Abstract
In the current practices of antiinfective therapy, ciprofloxacin is a very popular fluoroquinolone having a broad spectrum of activity and diverse therapeutic prospects. The reasons for its wide use include multiresistant pathogens susceptible only to ciprofloxacin. The available clinical evidence suggests the potentially enhanced efficacy of this drug in the treatment of various community acquired and nosocomial infections, e.g. respiratory tract, urinary tract, and skin infections and sexually transmitted diseases. As compared to other agents of its class, the pharmacokinetic profile of ciprofloxacin demonstrates equivalent or greater bioavailability, higher plasma concentrations, and increased tissue penetration, as reflected in the greater volume of distribution. Various molecular modifications of this drug have been made to further improve its characteristics. Several methods of analytical determination of ciprofloxacin and its metabolites in biological fluids employing various techniques have been reported. The present article is focused on the synthetic development, pharmacotherapeutic, and analytical evaluation vistas of ciprofloxacin.
Introduction
During the past 25 years, antimicrobial agents have been introduced at a rate exceeding our ability to integrate them into clinical practiceCitation1,Citation2. Since their introduction, fluoroquinolones have become a mainstay in the treatment of serious bacterial infectionsCitation3,Citation4. These are synthetic antibacterial agents structurally related to nalidixic acidCitation5. They depict several favorable properties such as excellent bioavailability, good tissue penetrability, and a relatively low incidence of adverse and toxic effectsCitation6. These drugs are potentially used in the treatment of urinary tract infection and prostatitis. They are also employed against bacterial enteric infections, biliary tract infections, sexually transmitted diseases, and prophylaxis in the immunocompromised neutropenic hostCitation7–9. One of the most successful and widely used compounds of the classCitation10, ciprofloxacin, was patented in 1983 by Bayer A.G. and subsequently approved by the United States Food and Drug Administration (US FDA) for use in the United States in 1987. Ciprofloxacin is marketed worldwide, with well over 300 different brand names, and since its introduction, the value of fluoroquinolones for the respective uses has been recognizedCitation11. The licensed uses for ciprofloxacin in the United States are quite limited, and it is to be considered as a drug of final resort when all other antibiotics have failed. There are 10 approved uses of this drug in the adult population and two approved uses in the pediatric population, as well as a variety of veterinary uses (as documented within the package inserts). Being not approved by the FDA, its other uses can be considered as off-label. Ciprofloxacin may interact with a number of other drugs, some herbal and natural supplements, and certain thyroid medicationsCitation12. Ciprofloxacin () has proved to be a blockbuster drug for Bayer A.G., generating billions of dollars in additional revenue. In 1999, ciprofloxacin was the 11th most prescribed drug in the United States, based on new prescriptions, and ranked 20th in total United States sales. In 1999, Bayer’s gross sales of ciprofloxacin in the United States were approximately $1.04 billion. The sale of ciprofloxacin increased dramatically following the anthrax scare of 2001. A deal between the US Government and Bayer Pharmaceuticals was announced to purchase 100 million tablets of ciprofloxacin. In 2004, ciprofloxacin and levofloxacin together commanded 65% ($3.3 billion) of global sales. Sales of ciprofloxacin for the first 9 months of 2008 were $242 million, as compared to $324 million for Bayer Aspirin. Some brands of ciprofloxacin are mentioned in .
Table 1. Major brands of ciprofloxacin in some countriesCitation13,Citation14.
Fluoroquinolones are classified on the basis of their spectrum of activity and pharmacokinetic profile. Ciprofloxacin is the most potent fluoroquinolone, active against a broad range of bacteriaCitation15, the most susceptible being the aerobic Gram-negative bacilli, especially the enterobacteriaceae and NeisseriaCitation16. It is a second-generation fluoroquinolone, and has depicted a considerable and myriad spectrum of activity for several infectious conditions. Ciprofloxacin is a very promising and efficacious drug, having potent antibacterial activity along with well-established safety aspects. Since its approval, ciprofloxacin has been extensively studied; more than 250 million patients have been treated successfully worldwide, and its safety profile is well documented in a commendable number of scientific publicationsCitation17. Ciprofloxacin offers a potential alternative for antimicrobial therapy in pediatric populations, such as children with cystic fibrosisCitation4. Data from more than 1500 pediatric patients treated with ciprofloxacin for infection related to cystic fibrosis have shown a safety profile in children and adolescents comparable to that in adult patientsCitation18. In vitro susceptibility data for commonly used fluoroquinolones show that the minimum inhibitory concentration (MIC) of ciprofloxacin is distinctly superior to other fluoroquinolones when tested against several Gram-negative bacterial strains. Against Escherichia coli, the MIC90 for ciprofloxacin was found to be 0.015–0.25, as compared to MICs90 of 0.12–0.39, 0.06–0.25, and 0.03–0.5 for ofloxacin, levofloxacin, and trovafloxacin, respectively. Similarly, against Klebsiella pneumoniae, the MIC90 was found to be 0.05–1.0 for ciprofloxacin when compared to 0.1–0.5 and 0.12–1.0 for levofloxacin and ofloxacin, respectivelyCitation19. In another investigation, Antonio et al. found that ciprofloxacin was more efficacious than some other fluoroquinolones such as ofloxacin and pefloxacin in preventing infections due to Gram-negative bacteria in a specific population of neutropenic patientsCitation20. Further, Yamane et al. reported that the activity of levofloxacin against Gram-positive bacteria was either equal to or less than that of ciprofloxacinCitation21. In another study, topical ciprofloxacin/dexamethasone therapy was found to be superior to oral amoxicillin/clavulanic acid in acute otitis media with otorrheaCitation22. Literature findings also suggest that ciprofloxacin, when given orally, is superior to ampicillin and chloramphenicol in curing typhoid in immunocompromised miceCitation23.
Ciprofloxacin, a commonly used broad-spectrum antibiotic, has also attracted significant interest of the scientific community due to its antiproliferative and apoptotic activities in several cancer cell lines. It was observed that it can induce time- and dose-dependent growth inhibition and apoptosis of various carcinoma, osteosarcoma, and leukemia cell linesCitation24. Aranha et al. found that ciprofloxacin may have a profound effect in bladder cancer management. In vitro studies on tumor cells derived from transitional cell carcinoma of the bladder revealed a dose- and time-dependent inhibition of cell growth by ciprofloxacin at concentrations that are easily attainable in the urine of patients. These studies provided strong experimental evidence for the use of this fluoroquinolone agent as a potential preventive and/or therapeutic agent for the management of transitional cell carcinoma of the bladderCitation25. They further demonstrated suppression of human prostate cancer cell growth by ciprofloxacin associated with cell cycle arrest and apoptosis. This fluoroquinolone showed antiproliferative and apoptosis-inducing activity on prostate cancer cells at the doses generally required for the treatment of antibacterial infections. It was also found that ciprofloxacin did not have any effect on non-tumorigenic prostate epithelial cells. The main advantage of this antibiotic is its relative non-toxicity as compared to current chemotherapy, which is not very effective, for the treatment of advanced hormone resistant prostate cancerCitation26. Doxorubicin and docetaxel, two standard antineoplastic agents in hormone-refractory prostate cancer (HRPC) therapy, and ciprofloxacin were evaluated singly and in several simultaneous and sequential drug combination schemes by Pinto and co-workers. Ciprofloxacin exhibited sensitization of HRPC cell lines to doxorubicin and docetaxel treatment in a schedule-dependent manner. It was inferred that ciprofloxacin may play a significant role as a chemosensitizing agent in prostate cancer treatmentCitation27. Bourikas et al. also demonstrated novel antiproliferative and immunoregulatory effects of ciprofloxacin on human intestinal epithelial cells in a concentration- and time-dependent mannerCitation28.
Herold et al. reported ciprofloxacin-induced growth inhibition and apoptosis in colon carcinoma cell lines, whereas hepatoma cells remained unaffected. The growth arrest was mediated through the inhibition of DNA synthesis, induction of mitochondrial injury, and subsequent apoptosis. Therefore, due to the encouraging effects of this topoisomerase inhibitor, ciprofloxacin can be explored as a good candidate for such therapiesCitation24. Moreover, ciprofloxacin is generally used in several large cancer treatment centers for antimicrobial prophylaxis in high-risk patients with prolonged neutropenia, including patients with acute leukemia undergoing remission induction chemotherapy, and recipients of bone marrow transplantationCitation29,Citation30. Also, orally administered ciprofloxacin is a safe and effective therapy of many serious infections in cancer patientsCitation31.
Various alterations have been made to improve the antibacterial activity of this drug molecule; however, the development has been primarily focused on the following aspectsCitation10,Citation32,Citation33:
Increased activity against resistant strains of microbes, anaerobes, and atypical organismsCitation34;
Reduced rate of emergence of resistanceCitation35;
Improved pharmacokinetics and pharmacodynamic profileCitation32.
