcat.[S]/(Km + [S] + [S]2/Kis), where Kis is the substrate inhibition constantCitation32. All points were weighted equally and values reported plus/minus the standard errors derived during this fitting process.
Inhibition studies were carried out over a range of substrate concentrations. At each concentration one substrate concentration was maintained at a saturating level (see above) and the other held at a sub-saturating level. These sub-saturating concentrations were chosen such that some were above and some below the Km for that substrate. The rate was measured at these substrate concentrations for a range of inhibitor concentrations. Since methylgalactopyranosides have previously been shown to be poor inhibitors of GALK1Citation29, a wide concentration range was used, with the highest concentrations (approximately 100 mM) dictated by the solubility of the compound in aqueous buffer.
The effects of pH were ascertained by measuring the kinetic parameters over the pH range 4.5–11. Since different buffer systems can also cause alterations in the rates of enzyme catalyzed reactions, the HEPES-OH buffer used in the experiments described above was substituted for a constant ionic strength buffer system of ACES (N-(2-acetamido)-2-aminoethanesulfonic acid) (100 mM)/Tris (50 mM)/ethanolamine (50 mM)Citation33. The turnover number was plotted against pH and the data fitted (using GraphPad Prism) to the equation kcat = kcat,lim(1 + [H+]/Ka1 + Ka2/[H+]), where kcat,lim is the limiting value of kcat and Ka1 and Ka2 are the acid dissociation constants of two different ionizable groups in the enzyme’s active siteCitation34.
Results
Expression and purification of human GALK2
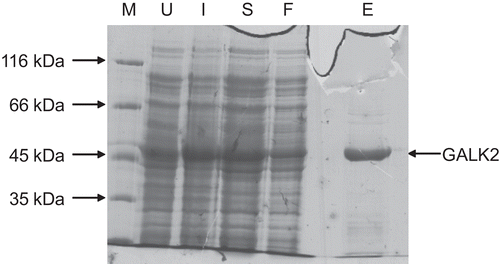
Recombinant human GALK2 could be expressed in, and purified from, E. coli (). The overnight (approximately 18 h) 20°C incubation following induction was required to give good yields (typically 1–2 mg per liter of culture). The use of alternative induction conditions (e.g. 3 h at 37°C, which has been reported for the bacterial expression of human galactokinase and UDP-galactose 4′-epimeraseCitation29,Citation35) resulted in little, or no, protein after purification (data not shown).
Figure 2. Purification of recombinant human GALK2. Samples taken during the purification procedure (see methods) were analyzed by 10% SDS-PAGE. The sizes of molecular mass markers (M) are shown on the left. U, extract from cells prior to induction; I, extracts from induced cells prior to harvesting by centrifugation; S, protein solution extracted from cells by sonication and clarified by centrifugation; F, material which passed through the nickel–agarose column; E, protein eluted from this column in the presence of 250 mM imidazole.
Activity and specificity of recombinant human GALK2
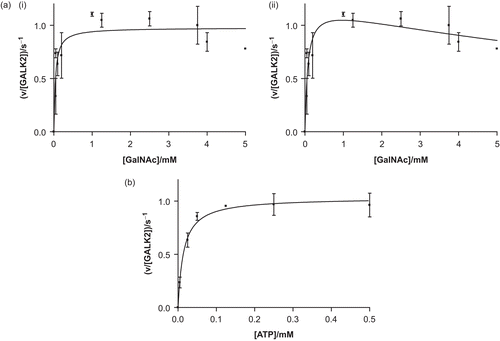
The purified recombinant protein was active and showed typical, saturatable kinetics when either substrate was varied (). Fitting of the data to the Michaelis–Menten equation resulted in values for the Michaelis constant for ATP (Km,ATP) of 14 ± 3 μM, the Michaelis constant for N-acetylgalactosamine (Km,GalNAc) of 40 ± 14 μM, and a turnover number (kcat) of 1.0 ± 0.1 s−1. These values compare well with those published for the enzyme purified from pig kidneyCitation9,Citation10. When the N-acetylgalactosamine concentration was varied, high concentrations (> 3.5 mM) appeared to result in a modest reduction in the rate. This may be due to substrate inhibition, a phenomenon which is well documented with galactose and galactokinaseCitation36,Citation37. Therefore, the data were also fitted to a modified form of the Michaelis–Menten equation which accounts for substrate inhibition. This fit results in a value for the substrate inhibition constant, Kis, of 13 ± 8 mM and modified values for Km and kcat of 73 ± 27 mM and 1.2 ± 0.1 s−1. Low levels of activity were detectable with galactosamine and galactose, but these were too small to permit meaningful kinetic analysis.