Mode of action
Fluoroquinolones inhibit the bacterial enzyme DNA gyrase, which nicks double-stranded DNA, introduces negative supercoils, and then reseals the nicked end. This is necessary to prevent excessive positive supercoiling of the strands when they separate to permit replication and transcriptionCitation36,Citation37. Ciprofloxacin inhibits the activity of DNA gyrase, an essential adenosine triphosphate-hydrolyzing topoisomerase II enzyme, or it prevents the detachment of gyrase from DNA. The topoisomerases exert their bactericidal activity by interacting with the DNACitation38. During the processes of replication and transcription, an enzyme called helicase unwinds the DNA double helix. The uncoiling process creates tension due to excess supercoiling of the remaining DNA double helix. This tension needs to be relieved if the process is to continue. The topoisomerase II enzyme allows the relaxation of supercoiled DNA by breaking both strands of the DNA chain, crossing them over, and finally resealing themCitation3.
Current synthetic developments
Extensive efforts have been undertaken for the synthetic development and to derive an array of significantly potent analog candidates of this drug. Molecular modification for lead optimization by bioisostearic replacements, homologation of the side chain or branching of the side chain, stereochemistry, and other useful techniques of analogous design and development of ciprofloxacin have given rise to agents with broad-spectrum activity and minimal toxic or side effectsCitation9. A number of derivatives of ciprofloxacin have been reported that have shown improved activity and potency. Some important examples are described below.
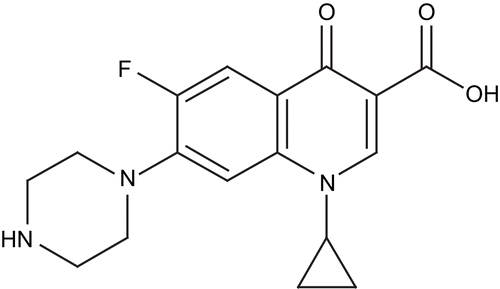
Shaharyar et al. synthesized a series of 1-[(sub)]-6-fluoro-3-[(sub)]-1,3,4-oxadiazol-2-yl-7-piperazino-1,4-dihydro-4-quinolones by the reaction of ciprofloxacin with an appropriate acid hydrazide in phosphorus oxychloride. All newly synthesized compounds were evaluated for antiproliferative activity against human lung tumor cell lines (A549). Among the synthesized compounds, 1-cyclopropyl-6-fluoro-3-(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)-7-(piperazin-1-yl)quinolin-4(1H)-one (a) was found to be the most active compoundCitation39 ().
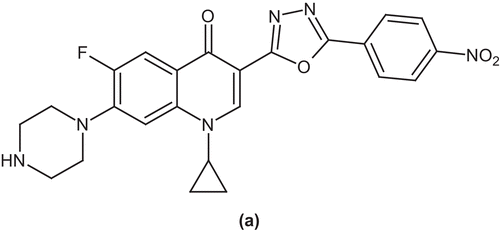
Vila et al. reported a number of ciprofloxacin derivatives and tested them against quinolone susceptible and resistant E. coli, Acinetobacter baumannii, Stenophotromonas maltophilia, and Staphylococcus aureus strains using a microdilution test. Among these derivatives, 4-methyl-7-piperazine ciprofloxacin (b) derivative showed a minimum inhibitory concentration for 50% of the organisms that was 16- and 8-fold lower than that of ciprofloxacin for A. baumannii and S. maltophilia, respectivelyCitation40 ().
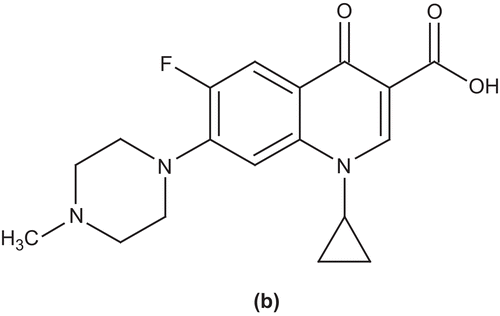
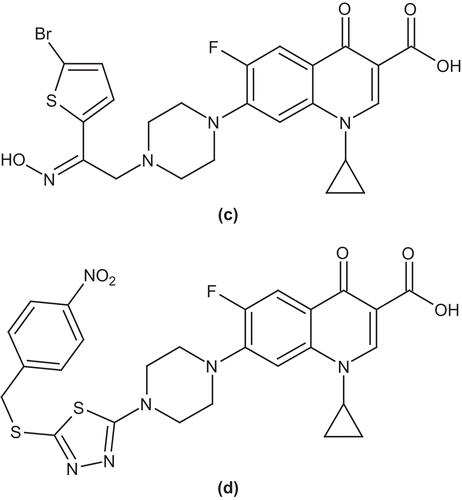
Foroumadi et al. synthesized and evaluated a series of N-[2-(5-bromothiophen-2-yl)-2-oxoethyl] and N-[2-(5-bromothiophen-2-yl)-2-oximinoethyl] derivatives of ciprofloxacin for antimicrobial activity against Gram-positive and Gram-negative microorganisms. Among the synthesized compounds, 7-(4-(2-(5-bromothiophen-2-yl)-2-(hydroxyimino)ethyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (c) showed significant activity against Gram-positive bacteria such as S. aureus, S. epidermidis, and Bacillus subtilisCitation41. In another study, they examined a series of N-(5-benzylthio-1,3,4-thiadiazol-2-yl) and N-(5-benzylsulfonyl-1,3,4-thiadiazol-2-yl) derivatives of piperazinyl quinolone and evaluated them for antibacterial activity against different microorganisms. Among the synthesized compounds, 7-(4-(5-(4-nitrobenzylthio)-1,3,4-thiadiazol-2-yl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (d) exhibited high activity against Gram-positive bacteriaCitation42 ().
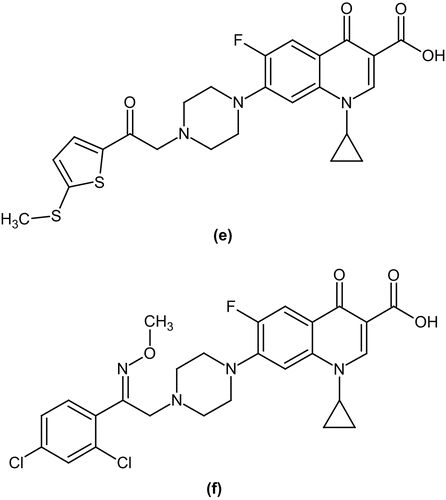
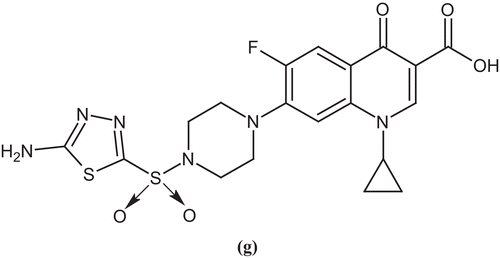
In the proceeding year, Foroumadi et al. examined a number of piperazinyl N-substituted ciprofloxacin derivatives for antibacterial activity against Gram-positive and Gram-negative bacteria. Among these derivatives, 1-cyclopropyl-6-fluoro-7-(4-(2-(5-(methylthio)thiophen-2-yl)-2-oxoethyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (e) showed significant improvement of potency against staphylococci, maintaining Gram-negative coverageCitation43. In the same year, they reported a number of N-(phenethyl)piperazinyl quinolone derivatives bearing a methoxyimino-substituent and evaluated them for antimicrobial activity against Gram-positive and Gram-negative microorganisms. In the series, 1-cyclopropyl-7-(4-(2-(2,4-dichlorophenyl)-2-(methoxyimino)ethyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (f) proved to be the most active analog against tested strainsCitation44 ().
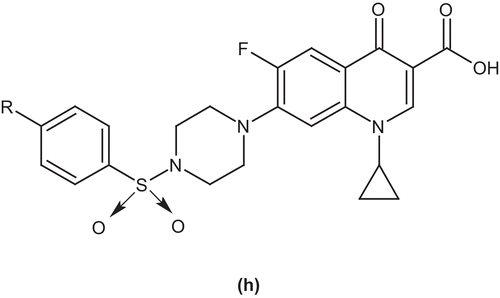
Talath et al. evaluated a series of 7-[4-(5-amino-1,3,4 thiadiazole-2-sulfonyl)]-1-piperazinyl fluoroquinolone derivatives. The compounds were evaluated for their in vitro antibacterial activity against some Gram-positive and Gram-negative bacteria and antitubercular activity against Mycobacterium tuberculosis H37Rv strain by the broth dilution assay method. The compound 7-(4-(5-amino-1,3,4 thiadiazole-2-ylsulfonyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (g) showed better antibacterial activity against Gram-positive bacteria as compared to the reference drug ciprofloxacinCitation45 ().
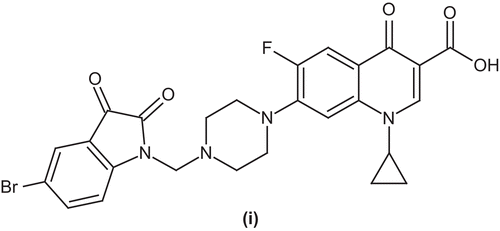
Nieto et al. characterized and tested a series of new benzenesulfonamide analogs of ciprofloxacin (h). Quantitative structure–activity relationship (QSAR) studies through Hansch analysis showed a linear correlation of the activity with electronic and steric parameters. Small electron-donor groups increased the in vitro activity against Gram-positive bacteria. Hydrophobic properties played a minor role when activity was measured as minimum inhibitory concentration. Finally, according to this QSAR study, the amino and methyl amino derivatives were found to be the most active analogs within this seriesCitation46 ().
Sriram et al. reported a number of 7-substituted ciprofloxacin derivatives and evaluated them for antimycobacterial activity in vitro and in vivo against M. tuberculosis and for inhibition of the supercoiling activity of DNA gyrase from M. smegmatis. Preliminary results indicated that compound 7-[4-{(5-bromo-2,3-dioxoindolin-1-yl)methyl}piperazin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (i) showed moderate activityCitation47 ().
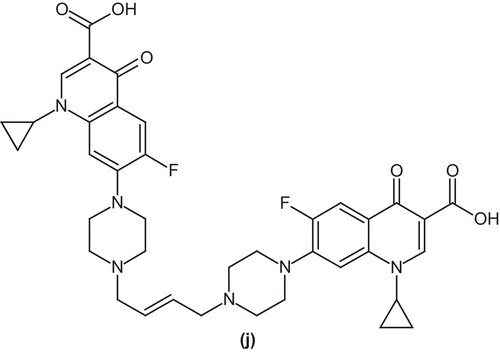
Kerns et al. screened a number of symmetric and asymmetric piperazinyl linked dimers of ciprofloxacin. A specific trans butenyl-linked dimer of ciprofloxacin (j) showed potent antibacterial activity against drug resistant strains of S. aureusCitation48 ().
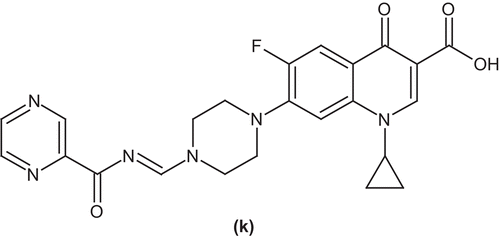
Imramovsky et al. studied a new type of potentially active molecule in which the connection of two active molecules across an easily released bridge is established. The synthesis is based on derivatives that originate from ciprofloxacin (k). The lipophilicity, hydrolysis (stability of the compound), and antitubercular activity as well as the structure lipophilicity and structure–activity relationship were studiedCitation49 ().
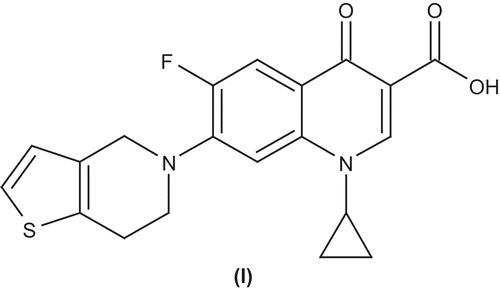
Srivastava et al. synthesized and evaluated antibacterial activities of a number of substituted 4,5,6,7-tetrahydro-thieno[3,2-c]pyridine quinolones (l). The activities were evaluated against a standard using the in vitro MIC assay method. Some of the compounds showed in vitro antibacterial activity comparable to that of ciprofloxacinCitation50 ().
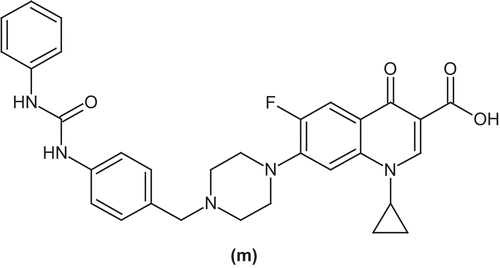
German et al. reported ciprofloxacin derivatives as substrate based inhibitors of bacterial efflux pumps. Bacterial efflux pump systems contribute to antimicrobial resistance in pathogenic bacteria. The co-administration of bacterial efflux pump inhibitors with antibiotics is being pursued to overcome efflux-mediated resistance to antibiotics. In this study, they evaluated some derivatives of ciprofloxacin bearing a bis aryl urea efflux pump inhibitor at the C-7 position (m)Citation51 ().
Al-Soud et al. examined a new class of dihaloquinolones bearing N-aldehydoglycosylhydrazides, mercapto-1,2,4-triazole, oxadiazoline, and α-amino ester precursors. These novel compounds are also conjugated with ciprofloxacin and have shown antimicrobial activityCitation52.
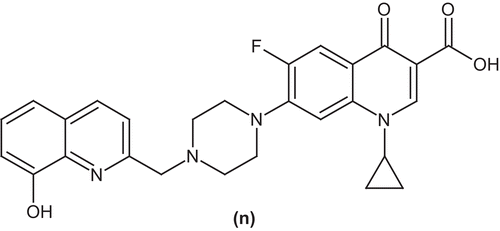
Zhao et al. investigated a number of ciprofloxacin derivatives and evaluated for antimycobacterial activity. A ciprofloxacin derivative (n) exhibited 98% inhibition of the growth of M. tuberculosisCitation53 ().
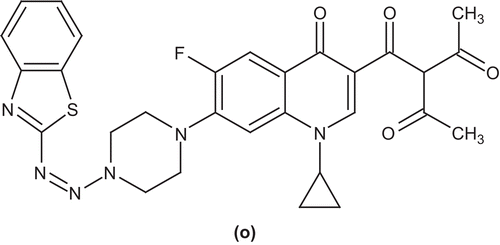
Yadav et al. afforded piperiazine-substituted ciprofloxacin derivatives by the reaction of ciprofloxacin with benzothiazole (o), thiazole, and diazonium chloride. The in vitro antibacterial activities of these compounds were determined by the conventional agar dilution method. The MIC of the test derivatives against the Staphylococcus strain indicated that most of the derivatives possessed better activity with respect to ciprofloxacinCitation54 ().
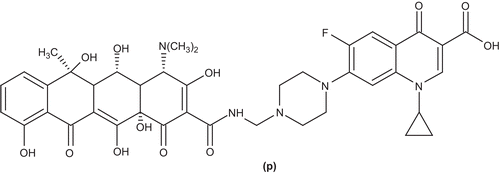
Sriram et al. synthesized new ciprofloxacin tetracycline conjugates by reacting appropriate tetracyclines, formaldehyde, and the secondary amino (piperazino) function of ciprofloxacin (p) using a microwave irradiation technique and evaluated them for anti-human immunodeficiency virus (HIV) and antimycobacterial activitiesCitation55 ().
Ye et al. reported ciprofloxacin derivatives primarily from 2-methyl-5-nitroimidazole and ciprofloxacin through nucleophilic substitution. The antibacterial activities of the synthesized compounds were evaluated. Nine new compounds were synthesized, some of which have exhibited appreciable antibacterial activityCitation56.
Bani et al. studied two new derivatives from the ciprofloxacin fluoroquinoline family, 7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-quinoline-3-methylcarbamate and 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-(3-oxopyrazolo)[4,3-c]quinoline, and tested them for antibacterial activity. Their molecular and crystal structures were determined. In vitro tests revealed lower activities than for ciprofloxacin. Characteristic structural features of these compounds are comparable to data for other known fluoroquinolinesCitation57.
Hu et al. discovered a novel antitumor lead compound derived from fluoroquinolone, in which the C-3 carboxyl group of ciprofloxacin was replaced with a heterocyclic ring to form the cyclopropyl fluoroquinolone aminothiadiazole scaffold and which was then reacted with aromatic aldehydes to give the Schiff base compounds. The structures of the new compounds were characterized by elemental analysis and spectral data, and their in vitro antitumor activity determined against SMMC-7721, HL60, and L1210 cell lines. The bioactive assay showed that 11 thiadiazole-substituted ciprofloxacin derivatives displayed potential cytotoxicity against the tested cancer cell linesCitation58.
It is generally believed that the action of fluoroquinolones increases with an increase in lipophilicity. However, in an experiment, Vazquez et al. determined the partition coefficients of a homologous series of ciprofloxacin in which a parabolic behavior was observed which evidenced that merely an increase in lipophilicity does not result in enhanced antimicrobial activityCitation59.
Several reports have also highlighted the interest in increasing the lipophilicity to improve the antitumor efficacy. Azema et al. synthesized novel 7-((4-substituted) piperazin-1-yl) derivatives of ciprofloxacin having various lipophilicities, and evaluated them in vitro as potential antitumor agents in five human cancer cell lines. The compounds exhibited potent activitiesCitation60.
Siddiqui et al. prepared a series of ciprofloxacin skeletal variants and screened them for antifungal, antimicrobial, and cytotoxic activities. The structures of these derivatives were confirmed by using spectral techniques such as infrared (IR), Citation1H nuclear magnetic resonance (NMR), and electron impact mass spectroscopy (EIMS). Cytotoxicity activities of these derivatives were determined in order to establish the effect of substituents on the biological activityCitation61.
Sharma et al. synthesized and evaluated 1-cyclopropyl-6-fluoro-1,4-dihydro-7-4-substituted-piperazin-1-yl-4-oxoquinoline-3-carboxylates. Compounds exhibited moderate to significant activitiesCitation62.