Figure 3. Recombinant human GALK2 (50 nM) is an active N-acetylgalactosamine kinase. In (a), the concentration of ATP was maintained at a constant level of 0.5 mM and N-acetylgalactosamine (GalNAc) was varied. The resulting data were fit (i) to the Michaelis–Menten equation (giving values of Km,GalNAc = 40 ± 14 μM and kcat = 1.0 ± 0.1 s−1) and (ii) to a modified version of this equation which accounts for substrate inhibition (giving values of Kis = 13 ± 8 mM, Km = 73 ± 27 mM, and kcat = 1.2 ± 0.1 s−1). In (b) the GalNAc concentration was maintained at 5 mM and the concentration of ATP was varied (giving values of Km,ATP = 14 ± 3 μM and kcat = 1.0 ± 0.1 s−1). Each point represents the mean of two or three separate determinations and the error bars the standard deviations of these means.
Inhibition by methylgalactopyranosides
Since 1-methylpyranosides do not undergo anomeric interconversion in water, the α- and β-methylgalactopyranosides () are stable compounds in aqueous solution. Both were tested as potential inhibitors of human GALK2. Although β-methylgalactopyranoside was shown to reduce the rate of reaction catalyzed by GALK2, this effect was slight, and consequently was difficult to measure (data not shown). In contrast, reproducible measurements could be produced with α-methylgalactopyranoside and, thus, further analysis was concentrated on this compound.
For a competitive inhibitor, a plot of reciprocal rate against inhibitor concentration (the so-called Dixon plot) results in a straight line at a constant substrate concentration. Repetition of the experiment with different substrate concentrations results in a family of straight lines which cross at a single point corresponding to the reciprocal Vmax and minus the competitive inhibition constant, KicCitation32,Citation38,Citation39. This was not observed under conditions where the GalNAc concentration was saturating, and three lower concentrations of ATP were used with a range of α-methylgalactopyranoside concentrations (). In contrast, for uncompetitive inhibition, plots of substrate concentration divided by rate plotted against inhibitor concentration gives straight lines for each substrate concentration which intersect at Km/Vmax and minus the uncompetitive inhibition constant, KiuCitation39. This was observed with GALK2 (), and thus it can be concluded that α-methylgalactopyranoside is an uncompetitive inhibitor with respect to ATP. Since Kiu is estimated to be approximately 150 mM, this compound is clearly a very poor inhibitor of GALK2.
Figure 4. The inhibition of GALK2 (50 nM) by α-methylgalactopyranoside. Inhibition was studied over a range of α-methylgalactopyranoside concentrations and three different ATP concentrations: 8 μM (inverted triangles, ▾), 15 μM (squares, ▪), and 60 μM (upright triangles, ▴). (a) In a Dixon plot the lines do not converge, suggesting that the inhibition is not competitive. (b) The converging lines on a plot of [ATP]/rate against [inhibitor] suggest that the mode of inhibition is uncompetitive with respect to ATP. In all cases the N-acetylgalactosamine concentration was 1.0 mM.
![Figure 4. The inhibition of GALK2 (50 nM) by α-methylgalactopyranoside. Inhibition was studied over a range of α-methylgalactopyranoside concentrations and three different ATP concentrations: 8 μM (inverted triangles, ▾), 15 μM (squares, ▪), and 60 μM (upright triangles, ▴). (a) In a Dixon plot the lines do not converge, suggesting that the inhibition is not competitive. (b) The converging lines on a plot of [ATP]/rate against [inhibitor] suggest that the mode of inhibition is uncompetitive with respect to ATP. In all cases the N-acetylgalactosamine concentration was 1.0 mM.](/cms/asset/4421913e-ef71-49d6-a963-7cc1546b1749/ienz_a_418122_f0004_b.gif)
Effects of pH on the turnover number
Careful analysis of the effects of pH on enzyme catalyzed reactions can reveal information about the chemical steps occurring in the enzyme’s mechanism. Specifically, they can tell us about the role of ionizable residues in binding to the substrates and in acid–base catalysis. The kcat showed a marked dependence on pH (), with maximal activity being observed between pH 7 and 8. Fitting of the data (as described in “Materials and methods”) to a simple model for pH effects on kcatCitation34 was successful (R2 = 0.7405), yielding pKa values of 6.5 and 10.3, although the standard errors in the estimation of the two Ka values were quite high (77% and 120% of the estimates).