Analytical aspects
In recent years, there has been rapid progress in quinolone research and development, resulting in the production of many clinically important fluoroquinolones, which have been subjected to diverse analytical or assay methods. Various physicochemical properties and analytical methods have been used for the determination of ciprofloxacin in pharmaceutical dosage forms and biological fluids.
Physical properties
Ciprofloxacin occurs as a white powder with bitter taste. It should be stored at 4°C in the dark to minimize photolytically induced degradation. It melts at 313–315°C. Ciprofloxacin is freely soluble in acetic acid, and slightly soluble in water, methanol, ethanol, or acetone. The octanol/water partition coefficient for ciprofloxacin was reported to be lower than 1Citation63. The pH–solubility profile shows that the dissociation and isoelectric constants for ciprofloxacin include pKa1 = 6.09, pKa2 = 8.62, and pI = 7.14 (isoelectric point, obtained by calculating the average of pKa1 and pKa2). This depicts that ciprofloxacin has two ionizable functional groups, the 6-carboxylic group and the N-4 of the piperazine substituent. Since carboxylic acid is normally a stronger acid than the ammonium group, the first ionization constant pKa1 (6.09) corresponds to the dissociation of a proton from the carboxyl group, while pKa2 (8.62) corresponds to the dissociation of a proton from the N-4 in the piperazinyl groupCitation64,Citation65. At the most physiologically relevant pH, the pKa2 value is 8.25, and significant dissociation of both the 6-carboxylic acid and the basic 10-(1-piperazino) groups occurs, leading to significant fractions of zwitterionic species.
Analytical methodologies
Some important analytical procedures for the determination of ciprofloxacin in pharmaceutical dosage forms and biological fluids have been presented and are discussed in the following text.
Ciprofloxacin content in serum was measured by high performance liquid chromatography (HPLC), in which the samples were analyzed by isocratic reversed phase chromatography on a C-18 column (5 × 100 mm). The mobile phase consisted of acetonitrile–100 mM sodium dihydrogen phosphate (20:80 v/v) adjusted to pH 3.9 with phosphoric acid. Sample elution was monitored with a fluorescence detector, using an excitation wavelength of 280 nm and a 418 nm long pass emission filterCitation66.
A simple and sensitive HPLC method was developed for the determination of enrofloxacin and ciprofloxacin in canine serum and prostatic tissue. Sample preparation consisted of mixing canine serum with a 1:1 dilution of acetonitrile and 0.1 M sodium hydroxide followed by ultrafiltration through a 10,000 molecular mass cut-off filter. Prostatic tissue was sonicated with the same solution prior to ultrafiltration. Separation of these two quinolones in the ultrafiltrate was accomplished by ion-paired liquid chromatography using a reversed-phase analytical column eluted with an acetonitrile–methanol–water solution. Enrofloxacin and ciprofloxacin were detected by a photometric ultraviolet (UV)-visible detector set at 278.6 nm and confirmed by a photodiode array detector operating from 230 to 360 nm. The limits of detection for enrofloxacin and ciprofloxacin were 4 and 2 ng/mL, respectivelyCitation67.
A rapid, accurate, and sensitive method has been developed for the quantitative determination of ciprofloxacin, with high in vitro activity against a wide range of Gram-negative and Gram-positive organisms. A Kromasil 100 C-8 250 mm × 4 mm analytical column was used for analysis. Detection was performed with a variable wavelength UV-visible detector at 275 nm, resulting in limits of detection of 0.01 ng per 20 µL injection for ciprofloxacin. A rectilinear relationship was observed up to 5 ng/µL for ciprofloxacin. Separation was achieved within 10 min. The method was applied to direct determination of the fluoroquinolone ciprofloxacin in human blood serum. Sample pretreatment involved only protein precipitation with acetonitrileCitation68.
Another method of analyzing ciprofloxacin was by using the HPLC technique in which the system consisted of a pump (model LC-600) programmed by a system controller, a UV-visible spectrophotometric detector, and a recorder. The separation was carried out using a Spherisorb C-18 pH stable column (Phase Separations), 15 cm long. The mobile phase consisted of citrate buffer–acetonitrile–methanol (85:10:5 v/v/v) with an apparent pH adjusted to 2.4 with perchloric acid. The flow rate was maintained at 1.5 mL/min, and the column effluent was monitored at 280 nm. Phenacetin was used as the internal standard. Relative standard deviations for within-day and day-to-day precision were within 5%Citation69.
A thin-layer chromatographic (TLC)–densitometric method has been developed for identification and quantification of ciprofloxacin and an ethylenediamine compound, a desfluoro compound, and fluoroquinolonic acid as ciprofloxacin degradation products in pharmaceutical preparations. By using chloroform–methanol–25% ammonia (43:43:14 v/v/v) as the mobile phase and silica gel 60 high-performance TLC plates as the stationary phase, individual constituents were separated, which were subjected to UV densitometric analysis at 330 nm for fluoroquinolonic acid and at 277 nm for the other compounds. This method gave well-developed peaks allowing easy qualitative and quantitative analysis. Dimethylsulfoxide (DMSO)–methanol (1:1) was used to extract drug constituents. This method showed high sensitivity (limit of detection 10–44 ng), a wide linearity range (3–20 µg/mL), and good precision (2.32–6.46% relative standard deviation) and accuracy (percentage recoveries 98.62–101.52%) for individual constituentsCitation70.
The biodistribution and pharmacokinetics of the fluorine-18-labeled [18F]ciprofloxacin in tissue were studied non-invasively in humans by means of positron emission tomography (PET). Special attention was paid to characterize the distribution of [18F]ciprofloxacin to select target tissues. Healthy volunteers (n = 12) were orally pretreated for 5 days with therapeutic doses of unlabeled ciprofloxacin. On the 6th day, subjects received a tracer dose (mean injected amount 700 ± 55 MBq, which contained about 0.6 mg of unlabeled ciprofloxacin) of [18F]ciprofloxacin as an intravenous bolus. Thereafter, PET imaging and venous blood sampling were initiated. Time–radioactivity curves were measured for liver, kidney, lung, heart, spleen, skeletal muscle, and brain tissues for up to 6 h after radiotracer administration. The first application of [18F]ciprofloxacin in humans has demonstrated the safety and utility of the newly developed radiotracer for pharmacokinetic PET imaging of the tissue ciprofloxacin distribution. Two different tissue compartments of radiotracer distribution could be identified. From the first compartment consisting of kidney, heart, and spleen, the radiotracer was washed out relatively quickly (half-lives (t1/2) 68, 57, and 106 min, respectively). The second compartment comprised liver, muscle, and lung tissue, which displayed prolonged radiotracer retention (t1/2 > 130 min). The highest concentrations of radioactivity were measured in the liver and kidney, the main organs of excretion (standardized uptake values (SUVs) 4.9 ± 1.0 and 9.9 ± 4.4, respectively). The brain radioactivity concentrations were very low (<1 kBq/g) and could therefore not be quantified. Transformation of SUVs into absolute concentrations (in micrograms per milliliter) allowed the researchers to relate the concentrations at the target site to the susceptibilities of bacterial pathogens. In this way, the frequent use of ciprofloxacin for the treatment of a variety of infections could be corroboratedCitation17.
Analytical procedures that apply chemiluminescence (CL) methods combined with flow injection have some advantages such as sensitivity, speed, ease of use, and use of simple instrumentation. The flow injection chemiluminescence methods have been described for determination of noscapine, propranolol, and ethamsylate in pharmaceutical preparations. A method for the determination of ciprofloxacin was described based on the enhancement by this compound of the weak CL from peroxynitrous acid. The CL reaction of fluoroquinolones with tris(2,2′-bipyridyl) ruthenium(II) [Ru(bipy)32+] and Ce(IV) were studied in sulfuric acid medium. This method has been used to determine ciprofloxacin hydrochloride in dosage forms and biological fluidsCitation71.
An indirect competitive enzyme-linked immunosorbent assay (ELISA) was developed to detect ciprofloxacin in food-animal edible tissues. Ciprofloxacin was converted by an active ester method into conjugates such as ciprofloxacin–bovine serum albumin (CPFX-BSA) and ciprofloxacin–human serum albumin (CPFX-HSA), which both allowed production of ciprofloxacin-specific rabbit antisera. In the ELISA, CPFX-HSA was coated over the microtiter plate, followed by incubation with standard ciprofloxacin and anti-ciprofloxacin antibody. The indirect competitive ELISA revealed that the antisera have no cross-reactivity with penicillin, gentamicin, neomycin, sulfadiazine, and chlortetracycline. The antisera cross-reacted with enrofloxacin and norfloxacin about 69.8 and 44.6% as much as they did with ciprofloxacin. This ELISA was highly sensitive (0.32 ng/mL) to ciprofloxacin determinations. The coefficients of variation varied from 3.7 to 9.2% over the range of ciprofloxacin concentrations studied. The linear detection range was between 1.6 and 1000 ng/mL. The results suggest that this ELISA is a specific, accurate, and convenient method for the screening of ciprofloxacin residues in food-animal edible tissuesCitation72.
Ciprofloxacin degradation by basidiomycetous fungi was studied by monitoring 14CO2 production from [14C]ciprofloxacin in liquid cultures. Gloeophyllum striatum was used to identify the metabolites formed from ciprofloxacin. After 8 weeks, mycelia had produced 17 and 10% 14CO2 from C-4 and the piperazinyl moiety, respectively, although more than half of ciprofloxacin (applied at 10 ppm) had been transformed into metabolites after 90 h. The structures of 11 metabolites were elucidated by HPLC combined with electrospray ionization mass spectrometry and 1H nuclear magnetic resonance spectroscopyCitation73.
A molecularly imprinted solid-phase extraction procedure was developed for the simultaneous identification of enrofloxacin and its active metabolite, ciprofloxacin, in milk samples. Water-compatible molecularly imprinted polymers synthesized in a water–methanol system showed a high degree of cross-reactivity for enrofloxacin and ciprofloxacin in aqueous environments. The imprinted particles were applied as selective sorbents in a solid-phase extraction process focusing upon complex milk matrices, which allowed the matrix compounds present in milk samples to be removed effectively. The extracts were sufficiently clean for further chromatographic analysis, and no interference originating from the biological matrix was observed. This method is simple and sensitive, and is therefore an alternative tool to the existing HPLC methods for analyzing residual enrofloxacin and ciprofloxacin in biological samplesCitation74.
Capillary zone electrophoresis (CZE) has been elaborated for separation, identification, and determination of ciprofloxacin and its impurities. For the separation, phosphate buffer pH 6.0 was supplemented with 0.075 M pentane-1-sulfonic acid sodium salt. The elaborated method was validated. The selectivity, linearity, limit of detection (LOD), limit of quantification (LOQ), precision, and accuracy of capillary zone electrophoresis were evaluated. The results obtained by CZE were also compared with those obtained by liquid chromatography. Regarding the validation results, the capillary electrophoresis (CE) method fulfills the current European Pharmacopoeia requirements. The evaluated CE method could be applicable to the analysis of different medicinal products containing ciprofloxacinCitation75.
In addition to the analytical methods discussed in the preceding text, several other methodsCitation76–81 have been reported by researchers which provide relevant scientific information for determination of ciprofloxacin.
Pharmacokinetic aspects
Pharmacokinetic characteristics, namely absorption, distribution, metabolism, and elimination of ciprofloxacin, are described below.
Absorption
Ciprofloxacin is readily absorbed, but its complete absorption is generally not achieved following oral administration. The absolute bioavailability of oral ciprofloxacin is within a range of 70–80%, with no substantial loss by first pass metabolismCitation82. An intravenous infusion of 400 mg ciprofloxacin given over 60 min every 12 h has been shown to produce an area under the serum concentration–time curve (AUC) equivalent to that produced by a 500 mg oral dose given every 12 hCitation13. The concentration of ciprofloxacin in brain tissue was 0.87 ± 0.08 mg/kg at a single dose of 200 mg (intravenously, i.v.), suggesting that a dose greater than 200 mg i.v. is required to ensure therapeutic concentrations in brain tissue. Several studies have described the pharmacokinetics of ciprofloxacin in cerebrospinal fluid (CSF). The penetration was excellent when compared with the corresponding serum concentrations, but the absolute CSF concentration was sometimes considered to be subtherapeuticCitation83. The relative constancy of the CSF level suggests a slow flux of ciprofloxacin across the blood–brain barrierCitation84. Although drug–food interactions may prolong the time required to reach maximum plasma concentration (tmax) and thus affect the area under the concentration–time curve, this does not significantly alter the bioavailability of the drugCitation85. The pharmacokinetic profile of ciprofloxacin is summarized in .
Table 2. Pharmacokinetic properties of ciprofloxacinCitation82,Citation86–88.
Distribution
Ciprofloxacin distribution in the tissues is superior to that of many other drugs of its class because there is little binding to plasma proteins. It has good penetration in various fluids and tissues of the body, except the central nervous system (CNS), after oral administration. A remarkable drug level is achieved in kidney, prostate, liver, and lung. Penetration into the cerebrospinal fluid is poor, except when the meninges are inflamed. The urinary drug concentration is higher than the minimum inhibitory concentration, so it is mainly used in urinary tract infectionsCitation9,Citation89. A small fraction of ciprofloxacin passes from the maternal to the fetal compartment, but this fraction is significantly small, indicating that there is a barrier to the transport of fluoroquinolones in the human placentaCitation90. The availability of ciprofloxacin at the interstitial target site is considered to be an important determinant for the effectiveness of antimicrobial therapy and to be the clinical outcome of an infection. In a study, concentrations in the interstitial fluid space at the target sites and AUCs were significantly lower than the corresponding concentrations in plasma for dosages of 400 and 500 mgCitation91.
Metabolism and elimination
Ciprofloxacin differs widely in the degree to which it is metabolized and eliminated in the liver or by renal excretion. The metabolism is inactivating, and is primarily by glucuronide conjugation at the 3-carboxylic group. The piperazine ring is readily metabolized, and this results in decreased antimicrobial activityCitation9. Elimination occurs by both renal and non-renal routes, but the primary route of elimination is via the renal route by glomerulus filtration and tubular secretionCitation86. Thus, in patients with renal impairment and in geriatrics, dosage adjustment is required. The secondary route of excretion is via the liverCitation82. Ciprofloxacin is poorly cleared by both peritoneal dialysis and hemodialysis.
Some change in pharmacokinetics is observed in diseased conditions, although diarrhea or cutaneous infections in human beings do not alter the oral absorption of ciprofloxacin. In the case of bacteremia, serum concentrations remain sufficient for effective treatment of Gram-negative infections, although differences can be observed in different analogs. The metabolism of ciprofloxacin to oxociprofloxacin is reduced in hepatic cirrhosisCitation92,Citation93.
Adverse effects
With a few exceptions, the adverse effects of ciprofloxacin have not too severe consequences when compared to the beneficial features. Toxicity is mild at therapeutic doses, and generally limited to gastrointestinal disturbances such as nausea, vomiting, and diarrheaCitation94. Although resistance to this class of antibiotics in pneumococci is rare, nevertheless some reports indicate that resistance to ciprofloxacin is increasingCitation95,Citation96. Recently, ciprofloxacin has been reported to be an effective therapeutic for anthraxCitation97,Citation98; however, a large dose is needed due to the blood–brain barrier (BBB), and heavy use of ciprofloxacin in such cases has been suspected to induce aseptic meningitisCitation99 and arthritis damageCitation100 and hence, there is a need to increase uptake by the brainCitation101. Ciprofloxacin is still effective as antibiotic prophylaxis for prostate biopsies, but there is an increase in infective complications and resistanceCitation102. Skin photosensitivity reactions have been reported during treatment with ciprofloxacinCitation85,Citation89. The greatest concern with ciprofloxacin use in children is potential bone and joint damageCitation4. Central nervous system effects are the second most common type of adverse events associated with ciprofloxacin therapy. Dizziness, insomnia, and mood alterations have frequently been observed during treatment. Seizures or hallucination have also been describedCitation85,Citation89,Citation94,Citation101. Rarely, anaphylaxis and agranulocytosis have been reportedCitation89. The use of ciprofloxacin to treat multidrug resistant tuberculosis in children has led to the emergence of invasive pneumococcal diseasesCitation103. Incidences of the most common drug-related adverse events occurring with the ciprofloxacin are given in .
Table 3. Incidences of adverse events with ciprofloxacinCitation87.
Drug interactions
Absorption of ciprofloxacin by the oral route of administration is decreased by the presence of antacids containing magnesium, aluminum, and other agents such as sucralfateCitation94. Ciprofloxacin also interacts with multivalent cation-containing products. Exceptionally, ranitidine does not alter the oral absorption of ciprofloxacinCitation4. These interactions of ciprofloxacin with antacids might be hazardous during the treatment of a serious infectionCitation104. A more disturbing interaction occurs between ciprofloxacin and theophylline or other methylxanthines such as caffeine. This interaction, which involves isoenzyme 1A2 of the cytochrome P-450 pathway, appears to be most pronounced, the occurrence of which can raise the serum theophylline concentration to a large extent. The clinical consequence of this interaction requires dose reduction and serum concentration monitoring of the xanthinesCitation105. Elevated serum levels of cyclosporine have been reported with concomitant use of ciprofloxacin. The serum concentration of antineoplastic drugs decreases due to the interaction with ciprofloxacinCitation4. A significant decrease in clearance along with increased serum concentration of ciprofloxacin is observed by interaction with azlocillin, cimetidine, and probenecid. Drugs that cause the urine to become alkaline, such as sodium bicarbonate, carbonic anhydrase inhibitors, and citrates, reduce the solubility of ciprofloxacin and may increase the possibility of crystalluriaCitation4,Citation104–106.
Clinical indications
Ciprofloxacin is effective in a broad range of infections including those difficult to treat. Because of the wide-spectrum bactericidal activity, oral efficacy, and good tolerability, it is being extensively employed for blind therapy of infections, but should not be used for minor cases or where Gram-positive organisms are primarily suspectedCitation4,Citation101,Citation107,Citation108. Some clinical indications of ciprofloxacin are summarized in .
Table 4. Summarized uses of ciprofloxacin in infectious diseasesCitation13,Citation14,Citation109–119.