Discussion
Recombinant human N-acetylgalactosamine kinase is active and displays similar properties to native mammalian enzymeCitation9,Citation10. The observation of possible substrate inhibition by GalNAc combined with the uncompetitive nature of α-methylgalactopyranoside inhibition argues strongly for an ordered ternary complex mechanism in which ATP is the first substrate to bind. Such a mechanism was also seen in some bacterial, pig, rat, human, and yeast galactokinasesCitation29,Citation36,Citation37,Citation40–42, although not in E. coli galactokinase (where a random ternary complex mechanism was observedCitation43) or plant galactokinases where galactose is the first substrate to bind in an ordered mechanismCitation44,Citation45. The greater potency of α-methylgalactopyranoside as an inhibitor, compared to β-methylgalactopyranoside, suggests (but does not prove) that the enzyme, like galactokinaseCitation46, has a preference for the α-anomer of its sugar substrate.
The interpretation of pH studies is complex, and care must be taken to distinguish specific effects resulting from the ionization of individual, catalytically important residues from generic effects on the protein (or the substrates) as a whole. The pH profile seen here is not the perfect bell-shaped curve that would be expected to arise from the ionization of two, catalytically important active site residues. Nevertheless, that a pH dependence was observed suggests that ionizable residues may be involved in catalysis, resulting in the observed alterations in kcat.
There is considerable debate in the field of GHMP kinases about the role of active site acids and bases in catalysis. The crystal structures of galactokinase, N-acetylgalactosamine kinase, and mevalonate kinaseCitation7,Citation8,Citation47–51 each show an aspartate residue in the active site appropriately positioned to abstract a proton from the substrate (see for a depiction of the location of Asp-190, the residue proposed to act as an active site base in GALK2). In each case a positively charged residue (in the GALK2, Arg-43) is located close to the putative active site base and is believed to maintain the ionization state of the carboxylate side chain. In theory, this arrangement, which involves one acidic and one basic residue, should result in a bell-shaped pH dependence of kcat. However, detailed pH and deuterium kinetic isotope studies on the yeast galactokinase, Gal1p, showed little effect of pH on kcatCitation42. Furthermore, the crystal structure of homoserine kinase reveals no obvious active site base, suggesting that GHMP kinases are able to catalyze their reactions by alternative mechanisms, perhaps involving transition-state stabilizationCitation52,Citation53. It has been postulated that all GHMP kinases proceed not via acid–base catalysis but through an approximation mechanismCitation8. In such a mechanism, the enzyme’s role is to hold the substrates in a conformation which facilitates conversion to the transition state. The reaction would begin with partial breaking and lengthening of the γ-phosphorus–oxygen bond in ATP. Subsequent movement of the γ-phosphate group toward the hydroxyl group on the sugar would displace the proton from the hydroxyl. The proton may be picked up by Asp-190 in the active siteCitation8. An approximation mechanism would also show marked pH effects, similar to the ones observed here.
Given the relatively large errors, the estimated pKa values determined for GALK2 should be treated with some caution (especially the higher value). However, assuming that they represent the unperturbed pKa values of single amino acid side chains, reasonable assignments would be to a histidine residue for the lower value and either a lysine or tyrosine for the higher valueCitation34. Neither value is consistent with an aspartate residue, unless its pKa has been raised by almost two orders of magnitude. Furthermore, such a raised pKa would make the residue less able to abstract a proton from the sugar. Thus, the data presented here are more consistent with an approximation mechanism. However, further studies on this fascinating group of enzymes will be required to resolve these matters.
Acknowledgements
We thank Peter McColgin and Andrew Frazer who carried out preliminary work on human GALK2 and Prof Hazel Holden (University of Wisconsin) who provided the GALK2 expression construct.
Declaration of interest: This work was funded in part by a grant from the Royal Society, UK (2004/R1).
References
- Timson DJ. GHMP kinases – structures, mechanisms and potential for therpeutically relevant inhibition. Curr Enzyme Inhib 2007;3:77–94.
- Bork P, Sander C, Valencia A. Convergent evolution of similar enzymatic function on different protein folds: the hexokinase, ribokinase, and galactokinase families of sugar kinases. Protein Sci 1993;2:31–40.