It is pertinent to mention here that, although effective in clinical trials, ciprofloxacin is not a drug of first choice in the treatment of presumed or confirmed pneumonia secondary to Streptococcus pneumoniaeCitation13,Citation14.
Conclusion
Significant pharmacological interventions of ciprofloxacin and current analytical methodologies for its determination or identification in various formulations and biological fluids have been discussed. Ciprofloxacin has emerged as a promising and efficacious drug, having a myriad spectrum of antibacterial activity. It is emphasized that further scientific and technological advancements in pharmacology, medicinal chemistry, and analytical techniques are required to accurately control the therapeutic and quality profile of this potent medicinal agent.
Acknowledgements
Prof. Om Prakash, Director, Institute of Pharmaceutical Sciences and Prof. R.P. Bajpai, Vice Chancellor, Kurukshetra University, Kurukshetra-136119, India, are duly acknowledged for providing necessary facilities.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dax SL. Antibacterial Chemotherapeutic Agents. London: Blackie Academic & Professional, 1997:298–345.
- Lee C, Ronald AR. Norfloxacin: its potential in clinical practice. Am J Med 1987;82:27–34.
- Patrick GL. An Introduction to Medicinal Chemistry. Oxford: Oxford University Press, 2003:379–435.
- Buck ML. Ciprofloxacin use in children: a review of recent findings. Pediatr Pharm 1998;4:12–18.
- Pandey SN. A Text Book of Medicinal Chemistry (Synthetic and Biochemical Approach). Bhelpur: Mahavir Press, 2003:547–85.
- Torriero AAJ, Ruiz-Diaz JJJ, Salinas E, Marchevsky EJ, Sanz MI, Raba J. Enzymatic rotating biosensor for ciprofloxacin determination. Talanta 2006;69:691–9.
- Guneysel O, Onur O, Erdede M, Dehizbasi A. Trimetoprim/sulfamethoxazole resistance in urinary tract infections. J Emerg Med 2009;36:338–41.
- Karachalios GN, Zografos G, Patrikakos V, Nassopoulou D, Kehagioglou K. Biliary tract infections treated with ciprofloxacin. Infection 2005;21:262–4.
- Abraham DJ. Burger’s Medicinal Chemistry Drug Discovery. Hoboken, NJ: John Wiley & Sons, 2003:582–7.
- Appelbaum PC, Hunter PA. The fluoroquinolone antibacterials: past, present and future perspectives. Int J Antimicrob Agents 2000;16:5–15.
- De Almeida MV, Saraiva MF, De Souza MVN, Da Costa CF, Vincente FRC, Lourenco MCS. Synthesis and antitubercular activity of lipophilic moxifloxacin and gatifloxacin derivatives. Bioorg Med Chem Lett 2007;17:5661–4.
- Cooper JG, Harboe K, Frost SK, Skadberg O. Ciprofloxacin interacts with thyroid replacement therapy. BMJ 2005;330:1002.
- Ciprofloxacin. http://en.wikipedia.org/w/index.php?title=Ciprofloxacin&oldid=287777716. Accessed 6 May 2009.
- Cipro. Bayer response 12 Feb 2009. http://www.fda.gov/cder/foi/label/2009/019537s69,19847s43,19857s50,20780s27lbl.pdf. Accessed 6 May 2009.
- Anquetin G, Greiner J, Mahmoud N, Santillana HM, Gozalbes R, Farhati K, et al. Design, synthesis and activity against Toxoplasma gondii, Plasmodium spp., and Mycobacterium tuberculosis of new 6-fluoroquinolones. Eur J Med Chem 2006;41:1478–93.
- Farhi D, Dupin N. The rise of fluoroquinolone-resistant Neisseria gonorrhoeae. Swiss Med Wkly 2008;138:223–4.
- Brunner M, Langer O, Dobrozemsky G, Muller U, Zeitlinger M, Mitterhauser M, et al. [18F] Ciprofloxacin, a new positron emission tomography tracer for noninvasive assessment of the tissue distribution and pharmacokinetics of ciprofloxacin in humans. Antimicrob Agents Chemother 2004;48:3850–7.
- Kubin R. Safety and efficacy of ciprofloxacin in paedatric patients—review. Infection 1993;21:413–21.
- Wimer SM, Schoonover L, Garrison MW. Levofloxacin: a therapeutic review. Clin Ther 1998;20:1049–68.
- Antonio DD, Piccolomini R, Iacone A, Fioritoni G, Parruti G, Betti S, et al. Comparison of ciprofloxacin, ofloxacin and pefloxacin for the prevention of the bacterial infection in neutropenic patients with haematological malignancies. J Antimicrob Chemother 1994;33:837–44.
- Yamane N, Jones RN, Frei R, Hoban DJ, Pignatari AC, Marco F. Levofloxacin in vitro activity: results from an international comparative study with ofloxacin and ciprofloxacin. J Chemother 1994;6:83–91.
- Joseph E, Dohar MD, Wall M, Peter S, Roland PS, Dupre SJ, et al. Topical ciprofloxacin/dexamethasone found superior to oral amoxicillin/clavulanic acid in acute otitis media with otorrhea. Otolaryngol Head Neck Surg 2005;133:127.
- Brunner H, Zeiler HJ. Oral ciprofloxacin treatment for salmonella typhimurium infection of normal and immunocompromised mice. Antimicrob Agents Chemother 1988;32:57–62.
- Herold C, Ocker M, Ganslmayer M, Gerauer H, Hahn EG, Schuppan D. Ciprofloxacin induces apoptosis and inhibits proliferation of human colorectal carcinoma cells. Br J Cancer 2002;86:443–8.
- Aranha O, Wood DP, Sarkar FH. Ciprofloxacin mediated cell growth inhibition, S/G2-M cell cycle arrest, and apoptosis in a human transitional cell carcinoma of the bladder cell line. Clin Cancer Res 2000;6:891–900.
- Aranha O, Grignon R, Fernandes N, Mcdonnell TJ, Wood DP, Sarkar FH. Suppression of human prostate cancer cell growth by ciprofloxacin is associated with cell cycle arrest and apoptosis. Int J Oncol 2003;22:787–94.
- Pinto AC, Moreira JN, Simoes S. Ciprofloxacin sensitizes hormone-refractory prostate cancer cell lines to doxorubicin and docetaxel treatment on a schedule-dependent manner. Cancer Chemother Pharmacol 2009;64:445–54.
- Bourikas LA, Kolios G, Valatas V, Notas G, Drygiannakis I, Pelagiadis I, et al. Ciprofloxacin decreases survival in HT-29 cells via the induction of TGF-beta1 secretion and enhances the anti-proliferative effect of 5-fluorouracil. Br J Pharmacol 2009;157:362–70.
- Ibukun EA, Eyitayo OA, Nwaka DUC, Aminat OA, Aderemi TA, Oludare FA, et al. Emergence of cross-resistance to fluoroquinolones in Gram-negative isolates from cancer infections in a tertiary hospital in Nigeria. J Am Sci 2008;4:14–20.
- Dekker AW, Rosenberg AM, Verhoef J. Infection prophylaxis in acute leukaemia: a comparison of ciprofloxacin with trimethoprim–sulfamethoxazole and colistin. Anna Intern Med 1987;106:7–11.
- Haron E, Rolston KVI, Cunningham C, Holmes F, Umsawasdi T, Bodey GP. Oral ciprofloxacin therapy for infections in cancer patients. J Antimicrob Chemother 1989;24:955–62.
- Balkhy HH, Memish ZA, Shibl A, Elbashier A, Osoba A. In vitro activity of quinolones against S. pneumoniae, H. influenzae and M. catarrhalis in Saudi Arabia. East Mediterr Health J 2005;11:36–44.
- Aydemir S, Tunger A, Cilli F. In vitro activity of fluoroquinolones against common respiratory pathogens. West Indian Med J 2006;55:9–12.
- Cassell GH, Mekalanos J. Development of antimicrobial agent in the era of new and reemerging infectious diseases and increasing antibiotic resistance. JAMA 2001;285:601–5.
- Rubin J, Walker RD, Blickenstaff K, Jones SB, Zhao S. Antimicrobial resistance and genetic characterization of fluoroquinolone resistance of Pseudomonas aeruginosa isolated from canine infections. Vet Microbiol 2008;131:164–72.
- Cozzarelli NR. DNA gyrase and the supercoiling of DNA. Science 1980;207:953–60.
- Schmidt FJ, Hofmann B, Hansen B, Scheuring S, Luckefahr M, Klootwijk M, et al. Relationship between ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin and moxifloxacin (BAY 12-8039) MICs and mutations in grlA, grlB, gyrA and gyrB in 116 unrelated clinical isolates of Staphylococcus aureus. J Antimicrob Chemother 1998;41:481–4.
- Walters JD, Zhang F, Nakkula RJ. Mechanism of fluoroquinolone transport by human neutrophils. Antimicrob Agents Chemother 1999;43:2710–15.
- Shaharyar M, Ashraf Ali EM, Abdullah MM. Synthesis and antiproliferative activity of 1-[(sub)]-6-fluoro-3-[(sub)]-1,3,4-oxadiazol-2-yl-7-piperazino-1,4-dihydro-4-quinolone derivatives. Med Chem Res 2007;16:292–9.
- Vila J, Sanchez-Cespedes J, Sierra JM, Piqueras M, Nicolas E, Freixas J, et al. Antibacterial evaluation of a collection of norfloxacin and ciprofloxacin derivatives against multiresistant bacteria. Int J Antimicrob Agents 2006;28:19–24.
- Foroumadi A, Emami S, Mehni M, Moshafi MH, Shafiee A. Synthesis and antibacterial activity of N-[2-(5-bromothiophen-2-yl)-2-oxoethyl] and N-[2-(5-bromothiophen-2-yl)-2-oximinoethyl] derivatives of piperazinyl quinolones. Bioorg Med Chem Lett 2005;15:4536–9.
- Foroumadi A, Emami S, Hassanzadeh A, Rajaee M, Sokhanvar K, Moshafi MH, et al. Synthesis and antibacterial activity of N-(5-benzylthio-1,3,4-thiadiazol-2-yl) and N-(5-benzylsulfonyl-1,3,4-thiadiazol-2-yl)piperazinyl quinolone derivatives. Bioorg Med Chem Lett 2005;15:4488–92.
- Foroumadi A, Oboudiat M, Emami S, Karimollah A, Saghaee L, Moshafi MH, et al. Synthesis and antibacterial activity of N-[2-[5-(methylthio)thiophen-2-yl]-2-oxoethyl] and N-[2-[5-(methylthio)thiophen-2-yl]-2-(oxoimino)ethyl] piperazinyl quinolone derivatives. Bioorg Med Chem 2006;14:3421–7.
- Foroumadi A, Ghodsi S, Emami S, Najjari S, Samadi N, Faramarzi MA, et al. Synthesis and antibacterial activity of new fluoroquinolones containing a substituted N-(phenethyl)piperazine moiety. Bioorg Med Chem Lett 2006;16:3499–503.
- Talath S, Gadad AK. Synthesis, antibacterial and antitubercular activities of some 7-[4-(5-amino-1,3,4 thiadiazole-2-sulfonyl)-piperazin-1-yl] fluoroquinolone derivatives. Eur J Med Chem 2006;41:918–24.
- Nieto MJ, Alovero FL, Manzo RH, Maria RM. Benzenesulfonamide analogs of fluoroquinolones: antibacterial activity and QSAR studies. Eur J Med Chem 2005;40:361–9.
- Sriram D, Yogeeswari P, Basha JS, Radhab DR, Nagaraja V. Synthesis and antimycobacterial evaluation of various 7-substituted ciprofloxacin derivatives. Bioorg Med Chem 2005;13:5774–8.
- Kerns RJ, Rybak MJ, Glenn WK, Vaka F, Cha R, Grucz RG, et al. Piperazinyl-linked fluoroquinolone dimmers possessing potent antibacterial activity against drug resistant strains of Staphylococcus aureus. Bioorg Med Chem Lett 2003;13:1745–9.
- Imramovsky A, Polanc S, Vinsova J, Kocevar M, Jampilek J, Reckova Z, et al. A new modification of antitubercular active molecules. Bioorg Med Chem 2007;15:2551–9.
- Srivastava BK, Solanki M, Mishra B, Soni R, Jayadev S, Valani D, et al. Synthesis and antibacterial activity of 4,5,6,7-tetrahydro-thieno[3,2-c]pyridine quinolones. Bioorg Med Chem Lett 2007;17:1924–9.
- German N, Wei P, Kaatz GW, Kerns RJ. Synthesis and evaluation of fluoroquinolone derivatives as substrate-based inhibitors of bacterial efflux pump. Eur J Med Chem 2008;43:2453–63.
- Al-Soud Y, Al-Masoudi NA. A new class of dihaloquinolones bearing N-aldehydoglycosylhydrazides, mercapto-1,2,4-triazole, oxadiazoline and α-amino ester precursors: synthesis and antimicrobial activity. J Braz Chem Soc 2003;14:790–6.
- Zhao YL, Chen YL, Sheu JY, Chen IL, Wang TC, Tzeng CC. Synthesis and antimycobacterial evaluation of certain fluoroquinolone derivatives. Bioorg Med Chem 2005;13:3921–6.
- Yadav P, Joshi YC. Synthesis and spectral studies of novel ciprofloxacin derivatives. Bull Chem Soc Ethiop 2008;22:459–64.
- Sriram D, Yogeeswari P, Senchani G, Banerjee D. Newer tetracycline derivatives: synthesis, anti-HIV, antimycobacterial activities and inhibition of HIV-1 integrase. Bioorg Med Chem Lett 2007;17:2372–5.
- Ye FQ, Ding YM, Chen L, Ye S, Chen ZX. Synthesis and antibacterial activity of ciprofloxacin derivatives. Acta Pharmaceutica Sinica 2005;40:132–135.
- Bani ZT, Kujund N, Kraja MB, Njevac AV, Prodi BK. Molecular structures of new ciprofloxacin derivatives. Journal of Molecular Structure 2002;611:73–81.
- Hu GQ, Wu XK, Wang X, Zhang ZQ, Xie SQ, Huang WL, Zhang HB. Synthesis and antitumor activity of C-3 heterocyclic-substituted fluoroquinolone derivatives (I): ciprofloxacin aminothiodiazole schiff-bases. Acta Pharmaceut Sin 2008;43:1112–15.
- Vazquez JL, Merino S, Domenech O, Berlanga M, Vinas M, Montero MT, et al. Determination of the partition coefficients of a homologous series of ciprofloxacin: influence of the N-4 piperazinyl alkylation on the antimicrobial activity. Int J Pharm 2001;220:53–62.
- Azema J, Guidetti B, Dewelle J, Le Calve B, Mijatovic T, Korolyov A, et al. 7-((4-Substituted)piperazin-1-yl) derivatives of ciprofloxacin: synthesis and in vitro biological evaluation as potential antitumor agents. Bioorg Med Chem 2009;17:5396–407.
- Siddiqui R, Najma S, Khalid MK, Nosheen A, Muhammad A, Saeed A. Effects of skeletal modifications of ciprofloxacin on antibacterial, antifungal and cytotoxic activities. J Chin Clin Med 2007;2:188–95.
- Sharma PC, Jain S. Synthesis and antibacterial activity of certain novel 1-cyclopropyl-6-fluoro-1,4-dihydro-7-4-substituted-piperazin-1-yl-4-oxoquinoline-3-carboxylates. Acta Pharm Sci 2008;50:35–40.
- Hirai K, Aoyama H, Irikura T, Iyobe S, Mitsuhashi S. Differences in susceptibility to quinolones of outer mermbrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother 1986;29:535–8.
- Melo MJP, Varanda FR, Dohrn R, Marrucho IM. Solubility of ciprofloxacin and moxifloxacin in different solvents: the effect of the HCl group. Enpromer 2005;1–5. http://www.enpromer2005.eq.ufrj.br/nukleo/pdfs/0984_solubility_of_antibiotics.pdf. Accessed 26 June 2009.
- Varanda F, De Melo MJP, Caco AI, Dohrn R, Makrydaki FA, Voutsas E, et al. Solubility of antibiotics in different solvents. 1. Hydrochloride forms of tetracycline, moxifloxacin, and ciprofloxacin. Ind Eng Chem Res 2006;45:6368–74.
- Lavda M, Clausnitzer CE, Walters JD. Distribution of systemic ciprofloxacin and doxycycline to gingival and gingival crevicular fluid. J Periodontol 2004;75:1663–7.
- Tyczkowska K, Hedeen KM, Aucoin DP, Aronson AL. High-performance liquid chromatographic method for the simultaneous determination of enrofloxacin and its primary metabolite ciprofloxacin in canine serum and prostatic tissue. J Chromatogr 1989;493:337–46.
- Samanidou VF, Demetriou CE, Papadoyannis IN. Direct determination of four fluoroquinolones, enoxacin, norfloxacin, ofloxacin, and ciprofloxacin, in pharmaceuticals and blood serum by HPLC. Anal Bioanal Chem 2003;375:623–9.
- Pham HH, Luo P, Genin F, Dash AK. Synthesis and characterization of hydroxyapatite-ciprofloxacin delivery systems by precipitation and spray drying technique. AAPS PharmSciTech 2002;3:1–9.
- Krzek J, Hubicka U, Szczepaniczyk J. High-performance thin-layer chromatography with densitometry for the determination of ciprofloxacin and impurities in drugs. J AOAC Int 2005;88:1530–6.
- Han-Wen S, Li-Qing L, Xue-Yan C, Hong-Mei S, Yun-Kai L. Determination of norfloxacin by flow injection analysis with chemiluminescence detection. Can J Anal Sci Spectrosc 2006;51:100–7.
- Duan J, Yuan Z. Development of an indirect competitive ELISA for ciprofloxacin residues in food animal edible tissues. J Agric Food Chem 2001;49:1087–9.
- Heinz-Georg W, Stadler M, Hans-Volker T, Dalhoff A, Karl W. Degradation of ciprofloxacin by basidiomycetes and identification of metabolites generated by the brown rot fungus Gloeophyllum striatum. Appl Environ Microbiol 1999;65:1556–63.