- Sherson S, Gy I, Medd J, Schmidt R, Dean C, Kreis M, et al. The arabinose kinase, ARA1, gene of Arabidopsis is a novel member of the galactose kinase gene family. Plant Mol Biol 1999;39:1003–12.
- Daugherty M, Vonstein V, Overbeek R, Osterman A. Archaeal shikimate kinase, a new member of the GHMP-kinase family. J Bacteriol 2001;183:292–300.
- Bajwa W, Torchia TE, Hopper JE. Yeast regulatory gene GAL3: carbon regulation; UASGal elements in common with GAL1, GAL2, GAL7, GAL10, GAL80, and MEL1; encoded protein strikingly similar to yeast and Escherichia coli galactokinases. Mol Cell Biol 1988; 8:3439–47.
- Luz JG, Hassig CA, Pickle C, Godzik A, Meyer BJ, Wilson IA. XOL-1, primary determinant of sexual fate in C. elegans, is a GHMP kinase family member and a structural prototype for a class of developmental regulators. Genes Dev 2003;17:977–90.
- Thoden JB, Timson DJ, Reece RJ, Holden HM. Molecular structure of human galactokinase: implications for type II galactosemia. J Biol Chem 2005;280:9662–70.
- Thoden JB, Holden HM. The molecular architecture of human N-acetylgalactosamine kinase. J Biol Chem 2005;280:32784–91.
- Pastuszak I, O’Donnell J, Elbein AD. Identification of the GalNAc kinase amino acid sequence. J Biol Chem 1996;271:23653–56.
- Pastuszak I, Drake R, Elbein AD. Kidney N-acetylgalactosamine (GalNAc)-1-phosphate kinase, a new pathway of GalNAc activation. J Biol Chem 1996;271:20776–82.
- Timson DJ, Reece RJ. Sugar recognition by human galactokinase. BMC Biochem 2003;4:16.
- Caputto R, Leloir LF, Trucco RE, Cardini CE, Paladini AC. The enzymatic transformation of galactose into glucose derivatives. J Biol Chem 1949;179:497–8.
- Cardini CE, Leloir LF. Enzymic phosphorylation of galactosamine and galactose. Arch Biochem Biophys 1953;45:55–64.
- Holden HM, Thoden JB, Timson DJ, Reece RJ. Galactokinase: structure, function and role in type II galactosemia. Cell Mol Life Sci 2004;61:2471–84.
- Zafra MF, Riquelme S, Castillo M, Garcia-Peregrin E. Effect of clofibrate on brain mevalonate-5-pyrophosphate decarboxylase. Neurochem Res 1987;12:787–90.
- Qiu Y, Li D. Inhibition of mevalonate 5-diphosphate decarboxylase by fluorinated substrate analogs. Biochim Biophys Acta 2006;1760:1080–7.
- Qiu Y, Li D. Bifunctional inhibitors of mevalonate kinase and mevalonate 5-diphosphate decarboxylase. Org Lett 2006;8:1013–16.
- Quistad GB, Cerf DC, Schooley DA, Staal GB. Fluoromevalonate acts as an inhibitor of insect juvenile hormone biosynthesis. Nature 1981;289:176–7.
- Rohmer M, Grosdemange-Billiard C, Seemann M, Tritsch D. Isoprenoid biosynthesis as a novel target for antibacterial and antiparasitic drugs. Curr Opin Invest Drugs 2004;5:154–62.
- Bosch AM, Bakker HD, van Gennip AH, van Kempen JV, Wanders RJ, Wijburg FA. Clinical features of galactokinase deficiency: a review of the literature. J Inherit Metab Dis 2002;25:629–34.
- Elsas LJ 2nd, Lai K. The molecular biology of galactosemia. Genet Med 1998;1:40–8.
- Petry KG, Reichardt JK. The fundamental importance of human galactose metabolism: lessons from genetics and biochemistry. Trends Genet 1998;14:98–102.
- Gitzelmann R. Galactose-1-phosphate in the pathophysiology of galactosemia. Eur J Pediatr 1995;154:S45–9.
- Mayes JS, Miller LR, Myers FK. The relationship of galactose-1-phosphate accumulation and uridyl transferase activity to the differential galactose toxicity in male and female chicks. Biochem Biophys Res Commun 1970;39:661–5.
- Slepak T, Tang M, Addo F, Lai K. Intracellular galactose-1-phosphate accumulation leads to environmental stress response in yeast model. Mol Genet Metab 2005;86:360–71.