- Yan H, Tian M, Row KH. Determination of enrofloxacin and ciprofloxacin in milk using molecularly imprinted solid-phase extraction. J Sep Sci 2008;31:3015–20.
- Michalska K, Pajchel G, Tyski S. Determination of ciprofloxacin and its impurities by capillary zone electrophoresis. J Chromatogr A 2004;1051:267–72.
- Kassab NM, Singh AK, Kedor-Hackmam ERM, Santoro MIRM. Quantitative determination of ciprofloxacin and norfloxacin in pharmaceutical preparations by high performance liquid chromatography. Braz J Pharm Sci 2005;41:507–13.
- Xiao JB, Yang CS, Ren FL, Jiang XY, Xu M. Rapid determination of ciprofloxacin lactate in drugs by the Rayleigh light scattering technique. Meas Sci Technol 2007;18:859–66.
- Ovando HG, Gorla1 N, Weyers A, Ugnia L, Magnoli A. Simultaneous quantification of ciprofloxacin, enrofloxacin and balofloxacin in broiler chicken muscle. Arch Med Vet 2004;1:93–8.
- Joos B, Ledergerber B, Flepp M, Bettex JD, Luthy R, Siegenthaler W. Comparision of high-pressure liquid chromatography and bioassay for determination of ciprofloxacin in serum and urine. Antimicrob Agents Chemother 1985;27:353–6.
- Nix DE, De Vito JM, Schentag JJ. Liquid-chromatographic determination of ciprofloxacin in serum and urine. Clin Chem 1985;31:684–6.
- Martin BS, Cornejo J, Iraguen D, Hidalgo H, Anadon A. Depletion study of enrofloxacin and its metabolite ciprofloxacin in edible tissues and feathers of white leghorn hens by liquid chromatography coupled with tandem mass spectrometry. J Food Protect 2007;70:1952–7.
- Egle JL. Principles of Pharmacology: Basic Concepts and Clinical Applications. New York: Chapman & Hall, 1996:1339–50.
- Leone M, Sampol-Manos E, Santelli D, Grabowski S, Alliez B, Durand A, et al. Brain tissue penetration of ciprofloxacin following a single intravenous dose. J Antimicrob Chemother 2002;50:607–9.
- Lipman J, Allworth A, Wallis SC. Cerebrospinal fluid penetration of high doses of intravenous ciprofloxacin in meningitis. Clin Infect Dis 2000;31:1131–3.
- Blondeau JM. Expanded activity and utility of the new fluoroquinolones: a review. Clin Ther 1999;21:3–39.
- Brunton LL, Lazo JS, Parker KL. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw-Hill, 2006:1111–26.
- Sharma PC, Saneja A, Jain S. Norfloxacin: a therapeutic review. Int J Chem Sci 2008;6:1702–13.
- Cheng D, Xu WR, Liu CX. Relationship of quantitative structure and pharmacokinetics in fluoroquinolone antibacterials. World J Gastroenterol 2007;13:2496–503.
- Scholar EM. Fluoroquinolones: past, present and future of a novel group of antibacterials. Am J Pharm Educ 2003;66:164–72.
- Polachek H, Holcberg G, Sapir G, Tsadkin-Tamir M, Polachek J, Katz M, et al. Transfer of ciprofloxacin, ofloxacin and levofloxacin across the perfused human placenta in vitro. Eur J Obstet Gynecol Reprod Biol 2005;122:61–5.
- Brunner M, Stab H, Moller JG, Schrolnberger C, Erovic B, Hollenstein U, et al. Target site concentrations of ciprofloxacin after single intravenous and oral doses. Antimicrob Agents Chemother 2002;46:3724–30.
- Wolfson JS, Hooper DC. Pharmacokinetics of quinolones: newer aspects. Eur J Clin Microbiol Infect Dis 1991;10:267–74.
- Apley MD, Upson DW. Lung tissue concentrations and plasma pharmacokinetics of danofloxacin in calves with acute pneumonia. Am J Vet Res 1993;54:937–43.
- Sarkozy G. Quinolones: a class of antimicrobial agents. Vet Med Czech 2001;46:257–74.
- Ho PL, Que TL, Tsang DNC, Ng TK, Chow KH, Seto WH. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob Agents Chemother 1999;43:1310–13.
- Chen DK, McGeer A, De Azaevedo JC, Low DE. Fluoroquinolone resistance in Streptococcus pneumoniae. N Engl J Med 1999;341:233–9.
- Liu L, Guo K, Lu J, Venkatraman SS, Luo D, Chey NK, et al. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG–TAT for drug delivery across the blood–brain barrier. Biomaterials 2008;29:1509–17.
- Brook I. The prophylaxis and treatment of anthrax. J Antimicrob Agents 2002;20:320–5.
- Kepa L, Oczko-Grzesik B, Stolarz W, Sobala SB. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci 2005;12:562–4.
- Mao Z, Ma L, Gao C, Shen J. Preformed microcapsules for loading and sustained release of ciprofloxacin hydrochloride. J Control Release 2005;104:193–202.
- Oliphant CM, Green GM. Quinolones: a comprehensive review. Am Fam Physician 2002;65:455–64.
- Feliciano J, Teper E, Ferrandino M, Macchia RJ, Blank W, Grunberger I, et al. The incidence of fluoroquinolone resistant infections after prostate biopsy are fluoroquinolones still effective prophylaxis. J Urol 2008;179:952–5; discussion 955.
- Gottberg AV, Klugman KP, Cohen C, Wolter N, De Gouveia L, Du Plessi M, et al. Emergence of levofloxacin-non-susceptible Streptococcus pneumoniae and treatment for multidrug-resistant tuberculosis in children in South Africa: a cohort observational surveillance study. Lancet 2008;371:1108–13.
- Dollery C. Therapeutic Drugs. Edinburgh: Churchill Livingstone, 1999:N137–40.
- Efthymiopoulos C, Blum B, Maroli A, Bramer SL. Theophylline and warfarin interaction studies with grepafloxacin. Clin Pharmacokinet 1997;33:39–46.
- Neu HC, Kumada T, Chin NX, Mandell W. The post-antimicrobial suppressive effect of quinolone agents. Drugs Exp Clin Res 1987;13:63–7.
- Lubasch A, Keller I, Borner K, Koeppe P, Lode H. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob Agents Chemother 2000;44:2600–3.
- Boy D, Well M, Kinzig SM, Sorgel F, Ankel FD, Naber KG. Urinary bactericidal activity, urinary excretion and plasma concentrations of gatifloxacin (400 mg) versus ciprofloxacin (500 mg) in healthy volunteers after a single oral dose. Int J Antimicrob Agents 2004;23:6–16.
- Drug card for ciprofloxacin (DB00537). Drug bank 19 Feb 2009. http://www.drugbank.ca/drugs/DB00537. Accessed 6 May 2009.
- Karachalios GN, Zografos G, Patrikakos V, Nassopoulou D, Kehagioglou K. Biliary tract infections treated with ciprofloxacin. Infection 2005;21:262–4.
- Launay E, Joram N, Jacqueline C, Miegeville AF, Caillon J, Potel G, et al. Efficacy of ciprofloxacin in an experimental model of Escherichia coli chorioamnionitis in rabbits. Antimicrob Agents Chemother 2009;53:1624–7.
- Capoor MR, Rawat D, Nair D, Hasan AS, Deb M, Aggarwal P, et al. In-vitro activity of azithromycin, newer quinolones and cephalosporins in ciprofloxacin-resistant Salmonella causing enteric fever. J Med Microb 2007;56:1490–4.
- Wexler HM, Molitoris E, Molitoris D, Finegold SM. In vitro activity of levofloxacin against a selected group of anaerobic bacteria isolated from skin and soft tissue infections. Antimicrob Agents Chemother 1998;42:984–6.
- Raina R, Prawez S, Dimitrova DJ, Pankaj NK, Verma PK. Disposition kinetics and urinary excretion of ciprofloxacin in goats following single intravenous administration. J Vet Sci 2008;9:241–5.
- Fontan MP, Rosales M, Fernandez F, Moncalian J, Fernandez CR, Alonso A, et al. Ciprofloxacin in the treatment of Gram positive bacterial peritonitis in patients undergoing CAPD. Perit Dial Int 1991;11:233–6.
- Zeiler HJ, Grohe K. The in vitro and in vivo activity of ciprofloxacin. Eur J Clin Microbiol 1984;3:393–43.
- Vali A, Faghihi G, Zaghian N, Koosha M. The efficacy of topical solution of 0.3% ciprofloxacin in treatment of mild to moderate acne vulgaris. Iranian Red Cresc Med J 2009;11:23–27.
- Bakker-Woudenberg IAJM, Ten Kate MT, Guo L, Working P, Mouyon JW. Improved efficacy of ciprofloxacin administered in polyethylene glycol-coated liposomes for treatment of Klebsiella pneumoniae pneumonia in rats. Antimicrob Agents Chemother 2001;45:1487–92.
- Sheng WH, Wang JT, Chen YC, Chang SC, Luh KT. In vitro activity of moxifloxacin against common clinical bacterial isolates in Taiwan. J Microbiol Immunol Infect 2001;34:178–84.