- Wierenga KJ, Lai K, Buchwald P, Tang M. High-throughput screening for human galactokinase inhibitors. J Biomol Screen 2008;13:415–23.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
- Platt A, Ross HC, Hankin S, Reece RJ. The insertion of two amino acids into a transcriptional inducer converts it into a galactokinase. Proc Natl Acad Sci USA 2000;97:3154–9.
- Timson DJ, Reece RJ. Functional analysis of disease-causing mutations in human galactokinase. Eur J Biochem 2003;270:1767–74.
- Horecker BL, Kornberg A. The extinction coefficients of the reduced band of pyridine nucleotides. J Biol Chem 1948;175:385–90.
- Marquardt D. An algorithm for least squares estimation of nonlinear parameters. SIAM J Appl Math 1963;11:431–41.
- Cornish-Bowden A. Fundamentals of Enzyme Kinetics. London: Portland Press, 2004.
- Ellis KJ, Morrison JF. Buffers of constant ionic strength for studying pH-dependent processes. Methods Enzymol 1982;87:405–26.
- Tipton KF, Dixon HB. Effects of pH on enzymes. Methods Enzymol 1979;63:183–234.
- Timson DJ. Functional analysis of disease-causing mutations in human UDP-galactose 4-epimerase. FEBS J 2005;272:6170–7.
- Ballard FJ. Purification and properties of galactokinase from pig liver. Biochem J 1966;98:347–52.
- Ballard FJ. Kinetic studies with liver galactokinase. Biochem J 1966;101:70–5.
- Dixon M. The determination of enzyme inhibitor constants. Biochem J 1953;55:170–1.
- Cornish-Bowden A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J 1974;137:143–4.
- Walker DG, Khan HH. Some properties of galactokinase in developing rat liver. Biochem J 1968;108:169–75.
- Lee L, Kinoshita S, Kumagai H, Tochikura T. Galactokinase of Bifidobacterium bifidum. Agric Biol Chem 1980;44:2961–6.
- Timson DJ, Reece RJ. Kinetic analysis of yeast galactokinase: implications for transcriptional activation of the GAL genes. Biochimie 2002;84:265–72.
- Gulbinsky JS, Cleland WW. Kinetic studies of Escherichia coli galactokinase. Biochemistry 1968;7:566–75.
- Dey PM. Galactokinase of Vicia faba seeds. Eur J Biochem 1983;136:155–9.
- Foglietti MJ, Percheron F. Purification and mechanism of action of a plant galactokinase. Biochimie 1976;58:499–504.
- Howard SM, Heinrich MR. The anomeric specificity of yeast galactokinase. Arch Biochem Biophys 1965;110:395–400.
- Thoden JB, Sellick CA, Timson DJ, Reece RJ, Holden HM. Molecular structure of Saccharomyces cerevisiae Gal1p, a bifunctional galactokinase and transcriptional inducer. J Biol Chem 2005;280:36905–11.
- Fu Z, Wang M, Potter D, Miziorko HM, Kim JJ. The structure of a binary complex between a mammalian mevalonate kinase and ATP: insights into the reaction mechanism and human inherited disease. J Biol Chem 2002;277:18134–42.
- Yang D, Shipman LW, Roessner CA, Scott AI, Sacchettini JC. Structure of the Methanococcus jannaschii mevalonate kinase, a member of the GHMP kinase superfamily. J Biol Chem 2002;277:9462–7.
- Thoden JB, Holden HM. Molecular structure of galactokinase. J Biol Chem 2003;278:33305–11.
- Hartley A, Glynn SE, Barynin V, Baker PJ, Sedelnikova SE, Verhees C, et al. Substrate specificity and mechanism from the structure of Pyrococcus furious galactokinase. J Mol Biol 2004;337:387–98.
- Zhou T, Daugherty M, Grishin NV, Osterman AL, Zhang H. Structure and mechanism of homoserine kinase: prototype for the GHMP kinase superfamily. Struct Fold Des 2000;8:1247–57.
- Krishna SS, Zhou T, Daugherty M, Osterman A, Zhang H. Structural basis for the catalysis and substrate specificity of homoserine kinase. Biochemistry 2001;40:10810–18.

![Figure 5. The effects of pH on the turnover number, kcat. The GALK2 concentration was 230 nM in these experiments. The line represents a fit to the equation kcat = kcat,lim(1 + [H+]/Ka1 + Ka2/[H+]).](/cms/asset/40afba64-ba34-42a4-81c2-328ad8ef9dbe/ienz_a_418122_f0005_b.gif)